Establish Albumin-creatinine Ratio Reference Value of Adults in the Rural Area of Hebei Province△
Qiao-jing Liang, Wen Huang*, Guo-juan Zhang, and Ning-li Wang*
1Department of Nephrology,2Beijing Institute of Ophthalmology, Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University, Beijing 100730, China
Establish Albumin-creatinine Ratio Reference Value of Adults in the Rural Area of Hebei Province△
Qiao-jing Liang1†, Wen Huang1†*, Guo-juan Zhang1, and Ning-li Wang2*
1Department of Nephrology,2Beijing Institute of Ophthalmology, Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University, Beijing 100730, China
albumin-creatinine ratio; albuminuria; chronic kidney disease
Objective To establish albumin-creatinine ratio (ACR) reference value of the rural population in Hebei province.
Methods This study enrolled 5154 participants. By excluding subjects with hypertension, diabetes, dyslipidemia, cardiovascular and cerebrovascular diseases, kidney diseases, and overweight condition, as well as those with an estimated glomerular filtration rate (eGFR)<60 ml/(min·1.73 m2), apparently healthy subjects (1168) were selected. Urine albumin was measured by using the immunoturbidimetic method, serum creatinine was measured by using Jaffe’s kinetic method on a morning spot-urine sample, and ACR was calculated. The 95th percentile of ACR in the healthy subjects was used as the normal upper limit.
Results The normal upper limit of ACR was 28.71 mg/g (3.25 mg/mmol) for males and 31.85 mg/g (3.60 mg/mmol) for females. Based on this ACR reference value, the age-gender standardized prevalence of albuminuria in the rural areas of Hebei province was 12.9%.
Conclusion The ACR reference value in the rural of Hebei province is higher than that of the Western population.M ICROALBUMINURIA is one of diagnosis criteria for kidney diseases, a predictor of endothelial dysfunction and a harbinger of markedly enhanced cardiovascular risk.1-11Kidney Disease Outcomes Quality Initiative (K/DOQI) clinical practice guidelines recommended that all patients with diabetes and/or hypertension should be screened for the presence of microalbuminuria.11Urine albumin excretion (UAE) on a 24-hour timed urine specimen is considered the “gold standard” for assessing albumin level and defining microalbuminuria.12However, 24-hour urine collections are cumbersome in routine clinical practice and subject to error due to inaccurate timing and/or incompleteness. An alternative method is urine albumin-creatinine ratio (ACR), which is a more convenient test for patients and may be less prone to errors due to improper collection methods and variations in 24-hour protein excretion compared with a random urine specimen. Witte et al13found that ACR in the first morning voids is consistent with UAE. They recommended ACR as a preferred screening strategy for diagnosing microalbuminuria.
Chin Med Sci J 2016; 31(1):23-30
Two sets of ACR cutoffs are currently and widely used:<30 mg/g, recommended by the American Diabetes Association (ADA);12and <17 mg/g for males and <25 mg/g for females, recommended by K/DOQI.14However, both of these reference value sets were mainly derived from Caucasian subjects,12,14and several studies have shown that ACR cutoffs have inherent racial disparities.14-16To date, there is no uniform ACR reference value for Chinese individuals. In this study, we investigate in the population of Handan villages, Hebei province, in order to establish the ACR reference value for the rural population in Heibei province.
SUBJECTS AND METHODS
Subjects
This is a substudy of the Handan Eye Study (HES).17The study protocol was approved and monitored by the Ethical Committee of Beijing Tongren Hospital, and written informed consent was obtained from all participants at the time of the examination. All study procedures adhered to the principles outlined in the Declaration of Helsinki for research involving human subject. Yongnian County, Handan, located in the south part of Hebei province (about 500 kilometers south of Beijing) has demographic characteristics similar to other rural Chinese locations according to the 2000 National Census.
The sample size was targeted to achieve an adequate precision to estimate prevalence and allow for risk factor analyses to be carried out. As the prevalence of the main diseases in this cross-sectional baseline survey was estimated to be 2% or higher, the present study can achieve a precision of 0.005 (d), considering a design effect of 1.5, with a sample of 4517.
