Adipolin/CTRP12对LPS致ARDS小鼠的保护作用及肺泡上皮钠离子通道的调节*
唐旭毛, 戚 迪, 王导新
(重庆医科大学附属第二医院呼吸内科,重庆400010)
Adipolin/CTRP12对LPS致ARDS小鼠的保护作用及肺泡上皮钠离子通道的调节*
唐旭毛, 戚 迪, 王导新△
(重庆医科大学附属第二医院呼吸内科,重庆400010)
目的:探讨adipolin/CTRP12对LPS致ARDS小鼠的作用及肺泡上皮钠离子通道(ENaC)的调节。方法:40只C57BL/6J小鼠按随机数字表分为对照组、LPS组、adipolin组和wortmannin(PI3K抑制剂)组,每组10只。苏木精-伊红(HE)染色观察肺组织病理改变;伊文氏蓝标记蛋白检测肺水清除率(AFC),BCA法测支气管肺泡灌洗液(BALF)中蛋白含量,评估肺组织通透性改变;ELISA检测BALF中白细胞介素(IL)-1β和肿瘤坏死因子(TNF)-α含量,髓过氧化物酶(MPO)试剂盒检测MPO活性,吉姆萨染色计数BALF总细胞数和多形核白细胞数,Western blot法检测肺组织α-ENaC蛋白表达和Akt磷酸化水平,实时荧光定量PCR检测肺组织α-ENaC的mRNA转录水平。结果:与control组相比,LPS组表现出典型的ARDS病理改变,肺损伤明显(P<0.05),湿干重比(W/ D)和BALF蛋白含量明显增高而AFC明显减弱(P<0.05),BALF细胞计数、MPO活性、IL-1β含量和TNF-α含量明显升高(P<0.05),而肺α-ENaC表达和Akt磷酸化水平显著下调(P<0.05)。与LPS组相比,adipolin组肺损伤明显减轻(P<0.05),W/D和BALF蛋白含量明显降低而AFC明显增强(P<0.05),且BALF细胞计数、MPO活性、IL-1β含量和TNF-α含量均较LPS组显著降低(P<0.05),伴α-ENaC表达和Akt磷酸化水平显著上调(P<0.05)。而PI3K抑制剂wortmannin组与adipolin组相比,其肺损伤明显加重(P<0.05),W/D和BALF蛋白含量明显增高,AFC明显减弱(P<0.05),BALF细胞计数、MPO活性、IL-1β含量和TNF-α含量明显升高(P<0.05),同时伴α-ENaC表达和Akt磷酸化水平显著下调(P<0.05)。结论:Adipolin/CTRP12可通过PI3K/Akt信号通路介导的α-ENaC上调机制,增强肺水肿液清除能力,从而对LPS所致ARDS小鼠发挥保护性调控作用。
急性呼吸窘迫综合征;Adipolin/CTRP12;上皮钠离子通道;PI3K/Akt信号通路
急性呼吸窘迫综合症(acute respiratory distress syndrome,ARDS)是以气体交换严重损害为特征的难治性低氧血症,其特征为以中性粒细胞为主的炎症细胞肺内浸润,大量富含蛋白的水肿液累积于肺泡腔,氧合指数显著降低[1]。因而有效地清除肺泡腔水肿液对维持正常气体交换功能及降低ARDS死亡率有重要意义[2]。肺上皮细胞钠通道(epithelial sodium channel,ENaC)由α、β和γ 3个亚基组成,广泛分布于肺I型[3-4]和II型[5-6]上皮细胞,是肺泡腔水肿液清除(alveolar fluid clearance,AFC)的关键作用环节[7]。
