慢性阻塞性肺疾病合并肺癌与单纯肺癌临床特征差异的分析
杜建飞 崔立春 耿会生 蒋冬梅 白莉
慢性阻塞性肺疾病合并肺癌与单纯肺癌临床特征差异的分析
杜建飞1崔立春1耿会生1蒋冬梅1白莉2
710016 西安,长安医院肿瘤科1
【摘要】目的探讨慢性阻塞性肺疾病(COPD)合并肺癌与单纯肺癌患者之间临床特征的差异,为肺癌疗效和预后判断提供依据。方法选取我院98例COPD合并肺癌患者及62例单纯肺癌患者为研究对象,分析二组的临床症状、原发肿瘤位置、性别、年龄、吸烟史、病理类型及TNM分期等资料。结果主要症状如咳嗽、咳痰、呼吸困难及咯血在COPD合并肺癌组与单纯肺癌组患者之间具有明显统计学差异,而胸痛及骨转移所致疼痛在两组之间未见明显统计学差异;COPD合并肺癌组患者一般以中央型、男性、吸烟为主,病理学以鳞状细胞癌多见,而单纯肺癌组患者则以周围型、女性、不吸烟及腺癌为常见,二者之间存在明显统计学差异,但是在年龄、TNM分期之间无明显统计学差异。结论COPD合并肺癌与单纯肺癌为相互独立性疾病,临床特征差异有利于二者的鉴别诊断,为疗效和预后判断提供依据。
【关键词】支气管肺癌;肺疾病,慢性阻塞性;临床特征
慢性阻塞性肺疾病(chronic obstructive pulmonary disease, COPD)是以不完全阻塞性肺通气障碍为临床特征的呼吸系统常见病、高发病。原发性支气管肺癌(简称肺癌)为呼吸系统最常见的恶性肿瘤。近年来随着我国人口老龄化,烟草消耗增长和大气严重污染,二者发病率均呈明显升高趋势。在临床表现上二者均为咳嗽、咳痰、气短等,无特异性表现,容易误诊及漏诊而延误诊断治疗。大量循证医学证据表明COPD与肺癌的发生存在着密切的联系[1-4]。
资料与方法
一、 一般资料
收集我院2012年至2015年收治的98例COPD合并肺癌患者和62例单纯肺癌患者,COPD诊断依据为中华医学会呼吸病学分会制定的COPD诊断标准。所有患者均经病理学证实,病理分型和TNM分期依据为国际肺癌研究协会2009 年第七版分期标准。
二、研究方法
根据患者影像学及肺功能检查结果将患者分为98例COPD合并肺癌组与62例单纯肺癌组,分析患者性别、年龄、吸烟史、临床表现、胸部CT表现、肺癌病理类型、TNM分期等。
三、统计学方法
采用SPSS 13.0 软件对结果进行统计学分析。计数资料比较采用χ2检验,以P<0.05为差异有统计学意义。
结 果
一、2组患者临床症状比较
咳嗽、咳痰、呼吸困难及咯血为COPD合并肺癌的常见临床症状,其发病率与单纯肺癌患者相比具有明显统计学差异(P<0.05),而胸痛及骨转移所致疼痛COPD组与单纯肺癌组未见明显统计学差异,见表1。
二、2组患者原发灶位置比较
