绵羊新孢子虫病和弓形虫病血清流行病学调查及风险因素分析
冯永杰, 王英华, 杨玉荣
绵羊新孢子虫病和弓形虫病血清流行病学调查及风险因素分析
冯永杰1, 王英华2, 杨玉荣1
目的调查河南、江苏和浙江省绵羊的新孢子虫病和弓形虫病的感染状况并进行风险因素分析,为绵羊寄生虫病防控和羊肉肉品安全监测提供基础依据。方法采用间接免疫荧光(IFAT)和改良凝集实验法(MAT)分别检测绵羊血清中的新孢子虫和弓形虫的抗体。统计分析风险因素的OR值及P值。结果绵羊新孢子虫的感染率为11.88%(62/522),弓形虫抗体的阳性率为17.81%(93/522)。地域和流产史是绵羊感染弓形虫的风险因素(P<0.05),但与新孢子虫的感染不相关(P>0.05);年龄和性别均与这两种原虫的感染不相关(P>0.05)。结论河南、江苏和浙江省绵羊新孢子虫病和弓形虫病的阳性率均较高,羊肉肉品安全现状堪忧。该地域绵羊的新孢子虫病流行情况首次报道。
绵羊;新孢子虫病;弓形虫病;风险因素;血清流行病学
Supported by the National Natural Science Foundation of China (No. 30800812) and the Scientific and Technological Project of Henan Province (No. B20140841)
弓形虫病和新孢子虫病可造成巨大的经济损失,常引起温血动物流产、死胎和弱胎等生殖障碍,也包括反刍类动物[1-2],新孢子虫感染是牛流产的最主要原因[2]。猫科动物和犬科动物分别为弓形虫(Toxoplasmagondii)和新孢子虫(Neosporacaninum)的终末宿主[1,3],含有卵囊的猫和犬粪便污染的草料、草场和饮用水是反刍动物感染的最主要途径[4-5]。新孢子虫病可引起犬和牛反复流产[6-7],患慢性新孢子虫病的牛或犬能够通过胎盘传染下一代[2,4]。已从草食动物如牛、马、鹿和绵羊体内分离到活体新孢子虫虫株[2]。但是,国内关于绵羊新孢子虫病的报道十分有限,新孢子虫病对绵羊等小反刍类动物造成的危害尚无法评估。弓形虫病是人畜共患的动物源性寄生虫病,属于我国二类疫病。从羊肉、羊奶及其奶制品中成功分离到活体弓形虫虫株已被报道[8-9]。目前为止,从我国羊分离出新孢子虫的研究未见报道,仅分离到1株弓形虫[10]。因此,本研究针对河南、江苏和浙江3省绵羊新孢子虫病和弓形虫病进行流行病学调查,分析感染的风险因素,为羊的原虫寄生虫病的流行病学防控提供参考依据。
1 材料与方法
1.1材料2014年10月到2015年5月,从河南、江苏和浙江3省(羊总饲养量约6 000万只)共采集农村散养湖羊绵羊血液样品522份(Epi Info7.1.5分析最低样本量为384只),分别来自河南67份,江苏247份,浙江208份。采集颈静脉血液,离心获得血清,血清-20 ℃保存。并对羊进行流行病学调查,详细记录地域、性别、年龄、流产史等信息。
1.2IFAT和MAT检测新孢子虫和弓形虫抗体绵羊血清的弓形虫抗体采用改良凝集法(modified agglutination test,MAT)检测[1,11]。采用间接免疫荧光(indirect fluorescent antibody test,IFAT)方法检测新孢子虫的抗体。每板均设置阳性、阴性和空白对照,被检血清抗体效价达到或超过1∶25者判定为弓形虫阳性[1],达到或超过1∶50判定为新孢子虫阳性[12-13],新孢子虫速殖子虫体表面呈现均一亮度的绿色为阳性[14]。IFAT采用的二抗为异硫氰酸荧光素(FITC)标记的兔抗绵羊IgG抗体(Abcam 公司,产品批号:ab150181)。经过福尔马林溶液固定纯化的含有全抗原的弓形虫速殖子购买于Kerafast 公司(产品批号:EH2002)。