新发现的脂肪因子adipolin/CTRP12与脂联素(adiponectin)同源,并具有胰岛素增敏效应[8]。研究发现adipolin能抑制脂肪组织炎症细胞浸润及炎症因子表达[9],从而发挥抗炎性保护作用。本课题组前期研究中证实,胰岛素可通过PI3K/Akt信号通路抑制肺炎症反应,并上调α-ENaC的表达,增强肺水肿液清除,从而多方面参与对ARDS的保护性调节效应[10-11]。因而,我们推测adipolin可减轻ARDS炎症反应并通过PI3K/Akt信号通路调节α-ENaC表达,增强肺水肿液清除能力,从而改善ARDS预后。
本研究旨在通过动物实验探讨adipolin在ARDS中的作用及其机制,并丰富对adipolin的生物学功能认识。
材料和方法
1 实验动物
40 只SPF级雄性C57BL/6小鼠,5~8周龄,体重18~22 g,购自重庆医科大学动物实验中心,并自由饮食、普通光照饲养于重庆医科大学动物实验中心。
2 试剂和仪器
脂多糖(lipopolysaccharide,LPS)、PI3K抑制剂渥曼青霉素(wortmannin)及adipolin购自Sigma;总RNA提取试剂盒HiScript 1st Strand cDNA Synthesis Kit、HiScript II One Step RT-qPCR SYBR Green Kit(Vazyme Biotech Co.)和DNA marker DL1000购自大连宝生物工程有限公司;RIPA裂解液、BCA蛋白浓度测定试剂盒及Western blot配胶试剂盒购自碧云天生物技术研究所;ELISA试剂盒购自北京四正柏生物科技有限公司;髓过氧化物酶(myeloperoxidase,MPO)检测试剂盒购自南京建成工程生物研究所;甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase,GAPDH)、α-ENaC、Akt及p-Akt单克隆抗体购自Abcam;ECL检测试剂盒购自南京凯基生物科技有限公司。37℃培养箱、60℃烤箱、摇床、水浴锅、电泳仪、低温高速离心机等实验仪器由重庆医科大学生命科学研究院提供。
3 实验方法
3.1 小鼠ARDS模型的建立 40只小鼠按随机数字表分为对照(control)组、LPS组、adipolin组和wortmannin组,每组10只。腹腔注射4%水合氯醛麻醉小鼠(0.2 mL/20 g)。固定小鼠,adipolin组、LPS组和wortmannin组小鼠通过气管插管,以5 μg/ g体重滴注无菌20%LPS(10 μg LPS溶于50 μL无菌生理盐水)建立ARDS模型。对照组给予等量无菌生理盐水。Wortmannin组在LPS注入前、后90 min分别腹腔注射渥曼青霉素(0.06 μg/g),adipolin组于LPS注射后20 min腹腔注射adipolin(2 mg/ kg),control组给予等量生理盐水。24 h后,麻醉并腹主动脉放血处死小鼠。