胸部CT表现可见,COPD合并肺癌组患者原发肿块以中央型常见,而单纯肺癌组原发肿瘤则以周围性为主,二者之间存在明显统计学差异(P<0.05),见表2。
三、2组患者一般情况及病理类型比较
COPD合并肺癌患者一般以男性、吸烟为主,病理学以鳞状细胞癌多见,而单纯肺癌组患者则以女性、不吸烟及腺癌常见,二者之间存在明显统计学差异,两组患者在年龄、TNM分期之间未见明显统计学差异(P>0.05), 表3。
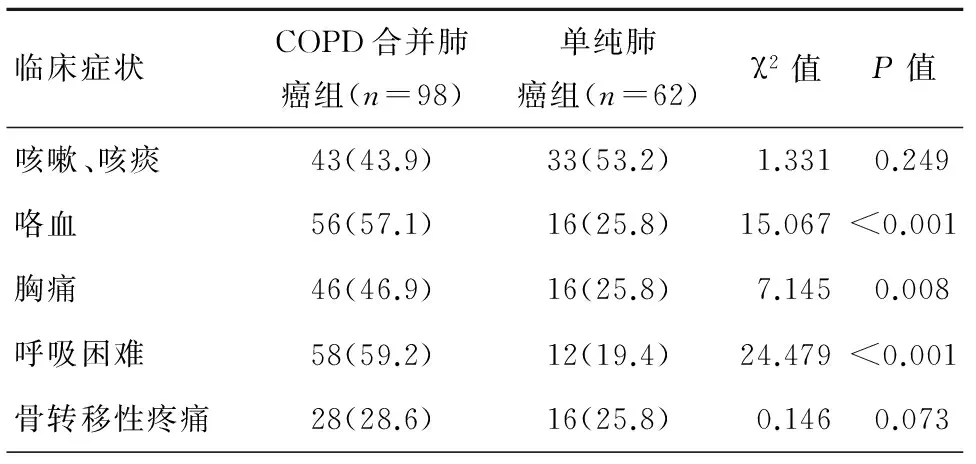
表1 2组患者临床症状比较[n(%)]
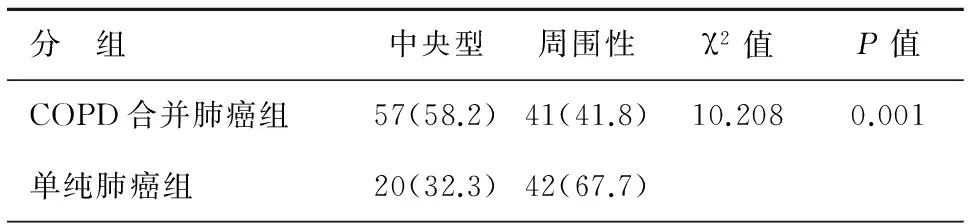
表2 2组患者肺癌原发病灶位置比较[n(%)]
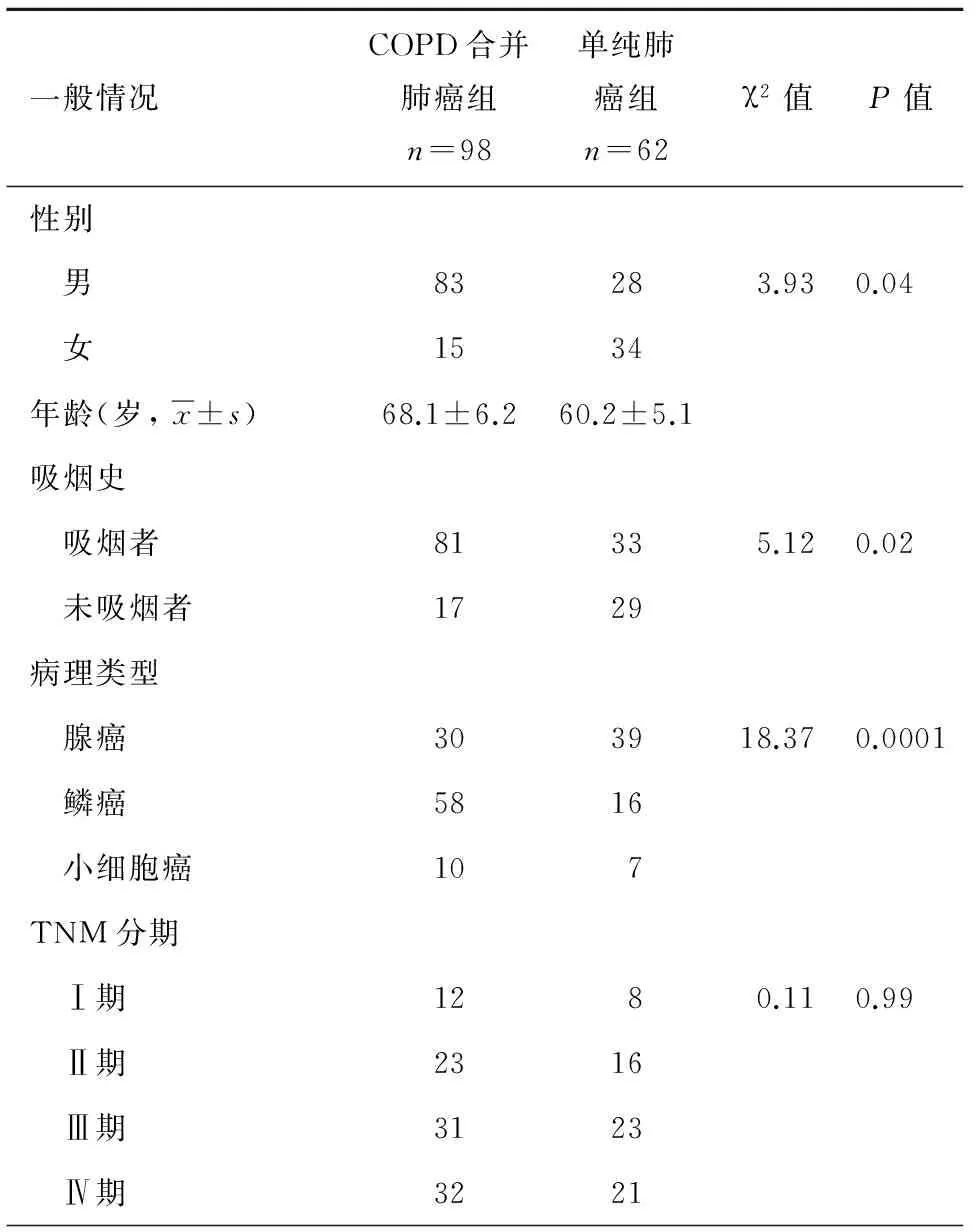
表3 2组患者一般情况及病理类型比较
讨 论
COPD和肺癌同为呼吸系统高发性疾病和高致死性疾病。2010年COPD已成为全球常见死亡病因的第三位[5]。2011年,我国登记的新发肺癌患者约65万人,而死于肺癌的患者约为52万人[6]。吸烟为公认的COPD与肺癌的共同致病因素之一[7-8]。研究认为吸烟可以增加男、女性患肺癌的风险,分别为17.4%和11.6%;而不吸烟者患肺癌男、女的风险仅为1.3%和1.4 %[9]。COPD和肺癌的发病率与患者在烟雾中的暴露具有明显的相关性[10-12]。烟草中尼古丁、焦油、亚硝胺、苯并芘等多种氧化剂刺激气道上皮使纤毛运动减弱,吞噬细胞吞噬清除能力降低,使气道净化能力减弱,同时也刺激平滑肌细胞、中性粒细胞、巨噬细胞及多种基质细胞在肺内聚集与活化。活化后的细胞可以分泌金属蛋白酶 、蛋白水解酶等降解、破坏肺泡和肺泡壁组织结构,进而导致肺组织失去原有结构和弹性,促进COPD的发生。另外,烟草中的有害成份作用于呼吸道黏膜上皮,导致细胞发育不良,化生、腺瘤样增生、DNA损伤最终导致恶性改变[13-14]。大量的研究表明,与不吸烟者相比,吸烟者发生肺癌的危险性平均增高4~10倍,且吸烟量与肺癌之间存在着明显的量-效关系,吸烟时间、吸烟量与肺癌的发生率呈正相关[15]。研究认为50%~70%肺癌患者发病与COPD有关,COPD患者支气管黏膜清除功能障碍而使致癌有害物长期存留[16];氧化剂与抗氧化剂功能失调而使氧自由基损伤DNA[17];慢性炎症持续刺激使DNA损伤转变为DNA突变而诱发恶性改变等多种机制共同作用而导致肺癌发生。本研究中,COPD合并肺癌组中吸烟者明显高于单纯肺癌组,且差别具有统计学意义。COPD合并肺癌原发病灶以中央型为主,而单纯肺癌组中则以周围型为主。病理分类COPD合并肺癌组以鳞癌为主,单纯肺癌组则以腺癌为主,二者之间存在明显统计学差异。而在小细胞癌的发病率中两组无明显差别,本研究结果再次验证了COPD为肺癌的独立危险因素,特别是在肺鳞状细胞癌患者中。对于气道梗阻的吸烟患者其患肺癌的风险为肺功能正常患者的5倍[18],肺癌在COPD患者中的高发病率表明二者之间可能存在着共同的发病机制。
