Association Between Geranylgeranyl Pyrophosphate Synthase Gene Polymorphisms and Bone Phenotypes and Response to Alendronate Treatment in Chinese Osteoporotic Women△
Lan-wen Han, Dou-dou Ma, Xiao-jie Xu, Fang Lü, Yi Liu, Wei-bo Xia, Yan Jiang, Ou Wang, Xiao-ping Xing, and Mei Li*Department of Endocrinology, Key Laboratory of Endocrinology of Ministry of Health, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, 100730 Beijing, China
Association Between Geranylgeranyl Pyrophosphate Synthase Gene Polymorphisms and Bone Phenotypes and Response to Alendronate Treatment in Chinese Osteoporotic Women△
Lan-wen Han†, Dou-dou Ma†, Xiao-jie Xu†, Fang Lü, Yi Liu, Wei-bo Xia, Yan Jiang, Ou Wang, Xiao-ping Xing, and Mei Li*
Department of Endocrinology, Key Laboratory of Endocrinology of Ministry of Health, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, 100730 Beijing, China
geranylgeranyl pyrophosphate synthase; tag single nucleotide polymorphisms; osteoporosis; alendronate
Objective To investigate the relationship between geranylgeranyl pyrophosphate synthase (GGPPS) gene polymorphisms and bone response to alendronate in Chinese osteoporotic women.
Methods A total of 639 postmenopausal women with osteoporosis or osteopenia were included and randomly received treatment of low dose (70 mg per two weeks) or standard dose (70 mg weekly) of alendronate for one year. The six tag single nucleotide polymorphisms of GGPPS gene were identified. Bone mineral density (BMD), serum cross-linked C-telopeptide of type I collagen (β-CTX), and total alkaline phosphatase (ALP) were measured before and after treatment. GGPPS gene polymorphisms and the changes of BMD and bone turnover markers after treatment were analyzed.
Results rs10925503 polymorphism of GGPPS gene was correlated to serum β-CTX levels at baseline, and patients with TT genotype had significantly higher serum β-CTX level than those with TC or CC genotype (all P<0.05). No correlation was found between polymorphisms of GGPPS gene and serum total ALP levels, as well as BMD at baseline. After 12 months of treatment, lumbar spine and hip BMD increased and serum bone turnover markers decreased significantly (P<0.01), and without obvious differences between the low dose and standard dose groups (all P>0.05). However, GGPPS gene polymorphisms were uncorrelated to percentage changes of BMD, serum total ALP, and β-CTX levels (all P>0.05).
Conclusion GGPPS gene polymorphisms are correlated to osteoclasts activity, but all tag single nucleotide polymorphisms of GGPPS gene have no influence on the skeletal response to alendronate treatment.O STEOPOROSIS is a systemic disease characterized by impaired bone strength and increased bone fracture risk, which remarkably increases the morbidity and mortality of the elderly.1,2Osteoporosis is significantly influenced by genetic factors, and many genetic loci are identified to be associated with bone mineral density (BMD) and bone fracture risk.3,4Recently, bone responses to anti-osteoporotic agents are indicated to be influenced by genetic variance of encoding genes of vitamin D receptor,5estrogen receptor,6low density lipoprotein receptor-related protein,7,8farnesyl diphosphate synthase (FDPS),9and so on.
Chin Med Sci J 2016; 31(1):8-16
Bisphosphonates (BPs) are widely used as potent inhibitors of bone resorption, which significantly increase BMD and reduce bone fracture risk.10,11However, clinical studies indicate the response rate to BPs is variant from 62% to 90% among different individuals with osteoporosis.12-14Identification of the non-responders to BPs is beneficial to improve the treatment effects and decrease medical costs. Alendronate, the most commonly used nitrogen-containing BPs, can reduce osteoclast activity through inhibiting FDPS and geranylgeranyl pyrophosphate synthase (GGPPS) of mevalonate pathway.15,16Common allelic variants of FDPS gene have been demonstrated to influence the response of women with osteoporosis to BPs.9,17GGPPS is another important target of BPs in osteoclasts, which belongs to the trans-prenyltransferase family.18The enzyme catalyzes the synthesis of geranylgeranyl pyrophosphate (GGPP) from farnesyl diphosphate and isopentenyl diphosphate. GGPP is an important molecule responsible for the C20-prenylation of proteins and for the regulation of a nuclear hormone receptor.18GGPPS encoding gene has 5 exons and locates on chromosome 1q43.19As the crucial target of alendronate in the mevalonate pathway of osteoclasts, genetic variance of GGPPS gene is speculated to affect the skeletal response to alendronate. However, little is known about the influence of GGPPS gene polymorphisms on phenotype of osteoporosis and effects of alendronate. Therefore, we evaluate the influence of GGPPS gene polymorphisms on BMD, bone turnover biomarkers, and bone response to alendronate treatment in Chinese postmenopausal women with osteoporosis or osteopenia.