3.2 小鼠肺组织病理切片染色并评分[12]取右下肺组织,4%多聚甲醛固定,制成5 μm的石蜡切片,苏木精-伊红(HE)染色,光镜观察。肺损伤评分标准:(1)肺泡出血;(2)肺泡水肿;(3)肺泡腔或血管内中性粒细胞浸润或聚集;(4)肺泡壁增厚和(或)透明膜形成及炎症细胞浸润。依据病变轻重评0~4分(0分为无病变或非常轻微;1分为轻度病变; 2分为中度病变;3分为重度病变;4分为极重度病变)。ARDS总评分为各项评定分数相加总和。
3.3 小鼠肺湿干重比(W/D) 取左肺,称量肺湿重,再置于60℃烤箱48 h至恒重后,称量其干重。根据公式计算肺W/D值。
3.4 小鼠支气管肺泡灌洗液(bronchoalveolar lavagefluid,BALF)细胞计数、蛋白含量、IL-1β含量、TNF-α含量及MPO活性测定 用1 mL PBS反复灌注小鼠肺组织 3次,并确保回收率大于 90%。收集BALF,4℃12 000 r/min离心20 min。取上清液,按照BCA蛋白测定试剂盒说明以及各试剂盒要求,测定BALF蛋白含量、IL-1β和TNF-α含量以及MPO活性。无菌PBS重悬下层沉淀后吉姆萨染色,光镜下计细胞总数及白细胞数[13]。
3.5 小鼠肺泡液体清除率(alveolar fluid clearance,AFC) 制备0.15 g/L伊文氏蓝标记的5%白蛋白无菌等渗生理盐水,以5 mL/kg气管滴注,并注入1 mL空气保证液体分布。于37℃机械通气1 h后,测定肺泡体内液体伊文氏蓝标记的白蛋白浓度,并计算AFC。肺泡液清除率公式为AFC(%)=(Vi-Vf)/ Vi×100%,Vf=Vi×Pi/Pf,其中Vi为注入肺泡内液体的量,Vf为肺泡内剩余的液体量,Pi为注入的伊文氏蓝标记的白蛋白浓度,Pf为最后伊文氏蓝标记的白蛋白浓度。
3.6 qPCR法测定小鼠肺组织α-ENaC的mRNA表达水平 按照总RNA提取试剂盒说明书提取小鼠肺组织总RNA,紫外分光光度计测总RNA浓度。再根据反转录试剂盒说明书,以小鼠肺组织总RNA为模板,反转录合成cDNA。随后按照扩增试剂盒说明书进行 PCR扩增。α-ENaC的上游引物为 5’-TACAACTCTTCCTACACTCGCCA-3’,下游引物为5’-CTGGTTGAAACGACAGGTAAAGAT-3’;内参照β-actin的上游引物5’-CGAGCGGGCTACAGCTTC-3’,下游引物为5’-GTCACGCACGATTCCCTCT-3’。qPCR检测α-ENaC的mRNA水平。PCR反应条件: 94℃5 min;94℃30 s,57℃ 30 s,72℃ 60 s,72 ℃ 10 min,共35个循环。用2-ΔΔCt法计算mRNA相对表达量。
3.7 Western blot法检测小鼠肺组织α-ENaC和p-Akt的蛋白水平 按RIPA试剂盒要求提取小鼠肺组织蛋白,BCA法测定蛋白浓度,行聚丙烯酰氨凝胶电泳分离蛋白,湿转法转移至聚偏二氟乙烯膜(PVDF 膜),5%脱脂奶粉或5%BSA封闭1 h后,加α-ENaC抗体(1∶500)、Akt(1∶1 000)、p-Akt(1∶800)或GAPDH抗体(1∶1 000)4℃孵育过夜。TBST洗膜10 min 3次,加入相应辣根过氧化物酶标记的II抗37℃孵育1 h,再以TBST洗膜3次,每次10 min,最后ECL法显像,Quantity One软件分析条带吸光度值。
