Percutaneous Removal of Benign Breast Lesions with an Ultrasound-guided Vacuum-assisted System: Influence Factors in the Hematoma Formation△
Hui-ping Huo, Wen-bo Wan, Zhi-li Wang, Hong-fei Li, and Jun-lai Li*
1Department of South Building Ultrasound,2Department of Ultrasound, Chinese People’s Liberation Army General Hospital, Beijing 100853, China
Percutaneous Removal of Benign Breast Lesions with an Ultrasound-guided Vacuum-assisted System: Influence Factors in the Hematoma Formation△
Hui-ping Huo1, Wen-bo Wan1, Zhi-li Wang1, Hong-fei Li2, and Jun-lai Li1*
1Department of South Building Ultrasound,2Department of Ultrasound, Chinese People’s Liberation Army General Hospital, Beijing 100853, China
benign breast lesion; ultrasound-guided vacuum-assisted system; hematoma;
influence factor
Objective To explore the influence factors in hematoma formation after removing benign breast lesions with an ultrasound-guided vacuum-assisted system.
Methods A total of 232 females with 312 benign breast masses received excisional biopsy with ultrasoundguided vacuum-assisted system. The pathology of patients, results of hematoma development and outcome, influence factors for hematoma occurrence (nodule size, nodule location, number of nodule, breast shape, menstrual period, efficacy time of bandage, and application of hemostatic agents during the procedure) were recorded.
Results Pathologic examination revealed fibroadenomas in 138 lesions, fibroadenosis in 127 lesions, intraductal papillomas in 39 lesions, inflammatory change in 4 lesions, retention cyst of the breast in 3 lesions, and benign phyllodes tumor in 1 lesion. Thirty hematomas were observed in patients (9.6%). Finally, 97.0% hematomas were absorbed completely within 6 months follow-up. The incidence rates of hematoma were increased by 24.7%, 10.0%, 63.2%, 13.9% in the nodule diameter larger or equal to 25 mm group, removal of larger or equal to two nodules once time from one patient group, menstrual period group, and larger and loose breast group, respectively (all P<0.05). However, the incidences were decreased by 60.6% in the bandage performed for 12-24 hours or beyond 24 hours group (P<0.05). The multiple logistic regression models revealed that nodule size (χ2=15.227, P<0.001), number of nodule (χ2=7.767, P=0.005), menstrual period (χ2=24.530, P<0.001), and breast shape (χ2=9.559, P=0.002) were independent risk factors associated with hematoma occurrence, but efficacy time of bandage was a protective factor associated with hematoma occurrence.
Conclusion The occurrence of hematoma after the minimally invasive operation was associated with nodule size, number of nodule, menstrual period, breast shape, and efficacy time of bandage.T HE epidemiologic statistics showed that 60% of all women would develop benign breast diseases during their adult lives.1Notably, benign breast mass is the most common benign breast diseases, including fibroadenoma and fibrocystic disease. Although benign breast diseases have little impact on occurrence of breast cancer, it increases psychological burden of patients and decreases quality of life. Therefore, it is necessary to pay special attention to diagnosis and treatment of benign breast mass. Furthermore, treatment of benign breast mass involves in technique of minimally invasive breast biopsy system, including vacuum-assisted percutaneous excisional biopsy, endoscopic lumpectomy, cryotherapy, et al.2-5As we known, ultrasonography (US) has been the primary device of diagnosis as well as treatment of benign breast mass. To a large extent, the widespread application of US is ascribed to its accuracy and noninvasive.6Combined with the advances in US technology, more and more minimally invasive operation has been explored. During the recent years, a growing interest has been placed on the application of ultrasound-guided vacuum-assisted hand-held device for removal of benign breast lesions.7,8Clinical data obtained from human study demonstrate the efficacy of ultrasoundguided vacuum assisted systems in excisional biopsy of benign breast lesions.9-11In addition, the results of long-term follow-up of ultrasound-guided vacuum-assisted systems excisions are comparable to conventional methods.12One of major complications after minimally invasive operation is hematoma, which involves in limiting the utility of ultrasound-guided vacuum-assisted systems in benign breast mass. However, the concrete influence factors and mechanism of hematoma induced by vacuum-assisted systems are unknown.