PATIENTS AND METHODS
Patients and treatment
This is a multicenter, open-label, prospective study. During 2008 and 2011, more than 2000 postmenopausal women were screened from 7 clinical centers in China (Beijing, Changsha, Shanghai, Chengdu, Xi'an, Guangzhou, and Harbin). A total of 639 postmenopausal women with osteoporosis or osteopenia were enrolled in this study. Inclusion criteria were as follows: (1) patients aged 41-75 years old, with menopause age older than 40 years and years since menopause (YSM) more than 1 year; (2) T-score of BMD at the lumbar spine or femoral neck less than -1; (3) at least 3 lumbar vertebras could be measured; (4) could ambulate at the least 30 minutes in each day. Exclusion criteria were as follows: (1) patients with severe liver or kidney diseases; (2) with severe gastrointestinal diseases; (3) intolerance to BPs; (4) with treatment history of BPs or parathyroid hormone 1-34 within recent 12 months; with treatment of estrogen, selective estrogen receptor modulators, active vitamin D analogues or calcitonin within recent 6 months; (5) with more than 2 weeks therapy history of corticosteroid or anticonvulsant therapy within recent 3 months; (6) with other metabolic or inherited bone diseases; (7) with autoimmune diseases.
Postmenopausal women with osteoporosis or osteopenia randomly received low-dose (70 mg every two weeks) or standard-dose (70 mg weekly) of alendronate (Shijiazhuang Ouyi Pharmaceutical Co. Ltd., Hebei, China) for 1 year. Patients took alendronate on an empty stomach at least 30 minutes before breakfast with 250 ml plain water, and remain upright for at least 30 minutes after dosing. All patients were supplemented with 600 mg elemental calcium plus 125 IU of vitamin D3daily (Caltrate D; Pfizer Inc., USA). Compliance to alendronate treatment was calculated according to the number of remaining tablets during the follow-up.
The study was approved by the Ethical Committee of Peking Union Medical College Hospital and all participants signed informed consents.
Genotyping
Genomic DNA was extracted from leukocytes of peripheral blood according to standard procedures. The Entrez Gene Database (http://www.ncbi.nlm.nih.gov/gene/) and HapMap (http://hapmap.ncbi.nlm.nih.gov/) were adopted to identify single nucleotide polymorphisms (SNPs) information of GGPPS gene in Chinese population. Six tag SNPs of GGPPS were selected as follows: rs2803851, rs2789367, rs10802624, rs10925503, rs3840452, and rs2789366, which could almost represent the whole genetic variance of this gene. The genotypes of rs3840452 of GGPPS gene were assayed by short tandem repeat. The genotypes of GGPPS gene of rs2803851, rs2789367, rs10802624, rs10925503, and rs2789366 were detected by TaqMan allelic discrimination assay (Applied Biosystems, USA). Primer sequences and the distributions of tag SNPs of GGPPS gene are shown inTable 1. The whole reacting volume was 6 μl, including 3 μl DNA sample, 2.5 μl TaqMan Universal PCR Master Mix (Applied Biosystems, USA), 0.125 μl TaqMan probe assay (including primers), and 0.375 μl ddH2O. Reactions were performed on a Real-time PCR system of ABI Prism7900 (Applied Biosystems) under standard condition.
Evaluation of bone response to alendronate
Skeletal response to alendronate was assessed by percentage changes of BMD and bone turnover biomarkers after treatment. BMD at the lumbar spine and proximal femur was measured by dual-energy X-ray absorptiometry (DXA) (Hologic or Lunar) at baseline and after 12 months of treatment. Cross calibration equations between two kinds of machine were as follows: Hologic BMD (g/cm2) = 0.802 –Lunar + 0.318 (r=0.991, P<0.001, SE=0.03 g/cm2).20BMD phantom scan was measured by each DXA instrument daily and no significant machine drift was detected during the whole period of study. The coefficients of variation (CV) of BMD were 0.8%-1.0% for the lumbar spine and total hip. Radiologists were responsible for the analysis of BMD data, quality control, and phantom scan, who were unaware of the study group assignments. According to the criteria of World Health Organization, osteoporosis was defined as BMD T-score ≤ -2.5 at the lumbar spine, femoral neck or total hip, and -2.5 < T-score ≤ -1.0 at above sites was considered as osteopenia.21Participants were defined as severe osteoporosis if they had fragility fracture history.
Serum calcium, phosphate, total alkaline phosphatase (total ALP, as a bone formation marker), cross-linked C-telopeptide of type I collagen (β-CTX, as a bone resorption marker) and 25 hydroxy-vitamin D (25OHD, as a marker of vitamin D nutritional status) levels were measured at baseline and after 12 months of treatment. Serum β-CTX and 25OHD levels were detected by electrochemiluminescence immunoassay (Roche Diagnostics Co. Ltd., Germany). The lowest detection limit of β-CTX and 25OHD was 0.01 ng/ml and 4.0 ng/ml, respectively. CV of intra-assay and inter-assay of β-CTX were 2.2%-4.6% and 2.5%-4.7%, respectively. The intra-assay and inter-assay CV of 25OHD were 4.1%-5.7% and 6.6%-9.9%, respectively.