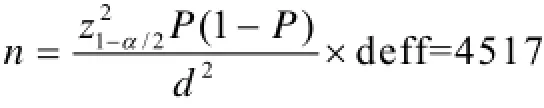
where Z is 95% confidence interval; P is estimated prevalence of the main disease.
Assuming a response rate of 90%, 6000 subjects would need to be recruited to achieve the targeted study size. Ultimately, 5154 participants completed the survey and examination. After providing informed consents, individuals were interviewed by 50 well-trained staffs. Physical and laboratory measurements were also taken by well-trained professionals adopting uniform standards.
Screening methods
Sociodemographic characteristics (age, sex, and education), medical history (hypertension, diabetes and cardiovascular diseases or stroke, recent drug or medication use), and lifestyle behavior (smoking status and alcohol intake) were obtained by using the validated questionnaires. Height and weight were measured according to standard protocols. Body mass index (BMI) was calculated from weight and height measurements as weight (kg) divided by square of height (m2). Overweight was defined as a BMI of 25 kg/m2or more. Blood pressure (BP) was measured three times in a sitting position after at least 5 measurements an average of three readings was then calculated.
Blood specimens were collected after an overnight fast of at least 8 hours. Serum creatinine (SCr) was measured with a kinetic Jaffe reaction. Fasting glucose (FG), triglycerides (TG), low density lipoprotein cholesterol (LDL-C), total cholesterol (TC), and high density lipoprotein cholesterol (HDL-C) were measured by enzymatic methods using an automatic clinical chemistry analyzer (Olympus AU2700, Tokyo, Japan). Dyslipidemia was defined as TC≥6.22 mmol/L or TG≥2.26 mmol/L or HDL-C≥1.04 mmol/L or LDL-C≥4.14 mmol/L.18
Fresh midstream urine was collected in the morning and women who were actively menstruating were excluded from the urine test. Urine albumin concentration (UALB) was measured by immunoturbidimetry (Audit Diagnostics, Cork, Ireland). Urine creatinine concentration (UCR) was measured by the same method used for SCr. Urine albumincreatine ratio (ACR)=UALB/UCR. The glomerular filtration rate (GFR) was estimated by using the Modification of Diet in Renal Disease (MDRD) Study equation for Chinese subjects.19
Inclusive criteria of healthy subjects
Subjects were classified into the healthy group if they met all the following conditions: (1) systolic BP (SBP)<140 mm Hg and diastolic BP (DBP)<90 mm Hg and without hypertension history and not taking any antihypertensive medication; (2) fasting glucose (FG)<7.0 mmol/L and without diabetes mellitus history and not taking any hypoglycemic agents; (3) TC<6.22 mmol/L, TG<2.26 mmol/L, HDL-C<1.04 mmol/L, and LDL-C<4.14 mmol/L13and without hyperlipidemia history and not taking any lipid-lowering medication; (4) BMI<25 kg/m2; (5) estimated GFR (eGFR) ≥60 ml/(min·1.73 m2) and without history of hematuria or proteinuria or any kidney disease; (6) without history of cardiovascular and cerebrovascular diseases.
Statistical analysis
The 95th percentile of ACR of the healthy group was used as the upper normal limit. ACR reference value was used to screen for albuminuria in the population. Albuminuria was defined that ACR value was higher than the ACR reference value. All continuous variables were presented as mean± standard deviation. Mean values of continuous variables were compared between groups by the unpaired t test, and categorical variables were compared by the Wald χ2test. A Wilcoxon rank-sum test was used to test ACR differences between males and females and among difference age groups. Spearman rank correlation analysis was carried out to reveal the correlation between ACR and age, BP, FG, LDL-C, TG, TC, BMI, eGFR. All statistical analyses were performed using SPSS software (version 17.0). A value of P less than 0.05 was considered statistically significant.
RESULTS
Characteristics of subjects
A total of 2321 males and 2833 females were enrolled in this study. Participants’ characteristics are summarized in Table 1. Hypertension prevalence differed significantly between males and females (P=0.000). Females, on average, had greater FG, TC, LDL-C, HDL-C, and BMI values, but lower age and eGFR values than males (all P<0.01).
Finally, 1168 participants were classified into the healthy group including 551 males and 617 females. Significant difference existed in age, HDL-C, eGFR, height, weight, BMI, and SBP between males and females (all P<0.01, Table 2).