Chin Med Sci J 2016; 31(1):31-36
In the present study, we evaluated the experiences of ultrasound-guided vacuum-assisted systems in treating benign breast masses, detected the complete excision and hematoma rates of ultrasound guided vacuum-assisted systems. Importantly, we have designed to investigate the influence factors for hematoma formation after the procedure.
PATIENTS AND METHODS
Patients
In this prospective study for exploring the influence factors in formation hematoma, a total of 232 female patients with benign breast mass received percutaneous excisional biopsy with ultrasound-guided vacuum-assisted system were selected in the center of Chinese People’s Liberation Army General Hospital from May 2013 to May 2015. The present study received approval from our institution’s ethics committee. Furthermore, all patients enrolled into the study were required to sign the informed consent document before the research. Among them, 312 benign breast lesions from 232 patients were detected and classified by ultrasound on the basis of the American College of Radiology Breast Imaging Reporting and Data System (ACR BI-RADS).13The mean largest diameter of the lesions was 1.7 ± 1.6 (range 0.4-3.0) cm. Except for category 2 and 3 lesions, category 4a or 4b lesions were further confirmed to be benign by core needle biopsy.
Ultrasound examination and imaging characteristics
The 7-gauge EnCor® system (SenoRx, Allso Viejo, CA) was used for ultrasound-guided vacuum-assisted system. Siemens Acuson Sequoia 512 ultrasound system (Acuson, Mountain View, CA) with a 15L8w linear array probe was used.
The involvement of ultrasound in examining lesions was performed by one doctor who specialized in breast imaging. The purpose of a careful breast ultrasound exam before excisional biopsy is to assess location and diameter of lesions. Furthermore, insertion site for probe to the lesions was definite, and the site was decided according to the shortest distance from lesions, including surrounding of areola, edge of breast or armpit.
Ultrasound-guided vacuum-assisted breast excisional biopsy procedure
The protocol for ultrasound-guided vacuum-assisted breast biopsy has been previously described in detail.2Firstly, anesthesia was performed before biopsy procedure. A total of 10 ml 1% lidocaine containing a 1:100 000 mixture of epinephrine was administrated into the cutaneous layer, mass around lesions and insertion course of probe. It is important to increase the safe distance between the mass and lesions. Secondly, probe was introduced into the breast through an about 5-mm skin incision, and then probe approached to lesions under real-time ultrasound visualization guidance. For avoiding damage to surrounding vessels and masses located near the lesions, probe was injected into the bottom or the side of lesions, and tubular sampling groove was pointed to lesions. The specimens were automatically sucked into tubular sampling groove through negative pressure. To remove the lesions completely, angle of probe was adjusted and sufficient surrounding normal breast tissue was extracted by rotating probe. The size of lesion taken out was about (0.3-0.5 cm)×(1.8-2.0 cm) for each time. The completion of lesions excision was determined by real-time ultrasound guidance. After blood was removed by vacuum-assistedhand-held device, manual compression to the skin incision site of lesions was performed for approximately 10-15 minutes to stop bleeding. No residual lesions and obvious blood were existed after an ultrasound examination, and then skin incision was dealt with a sterile elastic bandage for 24-48 hours. The pathological diagnosis was made according to postoperative paraffin pathological examination.
Follow-up visits
After 24-48 hours of the procedure, patients were required to have the first follow-up visit with ultrasound examination to assess the extent of hematoma development. Thereafter, follow-up visits were occurred at 1, 3, and 6 months after the procedure with ultrasound examination. In addition, the potential variables including nodule size, nodule location, number of nodule, breast shape, menstrual period, efficacy time of bandage, and application of hemostatic agents during operation were evaluated.
Statistical analysis
Statistical analyses were performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were generated for all variables. Continuous normal distribution variables were shown as mean ± standard deviation (SD), and categorical variables were shown in their occurrence frequency. Chi-square tests were performed to extract the significant risk factors associated with hematoma occurrence. A multiple logistic regression analysis with backward stepwise selection method was used to identify independent risk factors that were associated with hematoma occurrence. P<0.05 was considered statistically significant.
RESULTS
Pathological results
The age of 232 patients was 15-57 years (mean age 29.4±5.4 years). The mean largest diameter of the 312 benign breast lesions was 1.7±1.6 cm (range 0.4-3.0 cm). Pathological examination revealed fibroadenomas in 138 (44.2%) lesions, fibroadenosis in 127 (40.7%) lesions, intraductal papillomas in 39 (12.5%) lesions, inflammatory change in 4 (1.3%) lesions, retention cyst of breast in 3 (1.0%) lesions, and benign phyllodes tumor in 1 (0.3%) lesion.