1.3统计学分析数据采用软件Graph Pad Prism 4.0 进行统计分析。运用卡方检验或F检验判定绵羊的性别、日龄、流产史和地域与其感染这两种原虫之间存在的相关风险关系。当P<0.05则认为差异有统计学意义,即该因子是感染寄生虫的风险因子。
2 结 果
绵羊新孢子虫病的总感染率为11.88%(62/522,95% CI=14.76~21.34),弓形虫病的阳性率为17.81%(93/522,95% CI=9.36~14.95)。两种原虫的混合感染率为3.45%(18/522, 95% CI=2.16~5.42)。
风险因素分析发现(表1,表2),地理位置(OR=3.509)和流产史(OR=5.258)是绵羊感染弓形虫的风险因素(P<0.05),但与感染新孢子虫病不相关(P>0.05);年龄和性别因素均与绵羊感染这两类原虫不相关(P>0.05)。
表1绵羊新孢子虫病和弓形虫病的血清学流行病学
Tab.1Seroprevalence of T. gondii and N. caninum infection in sheep
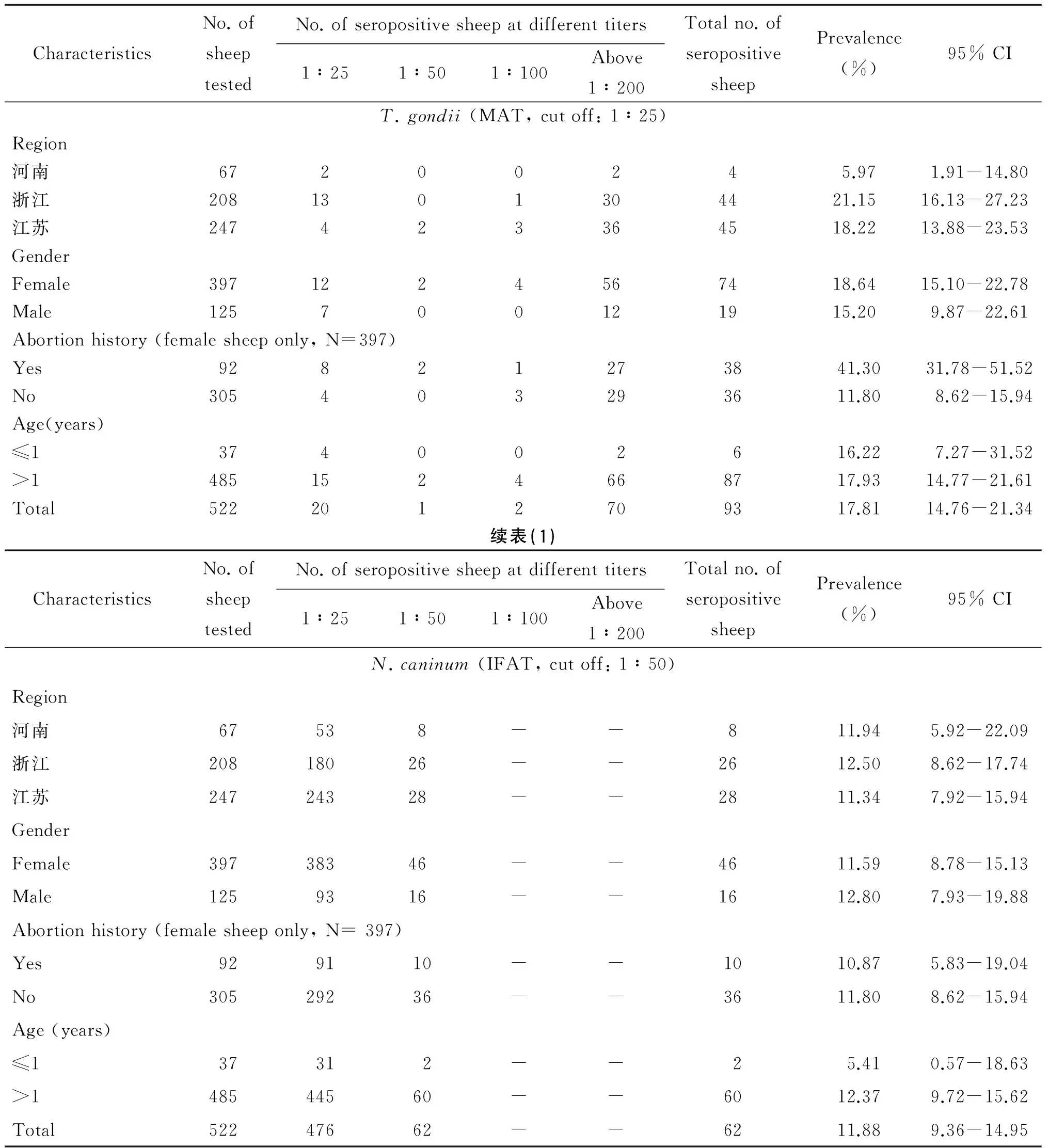
CharacteristicsNo.ofsheeptestedNo.ofseropositivesheepatdifferenttiters1∶251∶501∶100Above1∶200Totalno.ofseropositivesheepPrevalence(%)95%CIT.gondii(MAT,cutoff:1∶25)Region河南67200245.971.91-14.80浙江2081301304421.1516.13-27.23江苏247423364518.2213.88-23.53GenderFemale3971224567418.6415.10-22.78Male125700121915.209.87-22.61Abortionhistory(femalesheeponly,N=397)Yes92821273841.3031.78-51.52No305403293611.808.62-15.94Age(years)≤1374002616.227.27-31.52>14851524668717.9314.77-21.61Total5222012709317.8114.76-21.34续表(1)CharacteristicsNo.ofsheeptestedNo.ofseropositivesheepatdifferenttiters1∶251∶501∶100Above1∶200Totalno.ofseropositivesheepPrevalence(%)95%CIN.caninum(IFAT,cutoff:1∶50)Region河南67538--811.945.92-22.09浙江20818026--2612.508.62-17.74江苏24724328--2811.347.92-15.94GenderFemale39738346--4611.598.78-15.13Male1259316--1612.807.93-19.88Abortionhistory(femalesheeponly,N=397)Yes929110--1010.875.83-19.04No30529236--3611.808.62-15.94Age(years)≤137312--25.410.57-18.63>148544560--6012.379.72-15.62Total52247662--6211.889.36-14.95
表2地理位置,性别,年龄和流产史与绵羊感染新孢子虫、弓形虫的风险关系
Tab.2Odds ratio of geographical origin, gender, abortion history and age of sheep as risk factors for T. gondii and N. caninum antibody
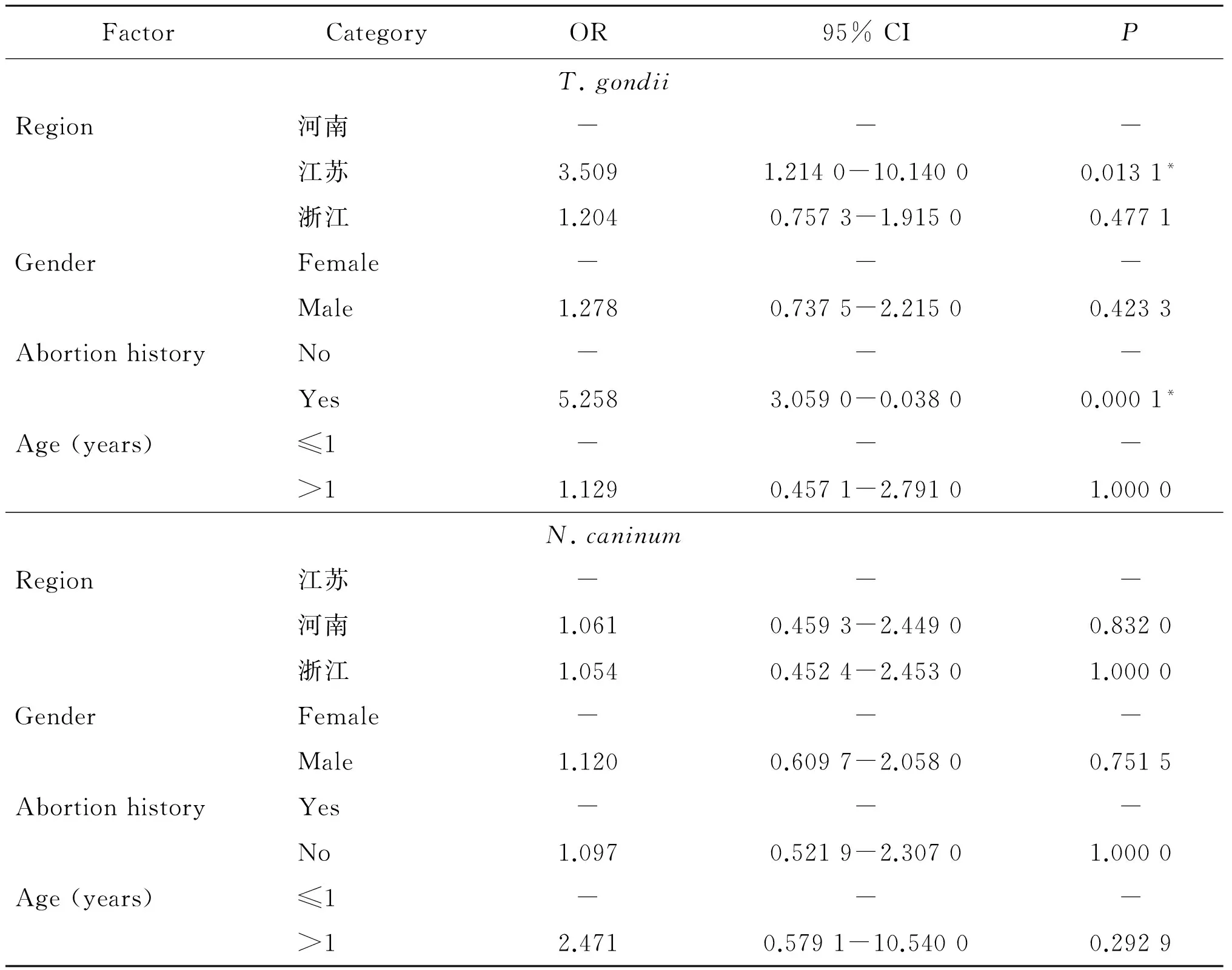
FactorCategoryOR95%CIPT.gondiiRegion河南---江苏3.5091.2140-10.14000.0131*浙江1.2040.7573-1.91500.4771GenderFemale---Male1.2780.7375-2.21500.4233AbortionhistoryNo---Yes5.2583.0590-0.03800.0001*Age(years)≤1--->11.1290.4571-2.79101.0000N.caninumRegion江苏---河南1.0610.4593-2.44900.8320浙江1.0540.4524-2.45301.0000GenderFemale---Male1.1200.6097-2.05800.7515AbortionhistoryYes---No1.0970.5219-2.30701.0000Age(years)≤1--->12.4710.5791-10.54000.2929
比值比:Odds ratio(OR); “*”差异有统计学意义“*” indicates significant differences.
3 讨 论
我国绵羊饲养量居世界第一,小反刍兽类的运输和迁徙已成为寄生虫病传播和扩散的最主要原因,其中绵羊和山羊的贡献显著[15]。涮羊肉,羊肉烧烤和手抓羊肉等烹饪习惯不能充分熟化羊肉,增加了人类感染羊源性寄生虫病的风险。
新孢子虫病可感染多种反刍类动物和非人灵长类[2,16]。新孢子虫病已被证实是导致绵羊流产的原因之一[17]。我国关于新孢子虫病的报道仅仅见于牛、狗、羊[18-20],调查地域也十分有限,羊新孢子虫病报道仅见青海省[20]。调查发现绵羊比山羊更加易感新孢子虫病[21]。本次调查,河南、江苏和浙江3省绵羊的新孢子虫总感染率(11.88%)与青海省(10.33%)[20]的阳性率基本持平,3个省份各自的感染率均在11%左右,可见绵羊新孢子虫的地域分布范围较广且分布水平相当。大于12月龄绵羊的新孢子虫抗体的阳性率(12.37%)约是小于12月龄(5.41%)的2倍,但风险因素分析发现年龄不是绵羊感染新孢子虫病的风险因子(P>0.05)。垂直传播和食入新孢子虫卵囊是反刍类动物感染新孢子虫病的主要传播途径[2]。然而,小于12月龄的绵羊获得新孢子虫抗体的具体原因有待研究。青海省的调查发现,狗的存在、放牧方式、牧场规模和卫生条件均是绵羊感染新孢子虫病的风险因素[20]。本次调查分析,地理位置,母羊流产史和性别与绵羊新孢子虫病均不相关(P>0.05)。由此推测新孢子虫可感染各个年龄阶段的绵羊,绵羊感染新孢子虫的主要原因是羊群周围的环境,净化羊群及草料储存环境(如隔绝犬等),阻断新孢子虫的传播是防控该病的有效途径。