COPD与肺癌之间存在着共同的发病机制,目前较为广泛认可的是慢性炎症在COPD与肺癌这两种疾病的发生中占据着重要的地位。活性氧[19]和氧自由基[20-21]是炎症反应与肿瘤之间联系的重要中介分子。正常肺脏内皮细胞凋亡需线粒体转录因子A调控,当患有COPD时线粒体转录因子A缺失,线粒体功能失调使正常肺脏内皮细胞凋亡失控而发生过度磷状细胞增生,最终导致肺癌发生[18]。核因子NF-κB 同样是慢性炎症与肿瘤之间联系的重要因子,慢性炎症时组织可过度表达转录因子NF-κB。NF-κB可刺激IL-1、IL-6、IL-8 和TNF-α等多种细胞因子表达,而这些细胞因子均为细胞周期分子,参与细胞凋亡的调控[22]。尽管目前认为慢性炎症在COPD与肺癌这两种疾病发生中占据着重要的地位,但是其如何诱导COPD向肺癌转变的机制仍不明确,未来的研究仍然应以二者的共同机制为主要研究方向。
参考文献
1任成山, 白莉, 钱桂生. 慢性阻塞性肺疾病合并肺癌临床特征及新理念[J/CD]. 中华肺部疾病杂志: 电子版, 2015, 8(2): 137-142.
2廖晨, 余祖滨, 张萍, 等. 慢性阻塞性肺疾病合并肺癌122例临床特征分析[J/CD].中华肺部疾病杂志, 2015, 8(1): 25-29.
3de-Torres JP, Wilson DO, Sanchez-Salcedo P, et al. Lung cancer in patients with chronic obstructive pulmonary disease. Development and validation of the COPD Lung Cancer Screening Score[J]. Am J Respir Crit Care Med, 2015, 191(3): 285-291.
4Griffin JP, Tolley EA, Zaman MK, et al. Chronic obstructive pulmonary disease with lung cancer: Prevalence, severity, and common pathogenesis[J]. J Cancer Res Ther, 2016, 4(1): 1-6.