Table 1. Primer sequences of Tag SNPs of GGPPS gene
Statistical analysis
Data of normal distribution were presented as mean ± standard deviation (SD), while those of abnormal distribution were expressed as median and quartiles. Genotype distributions of GGPPS gene were tested for Hardy-Weinberg using Chi-square test. Associations between genotypes of GGPPS gene and BMD, as well as serum levels of calcium, phosphate, total ALP, β-CTX, 25OHD were evaluated using analysis of variance adjusted for age, body mass index (BMI), and YSM. The response to alendronate treatment was estimated as the percentage changes of BMD and serum bone turnover biomarkers (total ALP and β-CTX), which were calculated with the following formula: (parameters after treatment-parameters at baseline)/ parameters at baseline × 100%. Association of the genotypes and percentage changes of BMD, total ALP, β-CTX after treatment was evaluated by analysis of variance and multiple linear regression adjusted for age, BMI, and YSM. The statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was considered when P value was less than 0.05.
RESULTS
Characteristics of study population at baseline
Demographic characteristics of the postmenopausal women with osteoporosis or osteopenia are shown in Table 2. No significant differences were found in age, YSM, BMI, BMD, serum 25OHD, total ALP, and β-CTX levels between the low dose and standard dose groups(all P>0.05).
GGPPS gene polymorphisms and bone phenotypes
Genotypes and allele distributions of the six tag SNPs of GGPPS gene are shown in Table 3. All SNPs were in Hardy-Weinberg equilibrium. At baseline, rs10925503 polymorphism of GGPPS gene was correlated to serum β-CTX levels, which were 0.45±0.02, 0.41±0.02, and 0.36±0.02 ng/ml in women with TT, TC, and CC genotypes, after adjusting for age, YSM, and BMI (all P<0.05). The other SNPs of GGPPS gene were uncorrelated to serum β-CTX levels (Fig. 1A). The serum bone formation marker (total ALP)levels did not present significant difference among genotypes of all tag SNPs of GGPPS gene (all P>0.05) (Fig. 1B). BMD at the lumbar spine and femoral neck had no obvious difference among all genotypes of GGPPS gene, after adjusting for age, YSM, and BMI (all P>0.05, Fig. 1C and 1D).
Patients with TT genotype of rs10925503 polymorphism of GGPPS gene had significantly higher serum β-CTX level than those with TC or CC genotype (P=0.012). The serum ALP levels, BMD at the lumbar spine and femoral neck did not present significant difference among genotypes of all tag SNPs of GGPPS (all P>0.05).
GGPPS gene polymorphisms and skeletal response to alendronate treatment
There were 540 (84.5%) women who completed the 12 months of treatment. And, 99 (15.5%) women withdrew from the treatment because of clinical adverse effects, protocol deviation, losing to follow-up and other reasons. After 12 months of treatment, no differences was found in BMD changes at the lumbar spine, femoral neck, and total hip between the standard-dose group (n=266; 5.07%, 2.93%, and 3.80%, respectively) and the low-dose group (n=274; 5.60%, 3.87%, and 3.28%, respectively; all P>0.05). In the low-dose and standard-dose groups, the serum levels of total ALP decreased by 24.6% and 29.1%, and serum β-CTX levels decreased dramatically by 57.0% and 69.7% after 12 months of treatment. However, after low dose or standard dose treatment of alendronate, the percentage changes of BMD at the lumbar spine and proximal femur presented no significant difference among different genotypes of GGPPS after adjusting for age, YSM, and BMI (Tables 4 and 5). No significant differences in percentage changes of serum ALP and β-CTX levels were found among different genotypes of GGPPS gene (Tables 4 and 5).