弓形虫病是人畜共患病,对畜牧生产和人类的健康已造成显著的危害。本次调查发现,河南、江苏和浙江3省绵羊的弓形虫阳性率(17.81%)处于我国已有报道的中等水平(2%~39%)。浙江和江苏绵羊的弓形虫阳性率均高于河南,江浙地区较为温暖湿润的气候条件和丰富的物种资源更加有利于弓形虫的生存和传播。然而,危险因素分析发现,仅江苏地区与河南的感染率存在显著差异(P<0.05),这说明河南和江苏两省的地域差异与弓形虫的感染存在相关关系,但是存在差异的原因需要进一步探索。绵羊的流产史与弓形虫阳性率之间相关性有统计学意义(P<0.01),患弓形虫病母羊流产率是健康母羊流产率的5.258倍。弓形虫感染是母羊流产的主要原因之一,但弓形虫是否可引起母羊反复流产,尚无确切证据,及时诊断和治疗怀孕母羊弓形虫病是提高羊繁殖率的有效方法。年龄和性别不是绵羊感染弓形虫的风险因素(P>0.05),这与已报道的研究相吻合[22],说明不同年龄和性别的绵羊均可受到弓形虫的侵袭,绵羊弓形虫病需要全面防控。因此,监测绵羊新孢子虫病和弓形虫病的流行情况不仅能有效控制寄生虫类疫病的扩散,而且为人畜等公共卫生安全提供基础保障。
(美国农业部的J.P.Dubey教授赠予新孢子虫和弓形虫的阳性、阴性对照血清,中国农业大学的刘群教授赠予包被新孢子虫速殖子的96孔板,在此一并感谢。)
[1] Dubey JP. Toxoplasmosis of animals and humans[M]. 2nd ed. Boca Raton: CRC Press, Taylor & Francis Group, 2010: 1-313.
[2] Dubey JP, Schares G. Neosporosis in animals-the last five years[J]. Vet Parasitol, 2011, 180(1/2): 90-108.
[3] Donahoe SL, Lindsay SA, Krockenberger M, et al. A review of neosporosis and pathologic findings ofNeosporacaninuminfection in wildlife[J]. Int J Parasitol Parasites Wildl, 2015, 4(2): 216-238.
[4] Dubey JP, Lindsay DS. Neosporosis, toxoplasmosis, and sarcocystosis in ruminants[J]. Vet Clin North Am Food Anim Pract, 2006, 22(3): 645-671.
[5] Panadero R, Painceira A, Lopez C, et al. Seroprevalence ofToxoplasmagondiiandNeosporacaninumin wild and domestic ruminants sharing pastures in Galicia (Northwest Spain)[J]. Res Vet Sci, 2010, 88(1): 111-115.