Hematoma development and outcome
After the first follow-up visit, 30 hematomas which mean diameter was not less than 10 mm were observed in patients (9.6%). Among these hematomas, the diameters of 3 hematomas were greater or equal to 30 mm. At the second follow-up visit, 21 hematomas were absorbed completely, and the rest 7 hematomas were also disappeared in 3 months of follow-up. After 6 months of the procedure, only 1 residual hematoma was seen, the size was about 0.9 cm×0.8 cm (Table 1).
Influence factors for hematoma occurrence
The major influence factors for the procedure induced hematoma as follows: (1) Nodule size, the incidence rate of hematoma in the nodule maximum diameter greater or equal to 25 mm group was significantly increased by 24.7% compared with the nodule maximum diameter less than 25 mm group (P<0.001); (2) Nodule location, the incidence rate of hematoma in the nodule located into the areola depth or the side of glands group was obviously increased by 7.2% compared with the nodule located other parts of the areola group (P=0.091); (3) Number of nodule, the incidence rate of hematoma of the removal of greater or equal to two nodules once time from one patient who had more than two lesions was markedly increased by 10.0% compared with the excision of single nodule from one patient (P=0.005); (4) Menstrual period, compared with the non-menstrual period, the incidence rate of hematoma was significantly increased by 63.2% in the menstrual period (P<0.001); (5) Breast shape, the incidence rate of hematoma was obviously increased by 13.9% in the larger and loose breast group compared to the small and compact breast group (P=0.002); (6) Efficacy time of bandage, the incidence rate of hematoma was markedly decreased by 60.6% in the bandage performed for 12-24 hours or beyond 24 hours group compared to the bandage performed less than 12 hours group (P<0.001); (7) Application of hemostatic agents during operation, compared with the non-drug group, the incidence rate of hematoma was decreased by 1.1% in the application of hemostatic agents group (P=0.951) (Table 2).
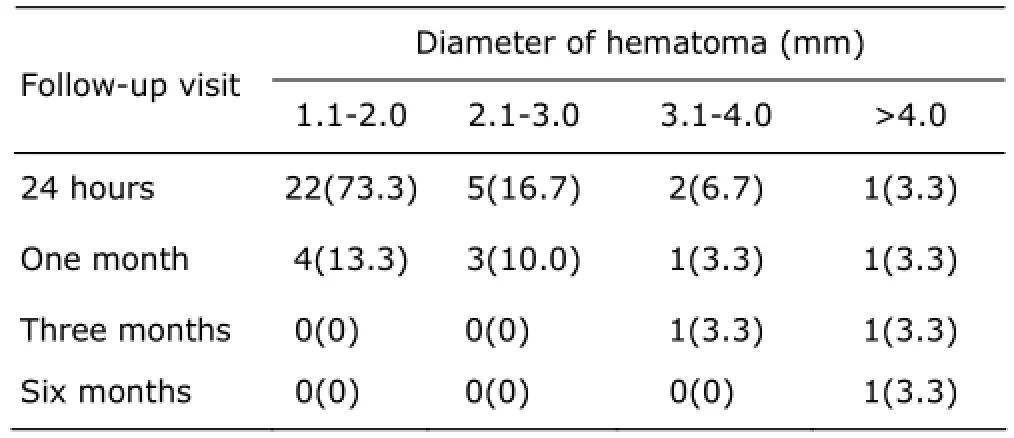
Table 1. During the indicated follow-up period, the number of residual hematoma with different diameters [n(%), n=30]

Table 2. Univariate anlaysis of influence factors associated with hematoma occurrence
The multiple logistic regression models revealed that the nodule size [odd ratio (OR)=18.78, 95% confidence interval (CI): 4.84-72.82, P<0.001], number of nodule (OR=4.85, 95% CI: 1.16-20.26, P=0.030), menstrual period (OR=130.63, 95% CI: 143.5-1189.38, P<0.001) and breast shape (OR=7.24, 95% CI: 1.92-27.31, P=0.003) were independent risk factors associated with hematoma occurrence. Specially, the efficacy time of bandage was a protective factor associated with hematoma occurrence (OR=0.012, 95% CI: 0.003-0.054, P<0.001).
