腹透患者心血管钙化的发生率、分布特点及相关影响因素
陈关芬 辛丽芹 杨丽华
腹透患者心血管钙化的发生率、分布特点及相关影响因素
陈关芬辛丽芹杨丽华
目的通过对腹膜透析患者的心脏瓣膜钙化及主动脉弓钙化进行评价,分析心血管钙化的发生率、分布特点及相关影响因素。方法选取174例腹膜透析患者,分别于统一时间段测量肢肱动脉血压,分别记录患者的血尿素氮、肌酐值;24 h的尿量、透出液量、尿素氮、肌酐值及透出液尿素氮、肌酐浓度值;并通过上述测定值计算评估透析充分性和残余肾功能。每隔6个月测定患者的生化指标。对腹膜透析患者心脏进行多普勒超声检查和患者胸部X线片检查,用Logistics回归分析评判心血管钙化危险因素。结果主动脉弓钙化及瓣膜钙化类别中钙化患者的年龄、透析龄及血磷值均高于非钙化患者(P<0.05);钙化患者的残余肾功能值显著低于非钙化患者(P<0.05);随着年龄/透析龄的增加,瓣膜/主动脉弓钙化的发生率显著上升(P<0.05)。年龄、透析龄和钙磷乘积均是影响心脏瓣膜钙化的独立危险因素(P<0.05),其中钙磷乘积危险系数最高;年龄、透析龄和血磷是影响主动脉弓钙化的独立危险因素(P<0.05),其中血磷危险系数最高;残余肾功能是能影响心脏瓣膜/主动脉弓钙化的独立因素(P<0.05),而且是保护因素。结论年龄和透析龄是影响心脏瓣膜钙化及主动脉弓钙化的独立危险因素,钙磷乘积是影响心脏瓣膜钙化危险系数最高的独立因素,血磷是影响主动脉弓钙化危险系数最高的独立因素。
腹膜透析患者;心脏瓣膜钙化;主动脉弓钙化;心血管钙化
腹膜透析作为终末期肾病替代治疗方式之一,可有效延长病患的生存时间,与血液透析相比,临床更能保持肾功能衰竭患者的心血管稳定性及保护残余肾功能(residual renal function, RRF),且腹透所需设备简单,操作便易,更适于家庭透析使用[1-4]。随着腹透技术的广泛应用,其带来的并发症也引起了医疗工作者的关注。Remón-Rodríguez等[5]发现腹透的主要并发症是心血管疾病(cardiovascular disease, CVD),发病率大于30%。CVD是慢性肾脏疾病(chronic kidney diseases, CKD)患者常见并发症,而心血管钙化是慢性肾脏疾病患者CVD的主要原因之一[6-8]。在正常的机体条件下,钙化是对矿物质化进行及诱导的精控步骤,不会产生血管钙化[9]。但是当机体发生某处病变时,钙化就会成为不可控的,一般认为血管粥样硬化及年龄对血管钙化起着正调控的作用[10]。然而具体是什么因素影响着终末期肾功能衰竭患者的心血管钙化,尚不完全清楚。因此本研究以腹透患者为研究对象,分别以心超检测心脏瓣膜,胸部X线检测和评估主动脉弓钙化清况,探讨腹膜透析患者心血管钙化的发生率及影响因素分析,以期对临床有一定的理论指导。