Table 2. Demographic characteristics of the study population at baseline§
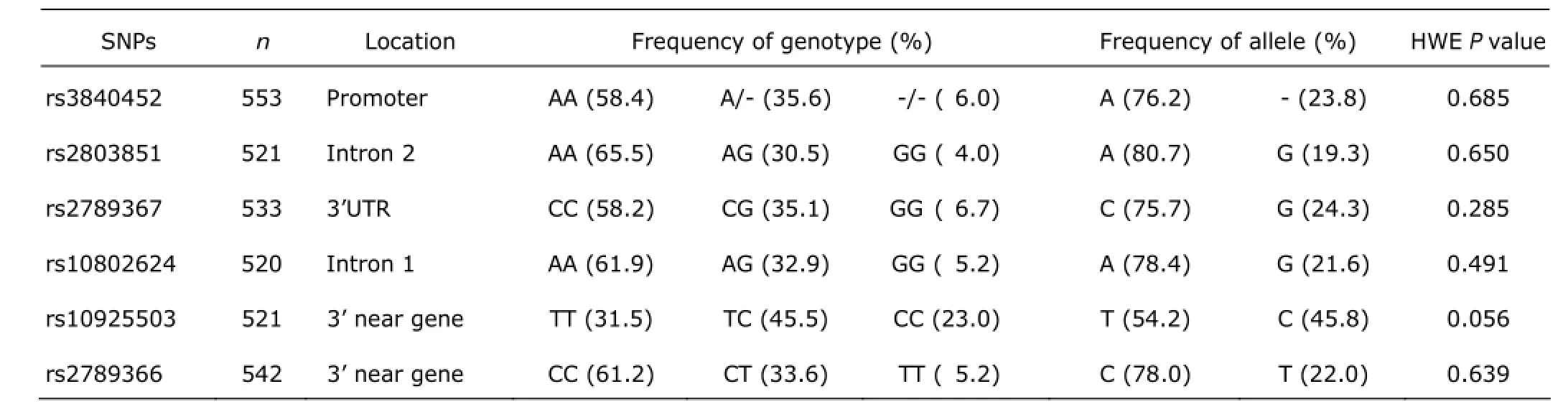
Table 3. Genotypes and allele distributions of the six tag SNPs of GGPPS gene
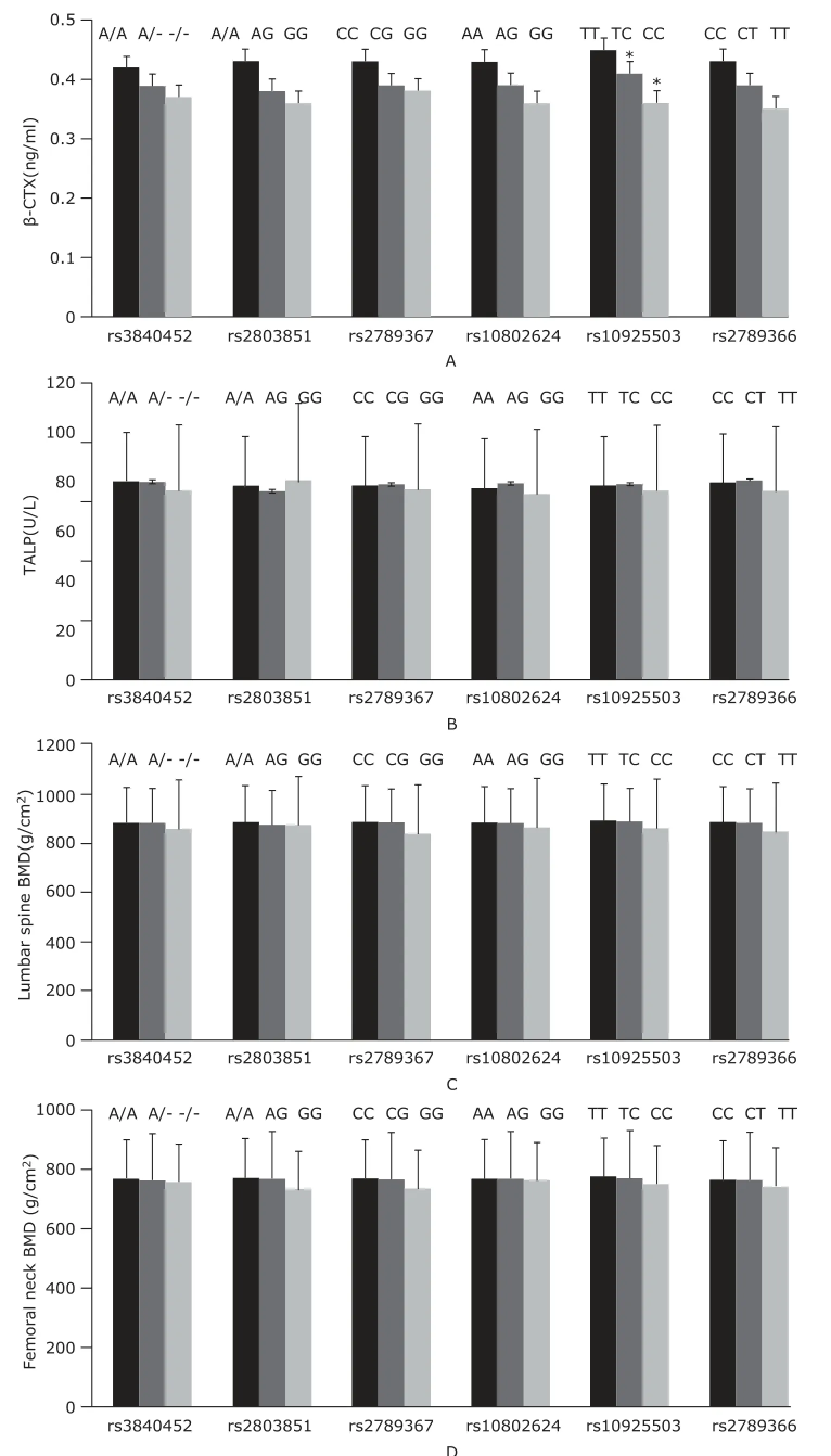
Figure 1. Baseline serum β-CTX (A) and ALP levels (B), BMD at the lumbar spine (C) and femoral neck (D) among different genotypes of tag SNPs of GGPPS gene.*P<0.05 compared with women with TT genotype.