4 统计学处理
采用SPSS 13.0统计软件进行统计分析,计量资料用均数±标准差(mean±SD)表示,组间比较采用单因素方差分析(one-way ANOVA),各组均数间两两比较采用SNK-q检验法,以P<0.05为差异有统计学意义。
结果
1 小鼠肺组织HE染色及损伤评分
HE染色病理切片光镜下可见control组肺组织无炎性细胞浸润,肺泡未见出血、水肿,肺泡间隔均一,无明显病理变化;LPS组可见大量炎症细胞浸润,肺泡间隔明显增厚,肺泡出血、水肿明显,肺组织可见明显炎症细胞聚集;adipolin组可见肺泡间隔轻度增厚伴少量炎性细胞浸润,肺泡轻度出血、水肿,肺泡腔未见透明膜;wortmannin组肺泡出血、水肿较明显,肺泡间隔中度增厚伴中量炎性细胞浸润,见图1。
2 小鼠肺组织W/D值的变化
与control组比较,LPS组、adipolin组和wortmannin组的W/D值明显增高(P<0.05),而adipolin组的W/D值较LPS组和wortmannin组明显降低(P<0.05),见图2。
3 各组小鼠BALF中的蛋白含量、TNF-α含量、IL-6含量及MPO活性的比较
LPS组、adipolin组和wortmannin组的蛋白含量、TNF-α含量、IL-1β含量及MPO活性均较control组明显增高(P<0.05),LPS组和wortmannin组总蛋白含量、TNF-α含量、IL-1β含量及MPO活性均较adipolin组明显增高(P<0.05),见图2、3。
4 小鼠BALF的细胞计数
LPS组、adipolin组和wortmannin组小鼠BALF细胞总数及白细胞数较control组明显增高(P<0.05),而adipolin组细胞总数及白细胞数较LPS组和wortmannin组明显减少(P<0.05),见图3。
5 不同处理对小鼠AFC的影响
LPS组、adipolin组和wortmannin组小鼠肺组织AFC明显低于control组(P<0.05),而adipolin组小鼠 AFC明显高于 LPS组和 wortmannin组(P<0.05),见图2。
6 小鼠肺组织α-ENaC mRNA及蛋白水平的检测
qPCR及 Western blot实验结果显示,LPS组、adipolin组和wortmannin组α-ENaC的mRNA及蛋白水平均较control组明显降低(P<0.05),LPS组和wortmannin组较adipolin组明显降低(P<0.05),见图4。
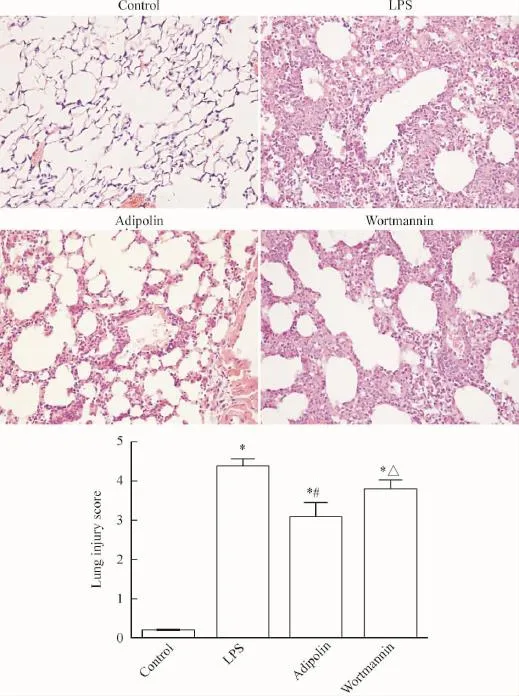
Figure 1.Pathological changes in the lung tissues and the lung injury scores in the mice(×400).Mean±SD.n=3.*P<0.05 vs control group;#P<0.05 vs LPS group;△P<0.05 vs adipolin group.图1 小鼠的肺组织病理改变和肺损伤评分