1 资料与方法
1.1一般资料选取2012年6月至2015年6月在云南省曲靖市第二人民医院和昆明医科大学第二附属医院腹膜透析治疗中心进行腹透的患者174例,其中男104例,女70例;平均年龄(63±14)岁;平均BMI(23.4±2.4)kg/m2;透析龄6~93个月,平均透析19个月;收缩压(145±12)mm Hg;舒张压(79±10)mm Hg;平均动脉压(101±8)mm Hg;残余肾功能(5.3±4.2)ml/min;腹膜Kt/V(3.5±2.6);残肾Kt/V(2.0±1.9);腹膜Ccr(48±26)L/周;残肾Ccr(29±19)L/周;iPTH(2.37±2.48)pg/ml;校正钙(2.55±0.26)mmol/L;血磷(1.7±0.4)mmol/L;血红蛋白(102±18)g/L;血糖(6.5±2.1)mmol/L;TG(2.0±1.2)mmol/L;TC(4.8±0.9)mmol/L;LDL(2.7±0.8)mmol/L;HDL(1.0±0.3)mmol/L。透析龄>12个月,患者年龄>18岁;排除外伤、创伤及严重感染患者,长期服用糖皮质激素,合并甲亢性心脏病及恶性骨肿瘤疾病等。研究方案经云南省曲靖市第二人民医院伦理委员会批准,进行的所有研究内容均知会受试人员并签署知情同意书。
1.2方法患者分别于统一时间段测量肢肱动脉血压3次,并求取平均动脉压。分别记录患者的血尿素氮、肌酐值;24 h的尿量、透出液量、尿素氮、肌酐值及透出液尿素氮、肌酐浓度值;并通过上述测定值计算评估透析充分性和残余肾功能。每隔6个月测定患者的生化指标[iPTH、校正钙、血磷、血红蛋白、血糖、三酰甘油(TG)、低密度脂蛋白(LDL)、高密度脂蛋白(HDL)、胆固醇(TC)],共计3次测量数据。对腹膜透析患者心脏进行多普勒超声检查,瓣膜钙化指主动脉瓣或二尖瓣有一个或多个瓣叶上有1 mm以上的强回声区。对腹膜透析患者胸部进行X线片检查,主动脉弓钙化有4个等级:0级(未见瓣膜钙化)、1级(钙化区域呈点状或薄片状)、2级(一个及以上区域的钙化增厚增密)和3级(钙化呈圆周样)。
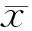
2 结果