5Burney PG, Patel J, Newson R, et al. Global and regional trends in COPD mortality, 1990-2010[J]. Eur Respir J, 2015, 45(5): 1239-1247.
6陈万青, 郑荣寿, 曾红梅, 等. 2011 年中国恶性肿瘤发病和死亡分析[J]. 中国肿瘤, 2015, 24(1): 1-10.
7Loeb LA. Tobacco Causes Human Cancers-A Concept Founded on Epidemiology and an Insightful Experiment Now Requires Translation Worldwide[J]. Cancer Res, 2016, 76(4): 765-766.
8Hecht SS, Carmella SG, Murphy SE, et al. Tobacco smoke toxicant and carcinogen biomarkers and lung cancer susceptibility in smokers[J]. J Thoracic Oncology, 2016, 11(2): S7-S8.
9Villeneuve PJ, Mao Y. Lifetime probability of developing lung cancer,by smoking status, Canada[J]. Can J Public Health, 1993, 85(6): 385-388.
10Mariani TJ. Respiratory disorders: Ironing out smoking-related airway disease[J]. Nature, 2016, 531(7596): 586-587.
11Terzikhan N, Verhamme KM, Hofman A, et al. Prevalence and incidence of COPD in smokers and non-smokers: the Rotterdam Study[J]. Eur J Epidemiol, 2016.
12Bastian LA, Gray KE, DeRycke E, et al. Differences in Active and Passive Smoking Exposures and Lung Cancer Incidence Between Veterans and Non-Veterans in the Women′s Health Initiative[J]. Gerontologist, 2016, 56 (Suppl 1): S102-S111.