[6] Pabon M, Lopez-Gatius F, Garcia-Ispierto I, et al. ChronicNeosporacaninuminfection and repeat abortion in dairy cows: a 3-year study[J]. Vet Parasitol, 2007, 147(1/2): 40-46.
[7] Dubey JP, Koestner A, Piper RC. Repeated transplacental transmission ofNeosporacaninumin dogs[J]. J Am Vet Med Assoc, 1990, 197(7): 857-860.
[8] Dubey JP, Casey SJ, Zajac AM, et al. Isolation and genetic characterization ofToxoplasmagondiifrom alpaca(Vicugnapacos) and sheep (Ovis aries)[J]. Trop Anim Health Prod, 2014, 46(8): 1503-1507.
[9] Dubey JP, Verma SK, Ferreira LR, et al. Detection and survival ofToxoplasmagondiiin milk and cheese from experimentally infected goats[J]. J Food Prot, 2014, 77(10): 1747-1753.
[10] Zhou P, Zhang H, Lin RQ, et al. Genetic characterization ofToxoplasmagondiiisolates from China[J]. Parasitol Int, 2009, 58(2): 193-195.
[11] Dubey JP, Desmonts G. Serological responses of equids fedToxoplasmagondiioocysts[J]. Equine Vet J, 1987, 19(4): 337-339.
[12] Soares HS, Ahid SM, Bezerra AC, et al. Prevalence of anti-Toxoplasmagondiiand anti-Neosporacaninumantibodies in sheep from Mossoro, Rio Grande do Norte, Brazil[J]. Vet Parasitol, 2009, 160(3-4): 211-214.
[13] Helmick B, Otter A, McGarry J, et al. Serological investigation of aborted sheep and pigs for infection byNeosporacaninum[J]. Res Vet Sci, 2002, 73(2): 187-189.