2.1所选腹膜透析患者的一般情况分析比较174例中,男104例(59.77%);平均年龄(63±14)岁;主动脉弓钙化及瓣膜钙化类别中钙化患者的年龄、透析龄及血磷值均显著高于非钙化患者(P<0.05);钙化患者的残余肾功能值显著低于非钙化患者(P<0.05);体重指数(BMI)、收缩压、舒张压、平均动脉压、腹膜Kt/V、残肾Kt/V、腹膜肌酐清除率(creatinine clearance rate,Ccr)、残肾Ccr、全段甲状旁腺激素(intact parathyroid hormone, iPTH)、校正钙、血红蛋白、血糖、TG、TC、LDL及HDL在钙化及非钙化患者差异无统计学意义(P>0.05)。见表1。
2.2腹膜透析患者主动脉弓/瓣膜钙化情况分析主动脉弓/瓣膜钙化类别中钙化组与非钙化组中年龄及透析龄存在差异性,因此根据不同的年龄/透析龄把瓣膜/主动脉弓钙化患者分别分为3组,并比较钙化发生率。随着年龄/透析龄的增加,瓣膜/主动脉弓钙化的发生率显著上升。见表2、3,图1、2。
2.3腹膜透析患者主动脉弓/瓣膜钙化危险因素分析年龄、透析龄和钙磷乘积均是影响心脏瓣膜钙化的独立危险因素,其中钙磷乘积危险系数最高,而血磷被排除;年龄、透析龄和血磷是影响主动脉弓钙化的独立危险因素,其中血磷危险系数最高,钙磷乘积不是影响主动脉弓钙化的独立因素;残余肾功是能影响心脏瓣膜/主动脉弓钙化的独立因素,而且是保护因素。见表4、5。
3 讨论
心血管疾病是慢性肾脏疾病患者常见并发症,而心血管钙化则是主要原因之一。Ogawa等[11]研究发现维持性血液透析患者中50.60%存在主动脉弓钙化;Inoue等[12]的研究结果类似,主动脉弓钙化发生率为54.30%;Choi等[13]研究发现终末期肾病患者血液透析后,有29.90%的患者发生心脏瓣膜钙化;Takahashi等[14]研究发现,有57.50%的血液透析患者发生心脏瓣膜钙化。本研究对象为腹膜透析患者,心脏瓣膜钙化及主动脉弓钙化发生率均低于文献中的血液透析患者;可能与血液透析患者的血流量较高有关,血液动力学不稳定,从而易对血管壁造成冲击力,最终损伤血管内皮细胞。
表1 所选腹膜透析患者的一般情况分析 ±s
注:与无钙化患者比较,*P<0.01
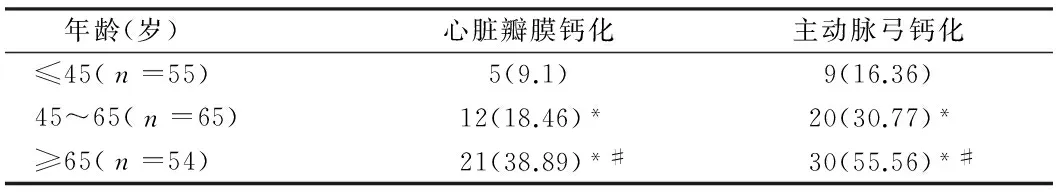
表2 不同年龄患者的心脏瓣膜/主动脉弓钙化发生率比较 例(%)
注:与≤45岁比较,*P<0.05;与45~65岁比较,#P<0.05

表3 不同透析龄患者的心脏瓣膜/主动脉弓钙化发生率比较 %
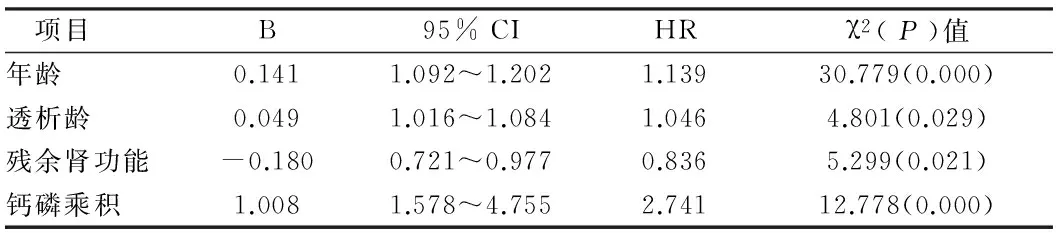
表4 心脏瓣膜钙化危险因素

图1 不同透析龄/年龄心脏瓣膜钙化情况
注:≥37月vs≤12月,OR=17.834,P=0.000;≥37月vs≤13~36月,OR=4.412,P=0.000;≥66岁vs≤45岁,OR=7.511,P=0.004;≥66岁vs≤46~65岁,OR=2.895,P=0.004