13Tuder RM, Petrache I. Pathogenesis of chronic obstructive pulmonary disease[J]. J Clin Invest, 2012, 122(8): 2749-2755.
14Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics[M]. Lung Cancer and Personalized Medicine. Springer International Publishing, 2016: 1-19.
15Wang Z, Seow WJ, Shiraishi K, et al. Meta-analysis of genome-wide association studies identifies multiple lung cancer susceptibility loci in never-smoking Asian women[J]. Hum Mol Genet, 2016, 25(3): 620-629.
16Kiri VA, Soriano J, Visick G, et al. Recent trends in lung cancer and its association with COPD: an analysis using the UK GP Research Database[J]. Prim Care Respir J, 2010, 19(1): 57-61.
17Lennon FE, Salgia R. Mitochondrial dynamics: biology and therapy in lung cancer[J]. Expert Opin Investig Drugs, 2014, 23(5): 675-692.
18Young RP, Hopkins RJ. Link between COPD and lung cancer[J]. Respir Med, 2010, 104(5): 758-759.
19Kasiappan R, Safe SH. ROS-Inducing Agents for Cancer Chemotherapy [J]. Reactive Oxygen Species, 2016, 1(1): 22-37.
20Kuranaga Y, Yamada N, Kashiwaya M, et al. Anti-Oncogenic gem-Dihydroperoxides Induce Apoptosis in Cancer Cells by Trapping Reactive Oxygen Species[J]. Int J Mol Sci, 2016, 17(1): 71.
21Lo KY, Wu SY, Sun YS. A microfluidic device for studying the production of reactive oxygen species and the migration in lung cancer cells under single or coexisting chemical/electrical stimulation[J]. Microfluidics and Nanofluidics, 2016, 20(1): 1-11.
22Shen HM, Tergaonkar V. NF-κB signaling in carcinogenesis and as a potential molecular target for cancer therapy[J]. Apoptosis, 2009, 14(4): 348-363.
(本文编辑:王亚南)
杜建飞,崔立春,耿会生,等. 慢性阻塞性肺疾病合并肺癌与单纯肺癌临床特征差异的分析[J/CD]. 中华肺部疾病杂志: 电子版, 2016, 9(3): 275-277.
DOI:10.3877/cma.j.issn.1674-6902.2016.03.009
基金项目:国家自然科学基金资助项目(81370139)
通讯作者:白莉,Email:blpost@126.com
中图法分类号:R563,R734.2
文献标识码:A
Corresponding author:Bai Li, Email:blpost@126.com
(收稿日期:2016-03-20)
Retrospective analysis of clinical features differences for lung cancer patients with or without chronic obstructive pulmonary disease
Dujianfei1,Cuilichun1,GengHuisheng1,JiangDongmei1,BaiLi2.1Desect1mentofOncology,ChanganHospital,Xi′an710016,China;2Desect1mentofRespiratoryDiseases,XinQiaoHospital,ThirdMilitaryMedicalUniversity,Chongqing400037,China
【Abstract】ObjectiveTo investigate the difference of clinical features for lung cancer patients with or without chronic obstructive pulmonary disease (COPD) and explore some evidences of outcome evaluation and prognosis. Methods98 cases of lung cancer patients with COPD and 62 cases without COPD were treated in Changan hospital. The clinical features of both groups were collected, include clinical symptoms, tumor location, gender, age, smoking history, histological type and TNM staging. ResultAmong the evaluable patients of two groups, the incidence rate of cough, sputum, dyspnea and hemoptysis, significant higher in COPD group (P<0.05). There were no significant difference the clinical symptom of chest pain and pain caused by bone metastases between two groups. The cancer characteristics of patients with COPD were generally central location, male, smoking, and squamous histopathology. On the other hand, patients without COPD were more likely peripheral location, women, not-smoking and adenocarcinoma histopathology. There were no significant differences for age, TNM stage between two groups. ConclusionLung cancer with or without COPD were independence disease and had different clinical characteristics. It may be helpful to treatment outcome evaluation and prognosis.
【Key words】Bronchus lung cancer;Chronic obstructive pulmonary disease;Clinical features
400037 重庆,第三军医大学新桥医院呼吸内科2