[14] Martins J, Kwok OC, Dubey JP. Seroprevalence ofNeosporacaninumin free-range chickens (Gallus Domesticcus) from the Americas[J]. Vet Parasitol, 2011, 182(2/4): 349-351.
[15] Vasileiou NG, Fthenakis GC, Papadopoulos E. Dissemination of parasites by animal movements in small rumi nant farms[J]. Vet Parasitol, 2015, 213(1/2): 56-60.
[16] Barr BC, Conrad PA, Sverlow KW, et al. Experimental fetal and transplacentalNeosporainfection in the nonhuman primate[J]. Lab Invest, 1994, 71(2): 236-242.
[17] Gonzalez-Warleta M, Castro-Hermida JA, Regidor-Cerrillo J, et al.Neosporacaninuminfection as a cause of reproductive failure in a sheep flock[J]. Vet Res, 2014, 45: 88.
[18] Yao L, Yang N, Liu Q, et al. Detection ofNeosporacaninumin aborted bovine fetuses and dam blood samples by nested PCR and ELISA and seroprevalence in Beijing and Tianjin, China[J]. Parasitology, 2009, 136(11):1251-1256.
[19] Yang YR, Zhang QF, Kong YG, et al. Low prevalence ofNeosporacaninumandToxoplasmagondiiantibodies in dogs in Jilin, Henan and Anhui provinces of the People’s Republic of China[J]. BMC Vet Res, 2014, 10: 295.
[20] Liu ZK, Li JY, Pan H. Seroprevalence and risk factors ofToxoplasmagondiiandNeosporacaninuminfections in small ruminants in China[J]. Prev Vet Med, 2015, 118(4): 488-492.
[21] Diakoua A, Papadopoulos E, Panousis N, et al.ToxoplasmagondiiandNeosporacaninumseroprevalence in dairy sheep and goats mixed stock farming[J]. Vet Parasitol, 2013, 198(3/4): 387-390.
[22] Bartova E, Machacova T, Sedlak K, et al. Seroprevalence of antibodies ofNeosporaspp. andToxoplasmagondiiin horses from southern Italy[J]. Folia Parasitol (Praha), 2015, 62: 043.
Seroprevalence and risk factors of sheep in neosporosis and toxoplasmosis
FENG Yong-jie1, WANG Ying-hua2, YANG Yu-rong1
(1.CollegeofAnimalScienceandVeterinaryMedicine,HenanAgriculturalUniversity,Zhengzhou450000,China;2.CenterforAnimalDiseaseControlandPreventionofHenanProvince,Zhengzhou450000,China)
The aim of this research was to investigate the neosporosis and toxoplasmosis for sheep in Henan, Jiangsu and Zhejiang provinces and analyze the risk factors of infection, and provide a foundation of prevention and control in this two parasitosis for sheep and monitoring in mutton safety. The indirect fluorescent antibody test(IFAT) and modified agglutination test(MAT) were used to detect the antibodies ofNeosporacaninumandToxoplasmagondii. Odds ratio(OR) andPvalue were analyzed to distinguish the correlativity of risk factors. The results showed that the seropositive ofNeosporacaninumwas 11.88%(62/522), and the infection rate of toxoplasmosis was 17.81%(93/522). Abortion history and region of sheep were the infection risk factors of toxoplasmosis(P<0.05), but not for neosporosis(P>0.05). Meanwhile, gender and age had no relationship of infection in sheep for this two protozoons(P>0.05). In conclusion, a high positive infection percentage for neosporosis and toxoplasmosis in sheep in Henan, Jiangsu and Zhejiang provinces. Improving and reinforcing the prevention and control measures were urgent, and safety of sheep meat should be focused. This was the first report of the infection of neosporosis in sheep from central and eastern China.
sheep; neosporosis; toxoplasmosis; risk factors; seroprevalence
Yang Yu-rong, Email: yangyu7712@sina.com
杨玉荣,Email: yangyu7712@sina.com
1.河南农业大学牧医工程学院,郑州450000;2.河南省动物疾病预防控制中心,郑州450000
R531.8
A
1002-2694(2016)07-0613-05
2016-03-29;
2016-06-16
DOI:10.3969/j.issn.1002-2694.2016.07.005
国家自然科学基金 (No. 30800812);河南省科技攻关项目(No.B20140841)