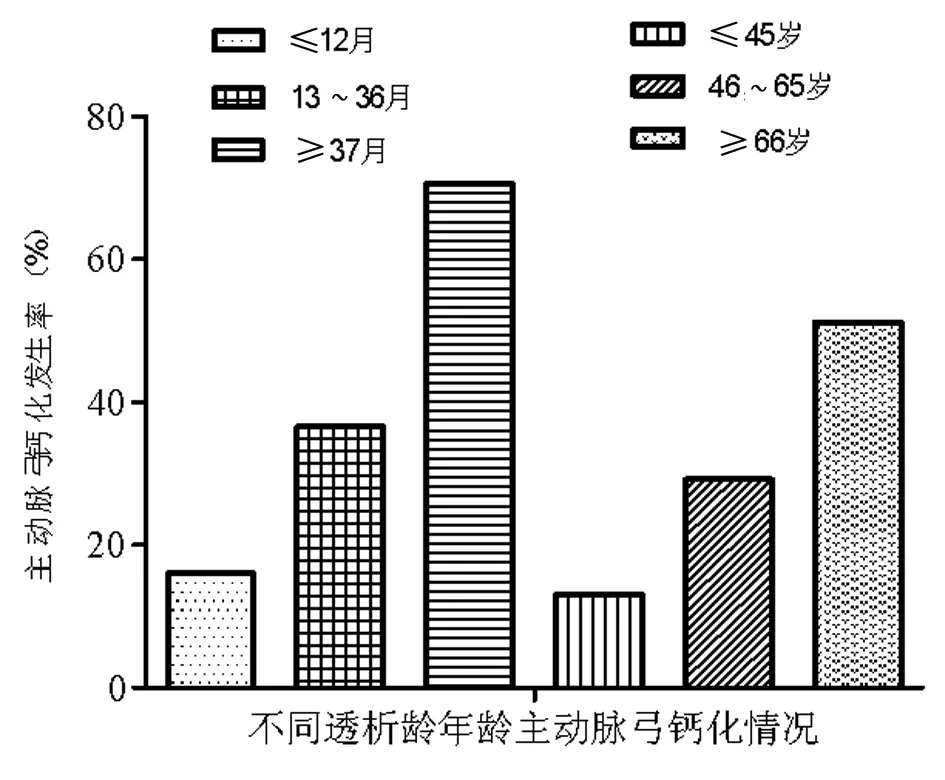
图2 不同透析龄/年龄主动脉弓钙化情况
注:≥37月vs≤12月,OR=12.534,P=0.000;≥37月vs≤13~36月,OR=4.098,P=0.001;≥66岁vs≤45岁,OR=7.102,P=0.002;≥66岁vs≤46~65岁,OR=2.624,P=0.005
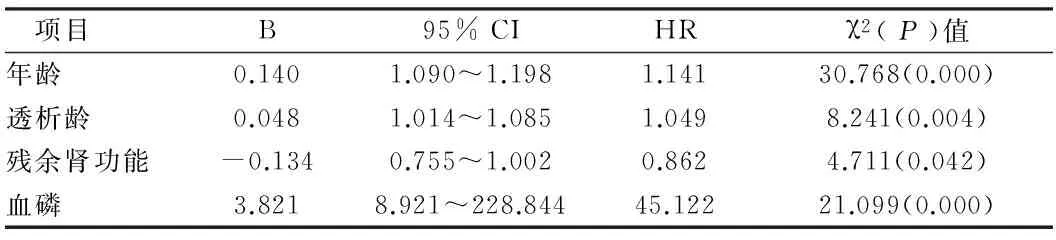
表5 主动脉弓钙化危险因素
Panuccio等[15]的研究结果表明,透析患者中心脏瓣膜钙化者的透析龄及年龄均显著高于心脏瓣膜无钙化者;Avila-Díaz等[16]的研究结果表明,腹透患者中的心脏瓣膜钙化进展较快者,年龄更高。在本研究中,Logistics回归分析表明年龄和透析龄是影响心脏瓣膜钙化及主动脉弓钙化的独立危险因素;而且心脏瓣膜钙化及主动脉弓钙化在不同的年龄和透析龄中表现出显著的差异性。年龄≥66岁心脏瓣膜钙化及主动脉弓钙化的发生率分别是小于45岁人群的4.77倍和3.93倍;透析时间大于37月心脏瓣膜钙化及主动脉弓钙化的发生率分别是透析时间小于12月人群的7.72 倍和4.41倍。
体外数据研究发现,在高浓度磷的诱导作用下,血管平滑肌细胞会逐渐向成骨软骨细胞转化[17, 18];Chen等[19]的研究数据表明,用尿毒症病人含磷的血清与血管平滑肌细胞在体外共培养,可使细胞钙化;Reynolds等[20]认为血钙和血磷在血管钙化中起协同作用;Shroff等[21]的研究表明在相同的钙磷乘积下,高磷的诱导血管平滑肌细胞钙化的效率要低于高钙。临床研究也证明终末端肾病患者钙磷发生与心血管钙化有显著的相关发生。在本研究中,Logistics回归分析表明除年龄和透析龄,钙磷乘积是影响心脏瓣膜钙化危险系数最高的独立因素,血磷是影响主动脉弓钙化危险系数最高的独立因素。因此在肾衰病人腹膜透析过程中,要注重防治高磷血和高钙血的发生,限制磷和钙的摄入,并尽量减少钙磷结合剂的使用。
综上所述,本研究通过对腹膜透析患者的一般情况、主动脉弓钙化、瓣膜钙化情况及相关危险因素进行分析,揭示了年龄和透析龄是影响心脏瓣膜钙化及主动脉弓钙化的独立危险因素,钙磷乘积是影响心脏瓣膜钙化危险系数最高的独立因素,血磷是影响主动脉弓钙化危险系数最高的独立因素。
1Kwong WK, Li KT.Peritoneal dialysis in Asia. Kidney Diseases,2015,1:S381-S385.