7 小鼠肺组织Akt和p-Akt蛋白水平的变化
Western blot实验结果显示LPS组、adipolin组和wortmannin组的p-Akt蛋白水平较control组明显降低(P<0.05),LPS组和wortmannin组较adipolin组明显降低(P<0.05)。而各组小鼠间Akt表达无明显差异,见图5。
讨论
ARDS主要由重症肺炎和脓毒血症发展而来,因其死亡率高、无特异性治疗手段[14-15],而一直是呼吸与危重症医学研究的重点和难点。ARDS主要病理特征为肺泡腔内大量富含蛋白的水肿液聚集,导致呼吸窘迫和顽固性低氧血症。因而有效地清除肺泡腔水肿液对维持正常气体交换及降低ARDS死亡率有重要意义[2]。研究发现,ENaC功能失调在新生儿呼吸窘迫综合症及高原性肺水肿中起关键作用[16],表明ENaC在肺泡水肿液的清除中起重要作用[7]。ENaC包括α、β和γ 3个同源亚基,其中α-ENaC基因敲除小鼠因肺水肿液清除能力低下而在出生后死于ARDS[17],提示α亚基在ARDS肺水清除作用中发挥决定性作用。新近发现的脂肪因子 adipolin/ CTRP12属于C1q肿瘤坏死因子相关蛋白家族,与adiponectin同源,主要表达于脂肪组织,不仅具有胰岛素增敏作用,还可抑制肥胖模型小鼠脂肪组织巨噬细胞浸润,并下调脂肪组织和巨噬细胞TNF-α、IL-1β及单核细胞趋化蛋白-1的表达[9],表现出抗炎作用。
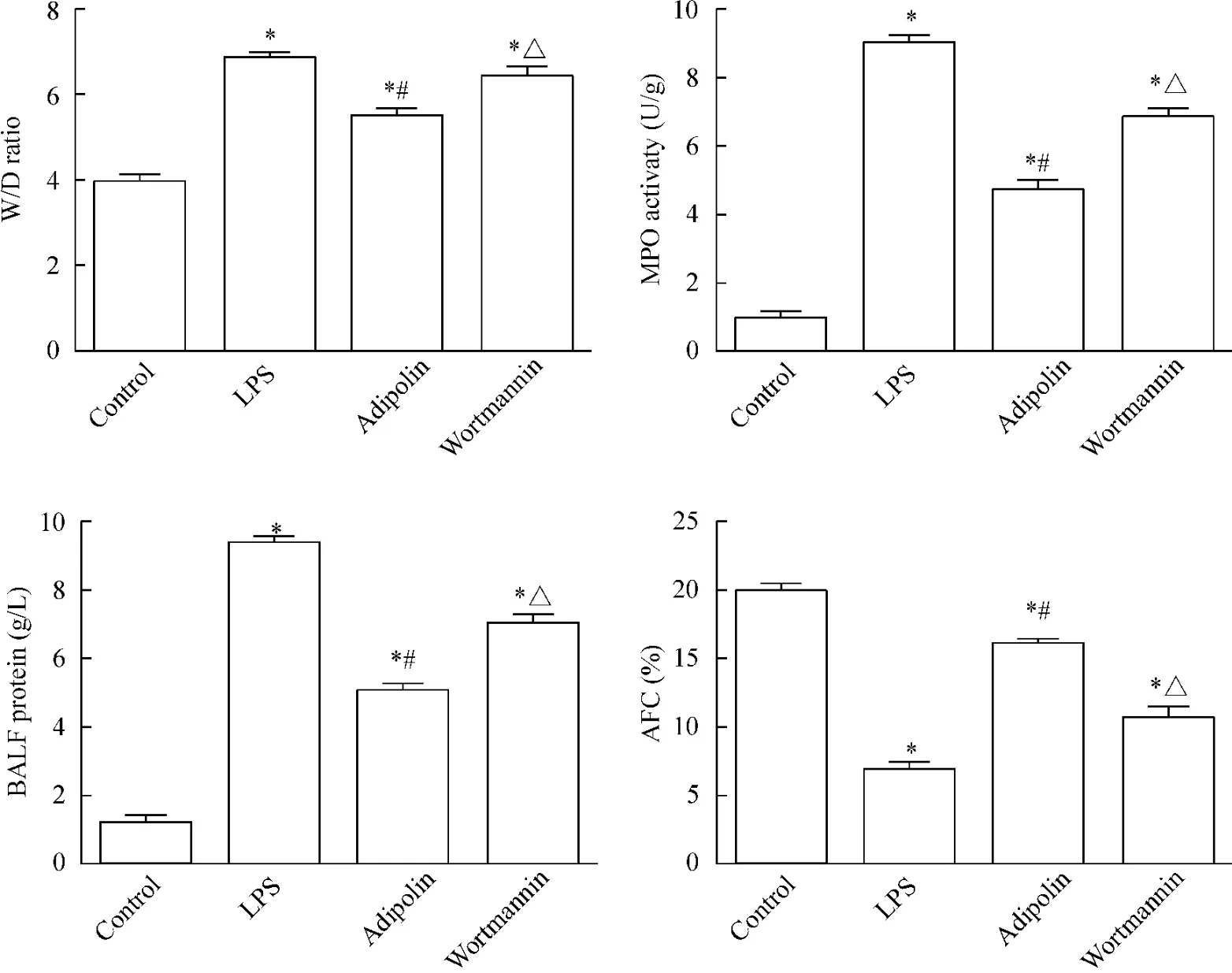
Figure 2.The lung wet/dry weight(W/D)ratio,MPO activity,the protein level in the BALF and AFC were detected.Mean±SD.n=3~4.*P<0.05 vs control group;#P<0.05 vs LPS group;△P<0.05 vs adipolin group.图2 肺组织湿/干重比、MPO活性、BALF中蛋白含量及AFC的测定
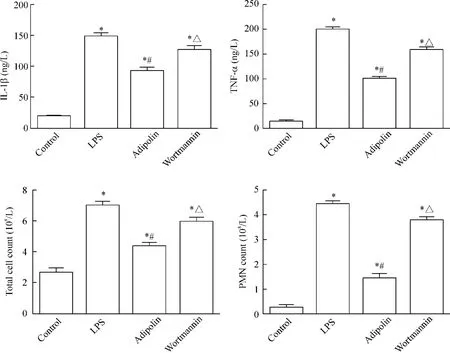
Figure 3.IL-1β level,TNF-α level,total cell counts and PMN counts in the BALF.Mean±SD.n=4.*P<0.05 vs control group;#P<0.05 vs LPS group;△P<0.05 vs adipolin group.图3 BALF中IL-1β含量、TNF-α含量、总细胞计数及白细胞计数的测定
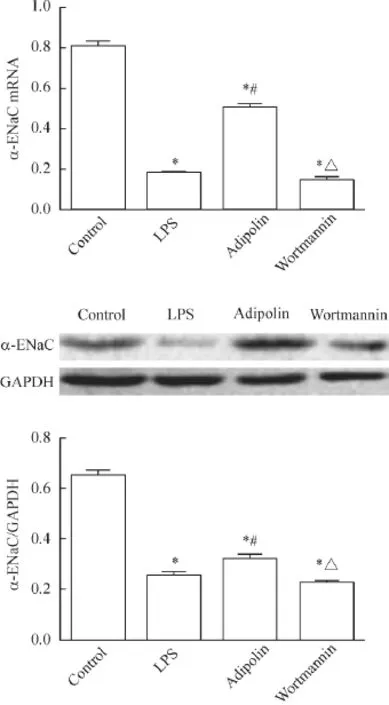