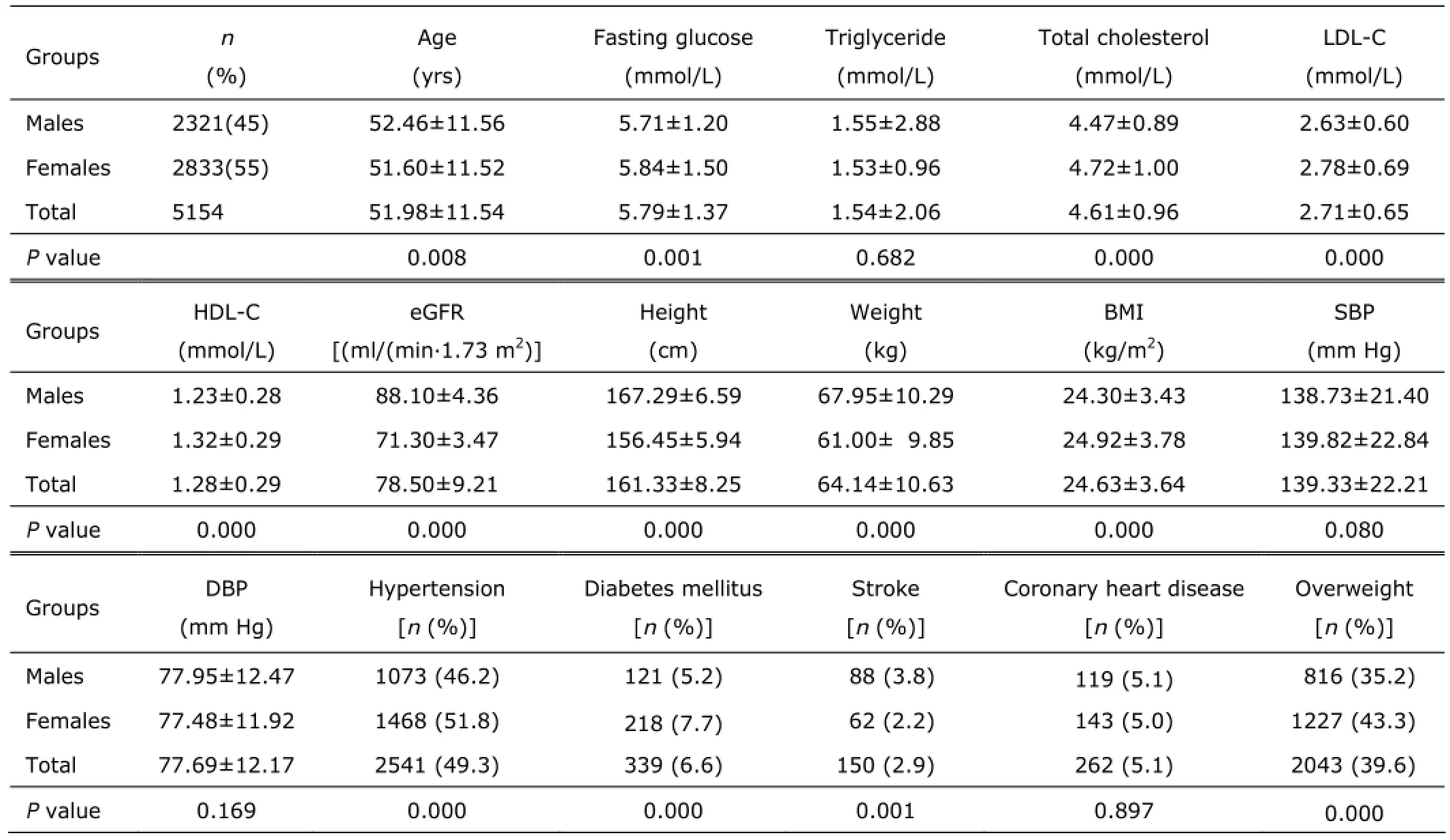
Table 1. Characteristics of participants§
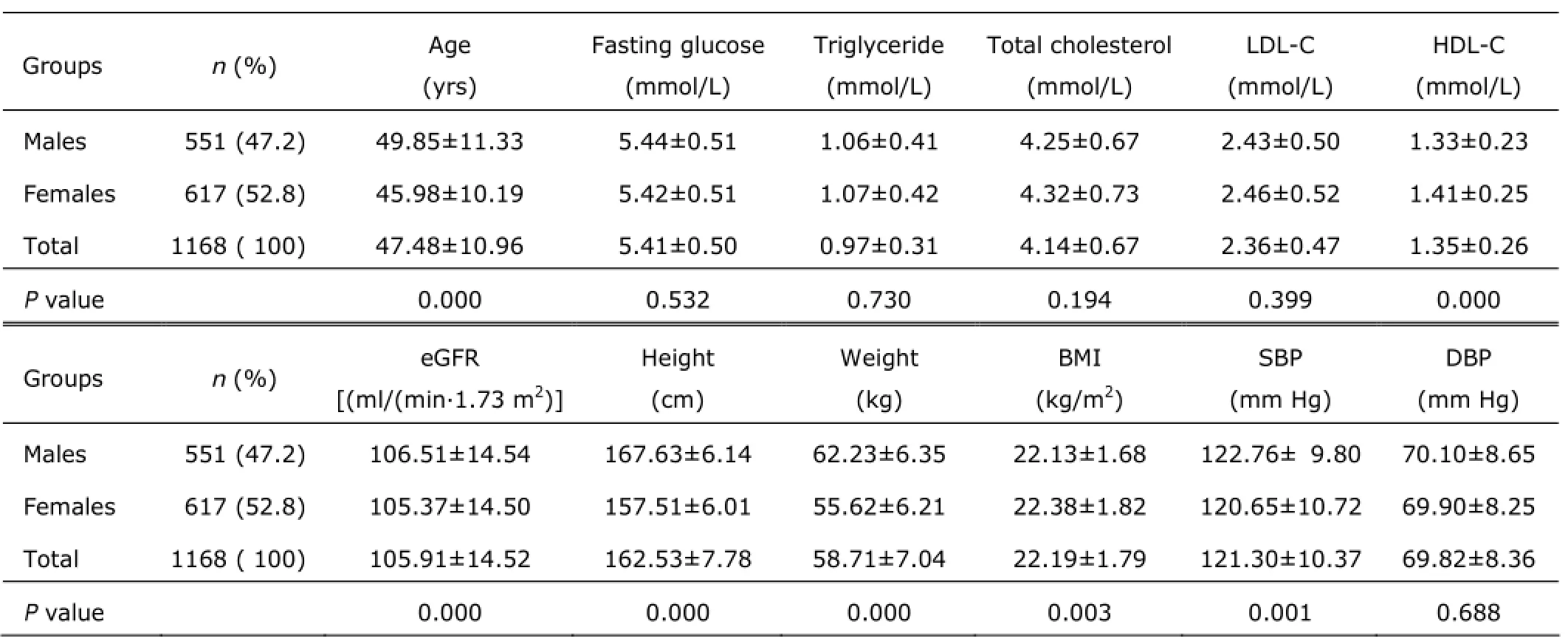
Table 2. Characteristics of healthy participants§
Reference value of urine ACR and albuminuria prevalence In the healthy group, the distribution of ACR values was skewed. It had a median of 7.02 mg/g and a 95th percentile of 31.37 mg/g. A Wilcoxon rank-sum test revealed that the males had significantly lower ACR value [M (P25, P75), 6.42 (3.06, 12.13) vs. 7.69 (3.92, 15.19) mg/g, χ2=−3.574, P=0.000] and greater UCR value [M (P25, P75), 810.03(505.01, 1175.03) vs. 693.04 (440.02, 1040.50) mg/L, χ2=−3.769, P=0.000] than the females. There was no significant difference in UALB between males and females [M (P25, P75), 4.70 (2.01, 9.03) vs. 4.91 (2.64, 9.73) mg/L, χ2=−1.025, P=0.305]. For males, the ACR median value was 6.42 mg/g, the 95th percentile was 28.71 mg/g; for females, the ACR median value was 7.69 mg/g and the 95th percentile was 31.85 mg/g. The normal upper limit of ACR was 28.71 mg/g (3.25 mg/mmol) for males and 31.85 mg/g (3.60 mg/mmol) for females. We calculated the albuminuria prevalence in this population by the ACR reference value. The albuminuria prevalence was 16.3%,with 15.2% for males and 17.2% for females. The age-gender standardized prevalence was 12.9%, with 11.9% for males and 13.2% for females.