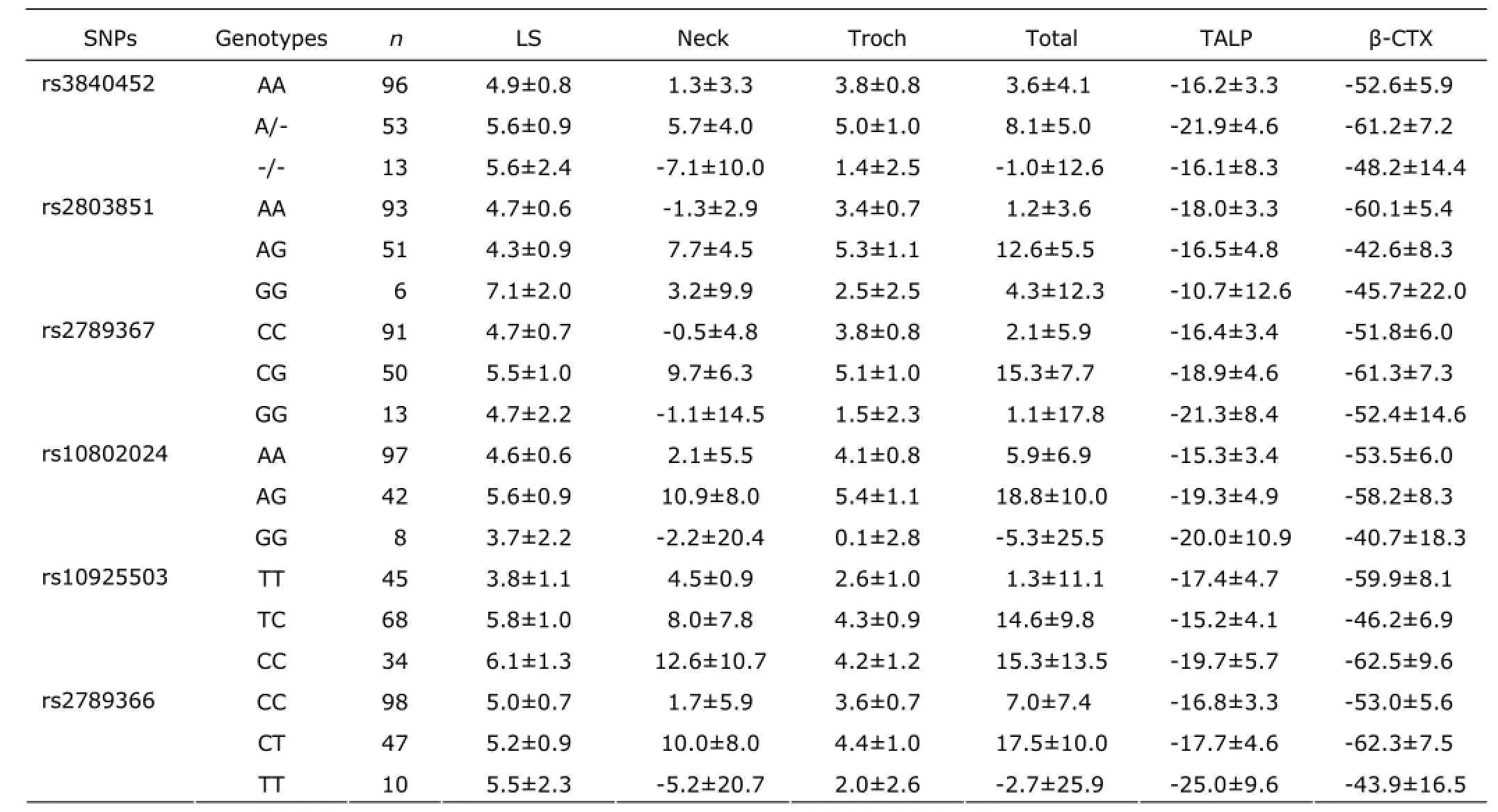
Table 4. The percentage change of BMD and bone turnover biomarkers among different genotypes after 12 months of low-dose of alendronate treatment (%)
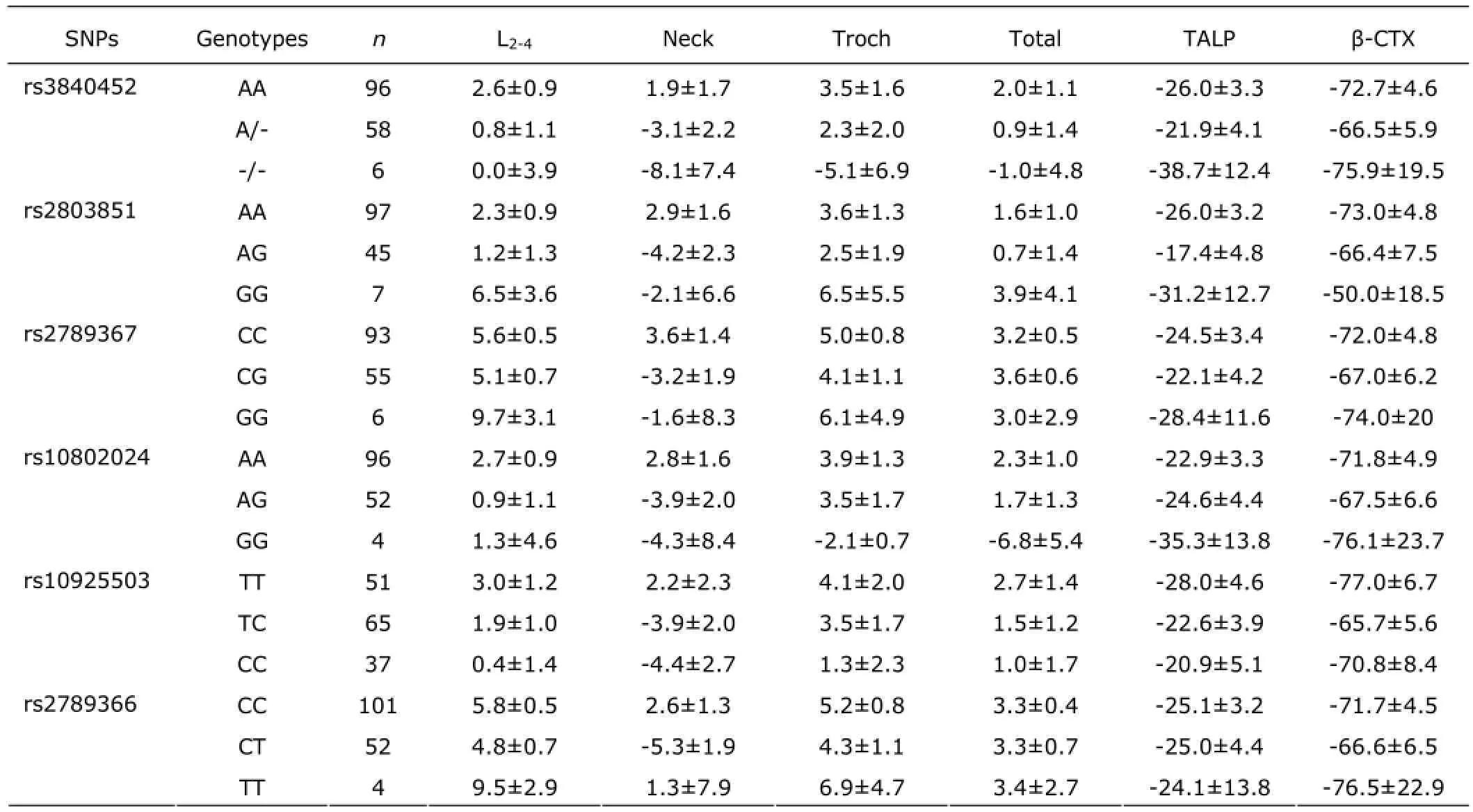
Table 5. The percentage changes of BMD and bone turnover biomarkers among different genotypes after 12 months of standard-dose of alendronate treatment (%)
DISCUSSION
Osteoporosis has a strong hereditary background and represents an important health problem among the elderly. Nitrogen-containing BPs are widely used in the treatment of osteoporosis and diseases with high bone remodeling, which have been demonstrated to reduce the production of farnesyl pyrophosphate (FPP) and GGPP through inhibition of crucial enzymes FDPS and GGPPS in the mevalonate pathway of osteoclasts.22,23The mechanism of alendronate suppression of osteoclast formation is also directly correlated to the inhibition of GGPPS.24-26Therefore, it is important to evaluate the correlation of genetic variation in GGPPS and bone phenotypes, as well as skeletal responsiveness to alendronate. However, the pharmacogenetic information on GGPPS and alendronate treatment is still scanty.
In this study, we found that the frequencies of GGPPS polymorphisms of rs3840452 in Chinese women were similar to those in Korean,27and genotypes frequencies of rs2803851, rs2789367, rs10802624, rs10925503 of GGPPS in Chinese women were different from those in Japanese, European and African according to data in http://hapmap. ncbi.nlm.nih.gov/. We only found rs10925503 polymorphism of GGPPS was correlated to baseline level of bone resorption marker, and patients with TT genotype had significantly higher serum β-CTX level than those with TC or CC genotype. The location of rs10925503 is close to 3’ untranslated region of GGPPS, a possible binding site of transcription factor, which could regulate expression of GGPPS. Therefore, rs10925503 polymorphism of GGPPS was correlated to the activity of osteoclasts. No association was found between GGPPS polymorphisms with bone formation marker and BMD at baseline. In a small sample of Korean women, -/-genotype of GGPPS rs3840452 was found to be correlated to higher femoral neck BMD than AA or A/- genotypes,27which was inconsistent with our results. As there were only 7 Korean women with -/- genotype, a bias of the small sample in that study could not be ruled out.