DISCUSSION
With the great progress in the diagnosis and treatment of benign breast diseases, more and more attention has been placed on the use of minimally invasive breast biopsy system.4One kind of remarkable equipment is vacuumassisted system with ultrasound guidance. Ultrasound guided vacuum-assisted system was employed for removal of benign breast lesions during recent years.14-16Notably, the previous studies have confirmed that ultrasound guided vacuum-assisted system is an effective, safe method with satisfactory cosmetic outcomes for removal of benign breast lesions.17-19However, one coin has two sides. Potential failures of ultrasound guided vacuum-assisted system were mainly due to complications after operation, which limit its widespread application in managing breast diseases. And the major complication of the operation is hematoma. The present study showed that the incidence rate of hematoma was 9.6%, which is similar to the result of Vargas et al14and less than the incidence rate reported by Povoski et al.20Meanwhile, we further investigated influence factors for hematoma formation.
We demonstrated that multiple factors, including the nodule size, nodule number, breast shape, menstrual period, and efficacy time of bandage during the operation, were involved in hematoma formation after the procedure. The nodule size, number of nodule, menstrual period, and breast shape were independent risk factors associated with hematoma occurrence. Specially, efficacy time of bandage was a protective factor associated with hematoma occurrence. For nodule size, with the increase in nodule size, bleeding area could be enlarged as a result of increase in operation residual cavity as well as abundance of surrounding blood vessels adjunct to the lesions, and then leading to the occurrence of hematoma. About nodule location, the arteries suppling blood to the areola area or the sides of glands were more vulnerable to be damaged during the operation. For number of nodule, the more nodules, the more needle tract and residual cavity lead to insufficient oppression, which increase the chance of hematoma with the decrease in the blood viscosity and clotting Moreover, we advice patients to avoid menstrual period to undergo mass excision as far as possible. Inaddition, there were two factors involved in formation of hematoma for patients with larger and loose breast; on the one hand, inadequate oppression to operative region due to cushion of the gland, on the other hand, insufficient time for compression as result of the greater degree on gland movement. As the results showed in this study, the incidence rate of hematoma was markedly reduced with extension of bandage time.21Understanding of the factors influencing hematoma formation is beneficial to master operation indications.
In the current study, complete absorption of hematomas was shown to occur in 97.0% of patients within 6 months after surgery with the minimally invasive breast biopsy system, which demonstrated efficiency and safety of the system.
To explore the extent and range of removal under the ultrasound guidance, a total of 312 benign breast lesions were immediately monitored from all directions by real-time ultrasound imaging. To prevent residual tissue and recurrence, the mean diameter of excision could be larger ranged from 1 to 2 mm than that of the lesions. Our result showed that there were not only no residual tissue and recurrence, but also no deformation and subsidence of breast formed within 6 months after the procedure. It is speculated that the range of excision plays a crucial role in formation of hematoma. In addition, the accuracy of ultrasonic resolution was considered an important element for distinguishing the normal from diseased tissues. Meanwhile, the less duration of procedure also lowered the chance of hematoma.
In conclusion, the ultrasound guided vacuum-assisted systems is feasible, safe, high successful for removal biopsy of benign breast lesions. Importantly, the incidence of hematoma was associated with nodule size, number of nodule, menstrual period, breast shape, and efficacy time of bandage.
REFERENCES
1. Slanetz PJ, Wu SP, Mendel JB. Percutaneous excision: a viable alternative to manage benign breast lesions. Can Assoc Radiol J 2011; 62:265-71.
2. Wang ZL, Liu G, Huang Y, et al. Percutaneous excisional biopsy of clinically benign breast lesions with vacuum-assisted system: comparison of three devices. Eur J Radiol 2012; 81:725-30.
3. Xu Y, Ming J, Zhou Y, et al. Mammotome-assisted endoscopic breast-conserving surgery: a novel technique for early-stage breast cancer. World J Surg Oncol 2014; 12: 99.
4. Lakoma A, Kim ES. Minimally invasive surgical management of benign breast lesions. Gland Surg 2014; 3:142-8.
5. Otterson MF, Redlich PN, McDonald A, et al. Sequelae of cryotherapy in breast tissue. Cryobiology 2003; 47:174-8.
6. Hooley RJ, Scoutt LM, Philpotts LE. Breast ultrasonography: state of the art. Radiology 2013; 268:642-59.
7. Fine RE, Boyd BA, Whitworth PW, et al. Percutaneous removal of benign breast masses using a vacuum-assisted hand-held device with ultrasound guidance. Am J Surg 2002; 184:332-6.