Relationships between ACR and age, BP, biochemical parameters in the healthy individuals
For 1168 healthy individuals, 322 were in the 30-39 age group, 302 in the 40-49 age group, 379 in the 50-59 age group, and 165 in the >60 age group, respectively. The ACR [M (P25, P75)] of these 4 groups was 5.98 (2.67, 11.14), 6.47 (3.35, 12.91), 7.96 (4.00, 15.52), and 7.67 (4.23, 15.31) mg/g, respectively. ACR differed significantly among different age groups (χ2=25.32, P=0.000). For females, there was significant ACR difference between the 30-39 age group and 50-59 age group [P
In addition, we found ACR had no significant correlations with SBP (r=0.067, P=0.023), DBP (r=0.015, P=0.610), eGFR (r=-0.021, P=0.476), FG (r=0.034, P=0.248), LDL-C (r=0.044, P=0.137), TG (r=0.040, P=0.167), and TC (r=0.053, P=0.069).
Relationships between ACR and BP, diabetes mellitus, weight, biochemical parameters in 5154 participants There was significant difference in ACR between 2541 hypertension patients and 2613 non-hypertension patients [M (P25, P75), 10.47 (4.63, 24.83) vs. 7.38 (3.59, 15.82) mg/g, P=0.000]. Table 3 showed ACR was increased with DBP increasing (P=0.000). The risk factor for albuminuria was high SBP (for males, OR: 1.172, 95%CI: 1.111, 1.237, P=0.000; for females, OR: 1.221, 95%CI: 1.167, 1.276, P=0.000) and DBP (for males, OR: 1.247, 95%CI: 1.131, 1.375, P=0.000; for females, OR: 1.237, 95%CI: 1.137, 1.346, P=0.000). However, spearman rank correlation analysis revealed that there was no obvious correlation between ACR and BP (SBP: r=0.160, P=0.000; DBP: r=0.106, P=0.000).
The 339 patients with diabetes had higher ACR value than 4815 non-diabetes subjects [M (P25, P75), 13.78(5.31, 34.70) vs. 8.49 (3.99, 18.98) mg/g, P=0.000]. Nevertheless, spearman rank correlation analysis revealed there was no significant correlation between ACR and FG (r=0.107, P=0.000).
There were significant differences between 2515 patients with dyslipidemia and 2639 subjects without dyslipidemia [M (P25, P75), 9.15 (4.42, 21.74) vs. 8.37 (3.89, 18.65) mg/g, P=0.001]. There was no significant difference between 3111 overweight patients and 2043 normal BMI subjects [M (P25, P75), 8.89 (4.16, 20.19) vs. 8.67 (4.00, 19.57) mg/g, P=0.609]. However, the correlation between ACR and LDL-C (r=0.074, P=0.000), TG (r=0.057, P=0.000), TC (r=0.083, P=0.000), BMI (r=0.005, P=0.711) was not significant.
Albuminuria incidence in the different age groups, hypertension and/or diabetes groups
All 5154 participants were classified into the 30-39 (n=897), 40-49 (n=1061), 50-59 (n=1925), >60 (n=1271) years old groups, respectively. The median value of ACR in these 4 groups was 7.10, 7.38, 9.57, and 10.52 mg/g, respectively, and incidence of albuminuria was 10.8% (97/897), 11.0% (117/1061), 18.2% (350/1925), and 21.6% (275/1271), respectively. The incidence of albuminuria was significantly increased with age increasing (χ2=76.46, P=0.000).