2Williams A. Hemodialysis and peritoneal dialysis// Pediatric Urology: Surgical Complications and Management.John Wiley & Sons,2015,27:305-314.
3Beduschi GC,Figueiredo AE,Olandoski M,et al.Automated Peritoneal Dialysis Is Associated with Better Survival Rates Compared to Continuous Ambulatory Peritoneal Dialysis:A Propensity Score Matching Analysis.PLoS One,2015,10:e0138382.
4Wiggins KJ,Craig JC,Johnson DW,et al.Treatment for peritoneal dialysis-associated peritonitis.Cochrane Database of Systematic Reviews,2008,4:CD005284-CD005284.
5Remón-Rodríguez C, Quirós-Ganga P, Portolés-Pérez J, et al. Results of the cooperative study of Spanish peritoneal dialysis registries: analysis of 12 years of follow-up. Nefrología Publicación Oficial De La Sociedad Espaola Nefrologia,2014,34:18-33.
6Hajhosseiny R,Khavandi K,Goldsmith DJ.Cardiovascular disease in chronic kidney disease: untying the Gordian knot.International Journal of Clinical Practice,2013,67:14-31.
7Lees JS,Mark PB,Jardine AG. Cardiovascular complications of chronic kidney disease. Medicine,2015,43:469-473.
8Gajjala PR,Fliser D,Speer T, et al. Emerging role of post-translational modifications in chronic kidney disease and cardiovascular disease.Nephrology Dialysis Transplantation,2015,30:1814-1824.
9Giachelli CM.The emerging role of phosphate in vascular calcification. Kidney International,2009,75:890-897.
10Adeva-Andany MM, Fernández-Fernández C, Sánchez-Bello R, et al. The role of carbonic anhydrase in the pathogenesis of vascular calcification in humans. Atherosclerosis,2015,241:183-191.
11Ogawa T,Ishida H,Akomatsu M,et al.Progression of aortic arch calcification and all-cause and cardiovascular mortality in chronic hemodialysis patients.International Urology & Nephrology,2010,42:187-194.
12Inoue T, Ogawa T, Ishida H, et al. Aortic arch calcification evaluated on chest X-ray is a strong independent predictor of cardiovascular events in chronic hemodialysis patients.Heart & Vessels,2012,27:135-142.
13Choi MJ,Kim JK,Kim SG,et al.Association between cardiac valvular calcification and myocardial ischemia in asymptomatic high-risk patients with end-stage renal disease.Atherosclerosis,2013,229:369-373.
14Takahashi H, Ishii H, Aoyama T,et al. Association of Cardiac Valvular Calcifications and C-Reactive Protein With Cardiovascular Mortality in Incident Hemodialysis Patients.A Japanese Cohort Study,2013,61:254-261.