Figure 4.The mRNA and protein levels of α-ENaC in the mouse lung tissues determined by RT-qPCR and Western blot.Mean±SD.n=4.*P<0.05 vs control group;#P<0.05 vs LPS group;△P<0.05 vs adipolin group.图4 α-ENaC mRNA和蛋白表达的检测
本实验所选小鼠的体重、年龄无明显差异,并且其血糖及胰岛素水平在实验期间亦无明显差异。采用气管插管,滴注LPS建立肺内源性ARDS小鼠模型,旨在观察adipolin干预对小鼠ARDS的影响及潜在机制。本实验发现adipolin组肺组织损伤减轻,W/D降低,BALF蛋白含量、炎症细胞数量降低,同时BALF中IL-1β、TNF-α水平及MPO活性在adipolin干预后降低,证明adipolin对ARDS有抗炎保护作用。
磷脂酰肌醇3-激酶(phosphatidylinositol 3-kinase,PI3K)及其靶蛋白——蛋白激酶B(protein kinase B/Akt)是机体应对损伤的一条重要内源性负反馈通路[18],并且参与了ARDS中α-ENaC的调控[19-21]。研究发现,adipolin能上调小鼠肝组织和脂肪组织Akt磷酸化水平;亦能上调鼠H4IIE细胞Akt磷酸化水平,并且此效应被PI3K抑制剂LY29004阻断[22]。本课题组的前期研究证实了胰岛素[10-11]可通过PI3K/Akt通路上调ENaC从而对LPS介导的小鼠ARDS发挥保护作用。而本实验通过对ARDS小鼠的AFC能力评估和肺组织α-ENaC的mRNA转录及α-ENaC蛋白表达水平检测证实,adipolin干预可明显上调肺组织α-ENaC的表达,增强肺水清除能力,减轻肺水肿,从而对ARDS发挥保护性作用。本实验中,渥曼青霉素能下调小鼠肺组织Akt磷酸化水平并阻断adipolin的保护效应,进一步说明了adipolin可通过激活PI3K/Akt信号通路,促进Akt磷酸化而上调α-ENaC。不足的是,本实验未深入研究adipolin激活PI3K/Akt后的下游信号通路,也未对adipolin的抗炎作用作进一步探讨。
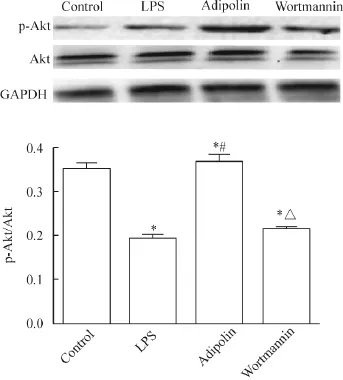
Figure 5.The protein level of p-Akt in the mouse lung tissues determined by Western blot.Mean±SD.n=4.*P<0.05 vs control group;#P<0.05 vs LPS group;△P<0.05 vs adipolin group.图5 p-Akt的蛋白水平检测
综上所述,adipolin可通过激活PI3K/Akt信号通路,促进 Akt磷酸化,介导 α-ENaC上调,增强AFC,从而发挥对LPS所致ARDS的保护作用。
[1] Ranieri VM,Rubenfeld GD,Thompson BT,et al.Acute respiratory distress syndrome:the Berlin Definition[J].JAMA,2012,307(23):2526-2533.