8. Yao F, Li J, Wan Y, et al. Sonographically guided vacuumassisted breast biopsy for complete excision of presumed benign breast lesions. J Ultrasound Med 2012; 31:1951-7.
9. Debi U, Thulkar S, Sharma S, et al. Role of directional vacuum assisted breast biopsy in previously equivocal biopsies for breast masses suspicious for malignancy. Malays J Pathol 2015; 37:25-33.
10. Ko KH, Jung HK, Youk JH, et al. Potential application of ultrasound-guided vacuum-assisted excision (US-VAE) for well-selected intraductal papillomas of the breast: single-institutional experiences. Ann Surg Oncol 2012; 19:908-13.
11. Wang ZL, Liu G, Li JL, et al. Sonographically guided percutaneous excision of clinically benign breast masses. J Clin Ultrasound 2011; 39:1-5.
12. Yom CK, Moon BI, Choe KJ, et al. Long-term results after excision of breast mass using a vacuum-assisted biopsy device. ANZ J Surg 2009; 79:794-8.
13. Chen SC, Yang HR, Hwang TL, et al. Intraoperative ultrasonographically guided excisional biopsy or vacuum-assisted core needle biopsy for nonpalpable breast lesions. Ann Surg 2003; 238:738-42.
14. Vargas HI, Vargas MP, Gonzalez K, et al. Percutaneous excisional biopsy of palpable breast masses under ultrasound visualization. Breast J 2006; 12:S218-22.
15. Krainick-Strobel U, Huber B, Majer I, et al. Complete extirpation of benign breast lesions with an ultrasound-guided vacuum biopsy system. Ultrasound Obstet Gynecol 2007; 29:342-6.
16. Wang ZL, Li JL, Su L, et al. An evaluation of a 10-gauge vacuum-assisted system for ultrasound-guided excision of clinically benign breast lesions. Breast 2009; 18:192-6.
17. Sperber F, Blank A, Metser U, et al. Diagnosis and treatment of breast fibroadenomas by ultrasound-guided vacuum-assisted biopsy. Arch Surg 2003; 138:796-800.
18. Mathew J, Crawford DJ, Lwin M, et al. Ultrasound-guided, vacuum-assisted excision in the diagnosis and treatment of clinically benign breast lesions. Ann R Coll Surg Engl 2007; 89:494-6.
19. Grady I, Gorsuch H, Wilburn-Bailey S. Long-term outcomeof benign fibroadenomas treated by ultrasound-guided percutaneous excision. Breast J 2008; 14:275-8.
20. Povoski SP, Jimenez RE. A comprehensive evaluation of the 8-gauge vacuum-assisted Mammotome(R) system for ultrasound-guided diagnostic biopsy and selective excision of breast lesions. World J Surg Oncol 2007; 5:83.
21. Jiang Y, Lan H, Ye Q, et al. Mammotome biopsy system for the resection of breast lesions: clinical experience in two high-volume teaching hospitals. Exp Ther Med 2013; 6:759-64.
for publication January 12, 2016.
*Corresponding author Tel/Fax: 86-10-66876050, E-mail: li_jl@yeah.net
△Supported by the National Major Scientific Equipment Special Project (2012YQ16020304).
 Chinese Medical Sciences Journal2016年1期
Chinese Medical Sciences Journal2016年1期
- Chinese Medical Sciences Journal的其它文章
- Pathology Verified Concomitant Papillary Thyroid Carcinoma in the Sonographically Suspected Thyroid Lymphoma: A Case Report△
- Life-threatening Spontaneous Retroperitoneal Haemorrhage: Role of Multidetector CT-angiography for the Emergency Management
- Respiratory and Cardiac Characteristics of ICU Patients Aged 90 Years and Older: A Report of 12 Cases
- Establish Albumin-creatinine Ratio Reference Value of Adults in the Rural Area of Hebei Province△
- Positive Rate of Different Hepatitis B Virus Serological Markers in Peking Union Medical College Hospital, a General Tertiary Hospital in Beijing△
- Association Between Geranylgeranyl Pyrophosphate Synthase Gene Polymorphisms and Bone Phenotypes and Response to Alendronate Treatment in Chinese Osteoporotic Women△