15Panuccio V,Tripepi R,Tripepi G,et al.Heart valve calcifications, survival, and cardiovascular risk in hemodialysis patients.American Journal of Kidney Diseases the Official Journal of the National Kidney Foundation,2004,43:479-484.
16Avila-Díaz M,Mora-Villalpando C,Ma PU,et al.De novo Development of Heart Valve Calcification in Incident Peritoneal Dialysis Patients. Archives of Medical Research,2013,44:638-644.
17Jono S,Peinado C,Giachelli CM.Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification.Journal of Biological Chemistry,2000,275:20197-20203.
18Steitz SA,Speer MY,Curinga G,et al.Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers.Circulation Research,2001,89:1147-1154.
19Chen NX,O'Neill KD,Danxia D, et al. Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells. Kidney International,2002,62:1724-1731.
20Reynolds JL,Joannides AJ,Skepper JN,et al.Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD.Journal of the American Society of Nephrology,2004,15:2857-2867.
21Shroff RC,McNair R,Skepper JN,et al.Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification.Journal of the American Society of Nephrology,2010,21:103-112.
The incidence rate,distribution characteristics and related influencing factors of cadiovascular calcification in peritoneal dialysis patients
CHENGuanfen*,XINLiqin*,YANGLihua.
*DepartmentofNephropathy,TheSecondPeople’sHospitalofQujingCity,Yunnan,Qujing655000,China
ObjectiveTo evaluate the cardiac valve calcification and aortic arch calcification in peritoneal dialysis patients, and to analyze the incidence rate,distribution characteristics and related influencing factors of cadiovascular calcification.MethodsOne hundred and seventy-four patients who underwent peritoneal dialysis in our hospital were enrolled in the study. The patient’s blood pressure of brachial artery was measured in unification time point,and blood urea nitrogen,creatinine values, 24-hour urine volume,dialysis liquid volume,urea nitrogen,creatinine and contents of urea nitrogen and creatinine in dialysis liquid were detected to evaluate the dialysis efficiency and residual renal function of patients. The biochemical indicators of patients were detected at an interval of 6 months,moreover, the patients received Doppler ultrasonography and chest X-ray examination,finally, the risk factors influencing cardiovascular calcification were evaluated by Logistics regression.ResultsThe average age [(63±14)years],dialysis duration and serum levels of phosphorus in patients with aortic arch calcification and heart valve calcification were significantly higher than those of patients without calcification (P<0.05), and residual renal function values were significantly lower than those of of patients without calcification (P<0.05). The incidence of cardiac valve calcification and aortic arch calcification was significantly increased along with the growth of patient’s age and dialysis duration (P<0.05). The patient's age, dialysis duration and calcium-phosphorus product were independent risk factors influencing cardiac valve calcification (P<0.05),in which, the danger coefficient of calcium-phosphorus product was the highest,moreover, patient’s age,dialysis duration and serum levels of phosphorus were independent risk factors influencing aortic arch calcification (P<0.05),in which, the danger coefficient of serum levels of phosphorus was the highest (P<0.05).Besides residual renal function of patients was independent risk factors influencing cardiac valve calcification and aortic arch calcification,furthermore,which was protection factor.ConclusionThe patient’s age and dialysis duration are independent risk factors influencing cardiac valve calcification and aortic arch calcification.The calcium-phosphorus product is independent factor influencing cardiac valve calcification, with the highest danger coefficient,besides, the serum level of phosph is independent factor influencing aortic arch calcification, with the highest danger coefficient
peritoneal dialysis patients;heart valve calcification;aortic arch calcification;cardiovascular calcification
655000云南省曲靖市第二人民医院肾病学科(陈关芬、辛丽芹);昆明医科大学第二附属医院(杨丽华)
R 459.51
A
1002-7386(2016)16-2409-04
2016-01-07)
doi:10.3969/j.issn.1002-7386.2016.16.002
项目来源:云南省科技计划项目(编号:2012FB162)