In 2290 hypertension cases without diabetic mellitus, 456 had abuminuria, and incidence rate was 19.9%. The albuminuria incidence in 88 participants who had diabetic mellitus with normal BP was 27.3%. In the cases who had hypertension combined with diabetic mellitus, the incidence rate was 27.9%. The albuminuria incidence for 2525 subjects without both diabetic mellitus and hypertension was 11.4%. The differences of albuminuria incidence among the 4 groups were significant (χ2=98.10, P=0.000). (Table 4)
Risk factors for albuminuria
All 5154 individuals were classified into the albuminuria group and non-albuminuria group according to ACR cutoffs recommended by our study. For males, there was significant difference in the parameters of age, FG, TC, LDL-C, SBP, DBP, incidences of hypertension and diabetes mellitus between the albuminuria and non-albuminuria groups (all P<0.05). For females, besides the above parameters, the two groups showed significant difference in TG, BMI, and annual income (all P<0.05). (Table 5)

Table 3. Relationship between ACR and DBP

Table 4. Relationships between albuminuria incidence and hypertension, diabetes [n (%)]

Table 5. Comparisons of parameters between the non-albuminuria and albuminuria groups§
Logistic regression analysis suggested age, BP, and FG were the risk factors of albuminuria. The OR value in the men was 1.275 (95%CI: 1.194, 1.362, P=0.000), 1.233 (95%CI: 1.155, 1.315, P=0.000), and 1.159 (95%CI: 1.109, 1.212, P=0.000), respectively; the OR value in the women was 1.146 (95%CI: 1.068, 1.231, P=0.000), 1.152 (95%CI: 1.109, 1.196, P=0.000), and 1.147 (95%CI: 1.097, 1.200, P=0.000), respectively.
DISCUSSION
Albuminuria is a marker of kidney injury, and also a predictor of cardiovascular diseases in the general population.20Screening for albuminuria is recommended in patients at increased risk for Chronic Kidney Disease (CKD), including those with hypertension, diabetes, cardiovascular diseases, and a family history of CKD.21ACR is a convenient test for microalbuminuria.22,23
It has been demonstrated that there were varying racial differences in ACR.14some studies revealed different urinary albumin and creatinine excretion rates among different races and advised that different threshold values is recommended for albuminuria screening. Deurenberg et al24revealed that for the individuals with the same BMI, body muscular mass of Asian population was lower and fat percent was higher compared to Caucasians. Cirillo et al16found that the population with lower muscular mass had higher ACR reference values. INTERMAP study recommended that ACR reference value of Japanese and Chinese should be higher than that of English and American.15
Previous studies have indicated that ACR differed between men and women. K/DOQI guidelines recommend that it is essential to establish gender-specific ACR reference value.11We found that ACR differed significantly between men and women. The ACR difference might result from a difference in urine creatinine clearance between men and women. James et al25revealed that men had 33% higher urine creatinine per weight than women. Creatinine is theproduct of skeletal muscular creatine and phosphagen. Each 20 g muscles generate 1 mg creatinine per day. The reference value appeared to be gender-specific, which can be attributed to the lower muscular mass in women compared with men.26
Houlihan et al27revealed that ACR was age-dependent and therefore they recommended to determine age specific ACR reference values. Our study showed that in the healthy participants, creatinine excretion was diminished and urinary albumin and ACR increased with age increasing. This condition may be attributed to the following reasons: with the increase of age, renal arteries become sclerosing, which leads to decline of renal blood flows and GFR; loss of body muscular mass brings serum creatinine level to a decline with the increasing age, resulting in decrease of urinary creatinine concentration.
Our study found that in the healthy population, significant ACR difference existed among different age cohorts. But difference was not found between the groups with different SBP, DBP, eGFR, FG, LDL-C, TG, and TC levels. For healthy males, there was significant ACR difference between the 30-39 and 50-59 age groups, 30-39 and >60 age groups, 40-49 and 50-59 age groups, 40-49 and >60 age groups. Therefore, we supposed that the age boundary of agespecific ACR cutoffs was 50-year-old. The 95th percentile of ACR of healthy men less than 50 years of age was 24.79 mg/g, and the value in healthy men older than 50 years of age was 33.77 mg/g.
There was significant ACR difference between the 30-39 and 50-59 age groups for healthy women. In order to define the age boundary for females, we reduced the age group interval to 5-year (α’=0.0023). Finally, significant ACR difference existed between the 30-34 age group and 55-59 age group. For female age-specific ACR cutoffs was not found.