As we know, according to changes of BMD, it was estimated that about 5%-10% of patients do not respond to anti-osteoporotic therapy.28Sequence variants in the human genome are important causes of difference in drug responses. Pharmacogenetic study in osteoporosis contributes to improve drug efficacy and safety through identification of genetic markers of different patients. Several studies indicated that GGPPS was the crucial target of BPs in mevalonate pathway of osteoclasts. BPs effectively blocked protein geranylgeranylation through inhibition of GGPPS, which led to ultrastructural changes of osteoclasts at lower concentrations and to apoptosis at high concentrations.29,30It would be of important clinical value to determine the individualized anti-osteoporosis therapy on the basis of the pharmacogenetic information of GGPPS and BPs. We detected all tag SNPs of GGPPS, which could almost represent the whole genetic variance of this gene. We evaluate the correlation between the genetic variation of GGPPS and bone response to alendronate in large sample of Chinese women. However, according to percentage changes of BMD and bone turnover biomarkers, no correlation was found between the genetic variation of GGPPS and bone responsiveness to alendronate, either in low-dose or in standard-dose group. Consistently, in Korean women, GGPPS rs3840452 polymorphism was also uncorrelated to the effects of alendronate.27
Our study has several limitations. Most of the ALP isoenzymes are derived from the bones and liver. Total ALP was measured rather than bone specific ALP in our study. The alendronate treatment period was rather short. We found serum 25OHD level was very low in this population, which was reported to affect the response to anti-resorbing drugs.31All patients were supplemented with only 125 IU of vitamin D3, which could not rule out the influence of vitamin D deficiency on the response to alendronate therapy. On the other hand, we found the correlation of rs10925503 polymorphism of GGPPS with β-CTX level, but the mechanisms involved in the genotype-related differences remained to be elucidated. We did not investigate the correlation between the GGPPS polymorphisms and other kinds of aminobisphosphonate, such as zoledronic acid or ibandronate, so the results could not represent other BPs and GGPPS.
In conclusion, rs10925503 polymorphism of GGPPS gene is correlated to activity of osteoclasts, but all tag SNPs of GGPPS have no influence on baseline BMD and skeletal response to alendronate. Therefore, GGPPS could only act as a candidate gene for bone resorption, instead of a pharmacogenetic gene for forecasting effects of alendronate.
ACKNOWLEDGMENTS
All the samples in the study come from seven clinical centers in China (Beijing, Changsha, Shanghai, Chengdu, Xi’an, Guangzhou, and Harbin). We acknowledge the support of clinical data collection for the research.
REFERENCES
1. Prior JC, Langsetmo L, Lentle BC, et al. Ten-year incident osteoporosis-related fractures in the population-based Canadian multicentre osteoporosis study−Comparing site and age-specific risks in women and men. Bone 2015;71:237-43.
2. Berglundh S, Malmgren L, Luthman H, et al. C-reactive protein, bone loss, fracture, and mortality in elderly women: a longitudinal study in the OPRA cohort. Osteoporos Int 2015; 26:727-35.
3. Mitchell BD, Streeten EA. Clinical impact of recent genetic discoveries in osteoporosis. Appl Clin Genet 2013; 6: 75-85.
4. Lee SH, Lee SW, Ahn SH, et al. Multiple gene polymorphisms can improve prediction of nonvertebral fracture in postmenopausal women. J Bone Miner Res 2013; 28: 2156-64.
5. Otrock ZK, Mahfouz RA, Charafeddine KM, et al. Vitamin D receptor genotypes and response to zoledronic acid therapy in thalassemia-induced osteoporosis. Ann Hematol 2008; 87:947-8.
6. Sai AJ, Gallagher JC, Fang X. Effect of hormone therapy and calcitriol on serum lipid profile in postmenopausal older women: association with estrogen receptor-α genotypes. Menopause 2011; 18:1101-12.
7. Kruk M, Ralston SH, Albagha OM. LRP5 polymorphisms and response to risedronate treatment in osteoporotic men. Calcif Tissue Int 2009; 84:171-9.
8. Zhou PR, Liu HJ, Liao EY, et al. LRP5 polymorphisms and response to alendronate treatment in Chinese postmenopausal women with osteoporosis. Pharmacogenomics 2014; 15:821-31.
9. Olmos JM, Zarrabeitia MT, Hernández JL, et al. Common allelic variants of the farnesyl diphosphate synthase gene influence the response of osteoporotic women to bisphosphonates. Pharmacogenomics J 2012; 12:227-32.
10. Favus MJ. Bisphosphonates for osteoporosis. NEJM 2010; 363:2027-35.
11. Russell RG. Bisphosphonates: the first 40 years. Bone 2011; 49:2-19.
12. Burnett-Bowie SM, Saag K, Sebba A. Prediction of changes in bone mineral density in postmenopausal women treated with once-weekly bisphosphonates. J Clin Endocrinol Metab 2009; 94:1097-103.