[2] Kolosova IA,Mirzapoiazova T,Moreno-Vinasco L,et al.Protective effect of purinergic agonist ATPγS against acute lung injury[J].Am J Physiol Lung Cell Mol Physiol,2008,294(2):L319-L324.
[3] Johnson MD,Widdicombe JH,Allen L,et al.Alveolar epithelial type I cells contain transport proteins and transport sodium,supporting an active role for type I cells in regulation of lung liquid homeostasis[J].Proc Natl Acad Sci U S A,2002,99(4):1966-1971.
[4] Borok Z,Liebler JM,Lubman RL,et al.Na transport proteins are expressed by rat alveolar epithelial type I cells [J].Am J Physiol Lung Cell Mol Physiol,2002,282 (4):L599-L608.
[5] Talbot CL,Bosworth DG,Briley EL,et al.Quantitation and localization of ENaC subunit expression in fetal,newborn,and adult mouse lung[J].Am J Respir Cell Mol Biol,1999,20(3):398-406.
[6] Yue G,Russell WJ,Benos DJ,et al.Increased expression and activity of sodium channels in alveolar type II cells of hyperoxic rats[J].Proc Natl Acad Sci U S A,1995,92(18):8418-8422.
[7] Matalon S,O’Brodovich H.Sodium channels in alveolar epithelial cells:molecular characterization,biophysical properties,and physiological significance[J].Annu Rev Physiol,1999,61:627-661.
[8] Ohashi K,Shibata R,Murohara T,et al.Role of anti-inflammatory adipokines in obesity-related diseases[J].Trends Endocrinol Metab,2014,25(7):348-355.
[9] Enomoto T,Ohashi K,Shibata R,et al.Adipolin/ C1qdc2/CTRP12 protein functions as an adipokine that improves glucose metabolism[J].J Biol Chem,2011,286(40):34552-34558.
[10]Deng W,Li CY,Tong J,et al.Regulation of ENaC-mediated alveolar fluid clearance by insulin via PI3K/Akt pathway in LPS-induced acute lung injury[J].Respir Res,2012,13:29.
[11]He J,Qi D,Wang DX,et al.Insulin upregulates the expression of epithelial sodium channel in vitro and in a mouse model of acute lung injury:role of mTORC2/SGK1 pathway[J].Exp Cell Res,2015,331(1):164-175.
[12]Wang Q,Zheng X,Cheng Y,et al.Resolvin D1 stimulates alveolar fluid clearance through alveolar epithelial sodium channel,Na,K-ATPase via ALX/cAMP/PI3K pathway in lipopolysaccharide-induced acute lung injury[J].J Immunol,2014,192(8):3765-3777.
[13]Qi D,He J,Wang D,et al.17beta-estradiol suppresses lipopolysaccharide induced acute lung injury through PI3K/Akt/SGK1 mediated up-regulation of epithelial sodium channel(ENaC)in vitro and in vitro[J].Respir Res,2014,15:159.
[14]Boyle AJ,Mac SR,McAuley DF.Pharmacological treatments in ARDS;a state-of-the-art update[J].BMC Med,2013,11:166.
[15]Spieth PM,Zhang H.Pharmacological therapies for acute respiratory distress syndrome[J].Curr Opin Crit Care,2014,20(1):113-121.
[16]Bhalla V,Hallows KR.Mechanisms of ENaC regulation and clinical implications[J].J Am Soc Nephrol,2008,19(10):1845-1854.