The 95th percentile was used as the upper normal boundary of ACR. The sample size would affect accuracy of results. When the cohort of healthy men was divided into the <50 years old group and ≥50 years old group, the sample size was reduced, which might affect the accuracy of ACR reference value. Further study is needed to establish more accurate age-specific reference value.
Crews et al28found that lower income was associated with a higher prevalence of albuminuria. According to the average annual income of Heibei rural family (3000 ¥) issued by the 2000 concus, participants were divided into two groups, family income<3000 ¥ group and ≥3000 ¥group respectively. ACR differed significantly between the two groups. Therefore, we suggested that individuals with income less than 3000 ¥ might have a higher risk of occurring diabetic mellitus and hypertension than those with income more than 3000 ¥. For that reason, individual who has an income <3000 ¥ might be with a higher ACR.
In this study we found that albuminuria prevalence in the population with both hypertension and diabetes was higher than those with hypertension or diabetes only. Logistic analysis showed that albuminuria risk factors included the increase of age, diabetes mellitus, and hypertension. It has been revealed that woman is an independent risk factor for albuminuria.29Our study found that there was no significant difference in albuminuria prevalence between males and females. Ma et al19revealed that when a single ACR cutoff was used, the prevalence of albuminuria was significantly lower among men compared with women, therefore women was an independent risk factor for albuminuria. No significant difference in the prevalence of albuminuria between men and women was noted when gender-specific ACR cutoffs were used. Gender-specific ACR cutoffs balanced ACR difference between males and females.
For this cohort, the healthy criteria are not accurate enough. Because oral glucose tolerance test or hemoglobin A1c is not detected in this study, the healthy group might include prediabetic individuals. Nevertheless, our results showed there was no significant difference in ACR among FG<5 mmol/L, 5 mmol/L≤FG<6.1 mmol/L, and 6.1 mmol/L≤FG<7.0 mmol/L groups.
We measured ACR using 1 spot-urine sample. It is well-known that intra-individual variability of albumin excretion is high, however ACR exhibits less variation than urine albumin does. Our study also found that BP, GFR, FG, and serum lipid had no influence on ACR at all. It is also impractical to recruit participants undergoing repeated urine analyses more than twice in a screening program. ACR reference values obtained from this cross-sectional study need to be validated by longitudinal observation.
In conclusion, the ACR reference value in the rural areas of Hebei province is higher than that of the Western population. The ACR reference value among the rural population in Hebei province was 28.71 mg/g for males and 31.85 mg/g for females. The age-gender standardized prevalence of albuminuria in Hebei province was 12.9%.
REFERENCES
1. Jay PG, Geoge LB. Micromaluminuria: marker of vascular dysfunction, risk factor for cardiovascular disease.Vasc Med 2002; 7:35-43.
2. Wang Q. The relationship between mircoalbuminuria and cardiovascular disease in diabetes. Cardiovasc Dis J Integr Tradit Chin West Med 2015; 3:189-91.
3. Ren DY, Ding XY. Study on correlation between microalbuminria and cerebral arterial atherosclerosis. J Mod Med Health 2016; 32:514-6.
4. Liu MM. Risk factors of albuminuria in diabetes mellitus. Clin J Mod Drug Appl 2016; 10:27-9.
5. Rao XP, Xu M, Liang M, et al. Microalbuminuria prevalence in metabolic abnormalities. Chin J Health Care Med 2015: 17:489-91.
6. Zhou L, Cai X, Li M. Plasma NT-proBNP is independently associated with albuminuria in type 2 diabetes. J Diabetes Complications 2016; Jan 23. pii: S1056-8727(16)00019-2.
7. Lambers HJ, Brinkman JW, Bakker SJ, et al. Update on microalbuminuria as a biomarker in renal and cardiovascular disease. Curr Opin Nephrol Hypertension 2006; 15:631-6.
8. Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421-6.
9. Viberti GC, Hill RD, Jarrett RJ, et al. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982; 1:1430-2.
10. Wachtell K, Ibsen H, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann Intern Med 2003; 139:901-6.
11. National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39:S1-S266.
12. Marshall SM. Screening for microalbuminuria: which measurement? Diabet Med 1991; 8:706-11.
13. Witte EC, Lambers HJ, de Zeeuw D, et al. First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol 2009; 20:436-43.