13. Emkey R, Delmas PD, Bolognese M, et al. Efficacy and tolerability of once-monthly oral ibandronate (150 mg) and once-weekly oral alendronate (70 mg): additional results from the monthly oral therapy with ibandronate for osteoporosis intervention (MOTION) study. Clin Ther 2009; 31:751-61.
14. Orwoll ES1, Binkley NC, Lewiecki EM. Efficacy and safety of monthly ibandronate in men with low bone density. Bone 2010; 46:970-6.
15. Tsubaki M, Komai M, Itoh T, et al. Nitrogen-containing bisphosphonates inhibit RANKL- and M-CSF-induced osteoclast formation through the inhibition of ERK1/2 and Akt activation. J Biomed Sci 2014; 21:10.
16. Dudakovic A, Wiemer AJ, Lamb KM, et al. Inhibition of geranylgeranyl pyrophosphate synthase induces apoptosis through multiple mechanisms and displays synergy with inhibition of other isoprenoid biosynthetic enzyme. JPET 2008; 324:1028-36.
17. Liu Y, Liu H, Li M, et al. Association of farnesyl diphosphate synthase polymorphisms and response to alendronate treatment in Chinese postmenopausal women with osteoporosis. Chin Med J 2014; 127:662-8.
18. Chen SH, Lin SW, Lin SR, et al. Moiety-linkage map reveals selective nonbisphosphonate inhibitors of human geranylgeranyl diphosphate synthase. J Chem Inf Model 2013; 53:2299-311.
19. Ericsson J, Greene JM, Carter KC, et al. Human geranylgeranyl diphosphate synthase: isolation of the cDNA, chromosomal mapping and tissue expression. J Lipid Res 1998; 39:1731-9.
20. Cummings SR, Bates D, Black DM. Clinical use of bone densitometry scientific review. JAMA 2002; 288:1889-97.
21. Kanis JA, Melton LJ, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res 1994; 9:1137-41.
22. Kavanagh KL, Guo K, Dunford JE, et al. The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc Natl Acad Sci USA 2006; 103:7829-34.
23. Tsubaki M, Kato C, Nishinobo M, et al. Nitrogen-containing bisphosphonate, YM529/ONO-5920, inhibits macrophage inflammatory protein 1 alpha expression and secretion in mouse myeloma cells. Cancer Sci 2008; 99:152-8.
24. Ling Y, Li ZH, Miranda K, et al. The farnesyl-dipho-sphate/ geranylgeranyl-diphosphate synthase of toxoplasma gondii is a bifunctional enzyme and a molecular target of bisphosphonates. J Biol Chem 2007; 282:30804-16.
25. Fisher JE, Rogers MJ, Halasy JM, et al. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci USA 1999; 96:133-8.
26. Coxon FP, Helfrich MH, Van’t Hof R, et al. Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Miner Res 2000; 15:1467-76.
27. Choi HJ, Choi JY, Cho SW. Genetic polymorphism of geranylgeranyl diphosphate synthase (GGSP1) predicts bone density response to bisphosphonate therapy in Korean women. Yonsei Med J 2010; 51:231-8.
28. Marini F, Brandi ML. Pharmacogenetics of osteoporosis: future perspectives. Calcif Tissue Int 2009; 84:337-47.
29. Benford, HL, McGowan NW, Helfrich MH, et al. Visualization of bisphosphonate-induced caspase-3 activity in apoptotic osteoclasts in vitro. Bone 2001; 28:465-73.
30. Halasy-Nagy JM, Rodan GA, Reszka AA. Inhibition of bone resorption by alendronate and risedronate does not require osteoclast apotosis. Bone 2001; 29:553-9.
31. Ishijima M, Sakamoto Y, Yamanaka M, et al. Minimum required vitamin D level for optimal increase in bone mineral density with alendronate treatment in osteoporotic women. Calcif Tissue Int 2009; 85:398-404.
for publication May 24, 2015.
†These authors contributed equally to this work.
*Corresponding author Tel: 86-10-69155088, Fax: 86-10-69155088, E-mail: limeilzh@sina.com
△Supported by National Natural Science Foundation of China (81570802) and National Key Program of Clinical Science (WBYZ2011-873).
 Chinese Medical Sciences Journal2016年1期
Chinese Medical Sciences Journal2016年1期
- Chinese Medical Sciences Journal的其它文章
- Pathology Verified Concomitant Papillary Thyroid Carcinoma in the Sonographically Suspected Thyroid Lymphoma: A Case Report△
- Life-threatening Spontaneous Retroperitoneal Haemorrhage: Role of Multidetector CT-angiography for the Emergency Management
- Percutaneous Removal of Benign Breast Lesions with an Ultrasound-guided Vacuum-assisted System: Influence Factors in the Hematoma Formation△
- Respiratory and Cardiac Characteristics of ICU Patients Aged 90 Years and Older: A Report of 12 Cases
- Establish Albumin-creatinine Ratio Reference Value of Adults in the Rural Area of Hebei Province△
- Positive Rate of Different Hepatitis B Virus Serological Markers in Peking Union Medical College Hospital, a General Tertiary Hospital in Beijing△