[17]Hummler E,Barker P,Gatzy J,et al.Early death due to defective neonatal lung liquid clearance in alpha-ENaC-deficient mice[J].Nat Genet,1996,12(3):325-328.
[18]高伟忠,但 伶,田泽丹,等.丙泊酚对肝缺血再灌注大鼠肺损伤及PI3K/Akt通路的影响[J].中国病理生理杂志,2013,29(3):488-492.
[19]Williams DL,Ozment-Skelton T,Li C.Modulation of the phosphoinositide 3-kinase signaling pathway alters host response to sepsis,inflammation,and ischemia/reperfusion injury[J].Shock,2006,25(5):432-439.
[20] Soundararajan R,Pearce D,Ziera T.The role of the ENaC-regulatory complex in aldosterone-mediated sodium transport[J].Mol Cell Endocrinol,2012,350(2):242-247.
[21]Mansley MK,Wilson SM.Effects of nominally selective inhibitors of the kinases PI3K,SGK1 and PKB on the insulin-dependent control of epithelial Na+absorption[J].Br J Pharmacol,2010,161(3):571-588.
[22]Wei Z,Peterson JM,Lei X,et al.C1q/TNF-related protein-12(CTRP12),a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes[J].J Biol Chem,2012,287(13): 10301-10315.
(责任编辑:林白霜,罗 森)
Adipolin/CTRP12 protects against LPS-induced ARDS by up-regulating alveolar epithelial sodium channel in mice
TANG Xu-mao,QI Di,WANG Dao-xin
(Department of Respiratory Medicine,Second Affiliated Hospital of Chongqing Medical University,Chongqing 400010,China.E-mail:wangdaoxin1@163.com)
AIM:To investigate the effect of adipolin/CTRP12 in LPS-induced acute respiratory distress syndrome(ARDS)and its potential regulation on alveolar epithelial sodium channel(ENaC)in mice.METHODS:C57BL/ 6J mice(n=40)were randomly divided into control group,LPS group,adipolin group and wortmannin(PI3K inhibitor) group with 10 mice in each group using random number table.The pathological changes of the lung tissues were evaluated by HE staining.The alveolar fluid clearance(AFC)was measured by Evans blue-marked albumin,and the concentrations of total protein in bronchoalveolar lavage fluid(BALF)were assessed by bicinchoninic acid(BCA)method.In BALF,the levels of IL-1β and TNF-α were determined by ELISA,and the activity of myeloperoxidase(MPO)was detected by an MPO assay kit.The total cell counts and polymorphonuclear neutrophil(PMN)counts in the BALF were analyzed by Giemsa staining.The mRNA levels of α-ENaC were assessed by qPCR,while the protein levels of α-ENaC and p-Akt were determined by Western blot.RESULTS:Compared with control group,the classic ARDS pathological changes were observed in the mice in LPS group,manifesting by severe pathological lung injury(P<0.05),increases in W/D weight ratio,total protein levels,cell counts,MPO activitiy,and IL-1β and TNF-α levels in the BALF,and decrease in AFC(P<0.05),accompanied by down-regulated levels of α-ENaC and p-Akt in the lung tissues(P<0.05).The deteriorating effects triggered by LPS were significantly reversed by administration of adipolin.However,PI3K inhibitor wortmannin canceled the beneficial effects of adipolin on LPS-induced ARDS,as evidenced by aggravated lung injury,increased levels of W/D weight ratio,protein levels,cell counts,MPO activity,and IL-1β and TNF-α levels in the BALF(P<0.05),and decreased levels of AFC,α-ENaC and p-Akt in the lung tissues.CONCLUSION:Adipolin protects against LPS-induced ARDS in the mice by up-regulating α-ENaC and enhancing AFC via PI3K/Akt signal pathway.
Acute respiratory distress syndrome;Adipolin/CTRP12;Epithelial sodium channel;PI3K/Akt signaling pathway
R363.2
A
10.3969/j.issn.1000-4718.2016.07.016
1000-4718(2016)07-1252-07
2016-02-19
2016-04-13
国家自然科学基金资助项目(No.81270141)
△Tel:023-63693094;E-mail:wangdaoxin1@163.com