14. Mattix HJ, Hsu CY, Shaykevich S. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. Jam Soc Nephrol 2002; 13:1034-9.
15. Dyer AR, Greenland P, Elliott P, et al. Evaluation of measures of urinary albumin excretion in epidemiologic studies. Am J Epidemiol 2004; 160:1122-31.
16. Cirillo M, Laurenzi M, Mancini M, et al. Low muscular mass and overestimation of microalbuminuria by urinary albumin/creatinine ratio. Hypertension 2006; 47:56-61.
17. Liang YB, Friedman DS, Wong TY, et al. Rationale, design, methodology, and baseline data of a population-based study in rural China: the Handan eye study. Ophthalmic Epidemiol 2009; 16:115-27.
18. Committee of China adult dyslipidemia prevention guide. China adult dyslipidemia prevention guide. Chin J Cardiol 2007; 35:390-419.
19. Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006; 17:2937-44.
20. Lee K, Kim J. Estimated glomerular filtration rate and albuminuria in Korean population evaluated for cardiovascular risk. Int Urol Nephrol 2016; 23:1-6.
21. Tazeen HJ, Nish C, Juanita H. Use of albumin creatinine ratio and urine albumin concentration as a screening test for albuminuria in an Indo-Asian population. Nephrol Dial Transplant 2007; 22:2194-200.
22. Na N, Jiang H. Research progress of urine albumin-creatinine ratio in type 2 diabetes mellitus. Chin J Clin 2015; 9: 3969-72.
23. Glassock RJ. Control of albuminuria in overt diabetic nephropathy: durability counts. Nephrol Dial Transplant 2016; 6:4-6.
24. Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat percent relationship. Obes Rev 2002; 3:141-6.
25. James GD, Jean ES. A longitudinal study of urinary creatinine and creatinine clearance in normal subjects. Race, sex, and age differences. Am J Hypertens 1988; 1:124-31.
26. Bakker AJ. Detection of microalbuminuria: receiver operating characteristic curve analysis favors albumin-to-creatinine ratio over albumin concentration. Diabetes Care 1999; 22:307-13.
27. Houlihan CA, Tsalamandris C, Akdeniz A, et al. Albumin to creatine ratio: a screening test with limitation. AJKD 2002; 39:1183-9.
28. Crews DC, William WM, Shoham DA. Low income and albuminuria among REGARDS (Reasons for Geographic and Racial Differences in Stroke) study participants. Am J Kidney Dis 2012; 60:779-86.
29. Li Y, Zhou LT, Liu FY. Epidemiologic study of chronic kidney disease in Changsha county of Hunan province. Chin J Nephrol 2010; 26:9-14.
for publication July 20, 2015.
†These authors contributed equally to this work.
*Corresponding author Wen Huang, E-mail: 25911412@163.com, Ning-li Wang, E-mail: wningli@vip.163.com
△Supported by the National Basic Research Program of China (973 Program) (2007CB512201), the Program of Health Policy for blindness prevention from China, the Key Technologies R&D Program (2006-10903) from the Science and Technology Bureau of Handan City, Hebei Province, China, a program from Beijing Tongren Hospital, and key discipline fund of Health Bureau, Handan City, Hebei Province, China.
 Chinese Medical Sciences Journal2016年1期
Chinese Medical Sciences Journal2016年1期
- Chinese Medical Sciences Journal的其它文章
- Pathology Verified Concomitant Papillary Thyroid Carcinoma in the Sonographically Suspected Thyroid Lymphoma: A Case Report△
- Life-threatening Spontaneous Retroperitoneal Haemorrhage: Role of Multidetector CT-angiography for the Emergency Management
- Percutaneous Removal of Benign Breast Lesions with an Ultrasound-guided Vacuum-assisted System: Influence Factors in the Hematoma Formation△
- Respiratory and Cardiac Characteristics of ICU Patients Aged 90 Years and Older: A Report of 12 Cases
- Positive Rate of Different Hepatitis B Virus Serological Markers in Peking Union Medical College Hospital, a General Tertiary Hospital in Beijing△
- Association Between Geranylgeranyl Pyrophosphate Synthase Gene Polymorphisms and Bone Phenotypes and Response to Alendronate Treatment in Chinese Osteoporotic Women△
