15-脱氧-前列腺素J2对单核巨噬细胞中巨噬细胞移动抑制因子表达的影响及作用机制
李伟阳,时雨濛,刘 欣,杨 琳,李丽英
首都医科大学细胞生物学系北京市肝脏保护与再生调节重点实验室,北京 100069
·论著·
15-脱氧-前列腺素J2对单核巨噬细胞中巨噬细胞移动抑制因子表达的影响及作用机制
李伟阳,时雨濛,刘欣,杨琳,李丽英
首都医科大学细胞生物学系北京市肝脏保护与再生调节重点实验室,北京 100069
摘要:目的研究15-脱氧-前列腺素J2(15 d-PGJ2)对小鼠单核巨噬细胞中巨噬细胞移动抑制因子(MIF)表达的影响及作用机制。方法以小鼠单核巨噬细胞系J774A.1为研究对象,分别给予以下处理:(1)脂多糖(LPS)组:1 μg/ml LPS 孵育1 h;(2)正常对照组:PBS孵育1 h;(3)阴性对照组:5 μmol/L 15 d-PGJ2孵育1 h;(4)15 d-PGJ2组:5 μmol/L 15 d-PGJ2预孵育1 h,1 μg/ml LPS 孵育1 h;(5)GW9662组:10 μmol/L GW9662预孵育1 h,5 μmol/L 15 d-PGJ2孵育1 h,1 μg/ml LPS孵育1 h;(6)Vehicle组:即GW9662对照组,使用GW9662的溶剂DMSO替代GW9662。采用免疫荧光和琼脂糖凝胶电泳技术检测小鼠单核巨噬细胞系J774A.1中MIF的表达,RT-qPCR和Western blot技术检测15 d-PGJ2对LPS诱导的J774A.1炎症反应模型中MIF mRNA和蛋白水平变化情况。观察GW9662对15 d-PGJ2调节MIF作用的影响,采用高内涵分析技术检测15 d-PGJ2是否调节巨噬细胞炎症反应时过氧化物酶体增殖物激活受体γ(PPAR-γ)的核转位。结果J774A.1在基因和蛋白水平均表达MIF。LPS组细胞中MIF mRNA表达上调到正常对照组的1.75倍(P=0.037),15 d-PGJ2组细胞中MIF mRNA表达上调被抑制,下调至LPS组的50%(P=0.026),MIF蛋白水平变化情况与mRNA水平相一致。GW9662逆转了15 d-PGJ2对MIF mRNA表达的下调(P=0.016)。高内涵自动成像系统分析结果显示,15 d-PGJ2处理组细胞中PPAR-γ的核质比上调至LPS组的1.39倍(P=0.003)。结论15 d-PGJ2可抑制单核巨噬细胞中MIF的表达,该作用可能依赖于PPAR-γ。
关键词:15-脱氧-前列腺素J2;巨噬细胞移动抑制因子;过氧化物酶体增殖物激活受体γ
ActaAcadMedSin,2016,38(3):247-252
巨噬细胞移动抑制因子(macrophage migration inhibitory factor,MIF)是一种具有多种生物学功能的细胞因子,位于“炎症瀑布”的上游,可以调节炎性因子的产生和释放,通过自分泌或旁分泌方式发挥作用,促使巨噬细胞聚集在损伤区域[1- 2]。研究发现,15-脱氧-前列腺素J2(15-Deoxy-Δ12,14-prostaglandin J2,15 d-PGJ2)能够在慢性肝损伤时抑制小鼠体内骨髓来源的单核巨噬细胞(bone marrow derived monocyte/macrophage,BMM)向损伤区域募集,并能够抑制体外培养小鼠单核巨噬细胞的生物学功能,但其作用靶点尚未明确[3- 4]。本研究以小鼠单核巨噬细胞系J774A.1为研究对象,以MIF为靶分子,在体外脂多糖(lipopolysaccharide,LPS)诱导的炎症模型中探讨15 d-PGJ2对MIF表达的影响及其机制。
材料和方法
材料小鼠单核巨噬细胞系J774A.1(中国医学科学院基础医学研究所细胞资源中心细胞库);DMEM培养基(美国Invitrogen公司),胎牛血清(德国Biochromag公司),胰蛋白酶、β-巯基乙醇、青霉素/链霉素(美国GIBICO/BRL公司),15 d-PGJ2(美国AnnArbor公司),LPS(美国Sigma公司);RNeasy Mimi Kit(德国Quagen公司),M-MLV逆转录试剂盒(美国Invitrogen公司),SYBR Green PCR Master Mix,Taqman Universal PCR Master Mix(美国ABI公司);抗GAPDH 单克隆抗体(美国Cell Signaling Technology公司),抗MIF多克隆抗体、抗F4/80多克隆抗体、抗PPAR-γ多克隆抗体(美国Santa Cruz Biotechenology公司)。
细胞培养J774A.1接种于含10%胎牛血清、1%青霉素/链霉素的DMEM培养液中,置于37 ℃、5% CO2细胞培养箱中培养,接种密度1×106/r=10 cm细胞培养皿或3×105/r=6 cm细胞培养皿,细胞汇合到90%时进行传代,取对数生长期细胞进行实验。
细胞的药物处理细胞汇合到80%时给予药物处理。细胞饥饿12 h,分别给予以下处理:(1)LPS组:1 μg/ml LPS 孵育1 h;(2)正常对照组:LPS的溶剂PBS孵育1 h;(3)阴性对照组:5 μmol/L 15 d-PGJ2孵育1 h;(4)15 d-PGJ2组:5 μmol/L 15 d-PGJ2预孵育1 h,1 μg/ml LPS 孵育1 h;(5)GW9662组:10 μmol/L GW9662预孵育1 h,5 μmol/L 15 d-PGJ2孵育1 h,1 μg/ml LPS孵育1 h;(6)Vehicle组:即GW9662对照组,使用GW9662的溶剂DMSO替代GW9662。
细胞免疫荧光细胞接种于96孔板中,1×104/孔,贴壁后用预冷的PBS清洗,4%多聚甲醛室温避光固定15 min;用0.5% Triton X- 100进行膜通透处理,2% BSA封闭;MIF(1∶50)抗体孵育,4 ℃过夜;F4/80(1∶200)抗体孵育,37 ℃ 1 h;加入相应荧光标记二抗,37 ℃ 1 h,避光;DAPI染细胞核后高内涵自动成像系统进行扫描。阴性对照组不加一抗,其余处理与实验组相同。
RT-qPCR处理后的细胞用预冷PBS清洗,加入350 μl 裂解液,收集裂解底物提取全细胞RNA(QIAGEN®RNeasy Mini Kit),定量(NanoVue)后取0.5 μg逆转录(不加逆转录酶作为阴性对照,即NO-RT),cDNA稀释后进行PCR反应,引物序列见表1。检测的临界点设定在PCR扩增过程中,荧光信号由本底进入指数增长阶段的拐点所对应的循环数(Ct)作为模板初始浓度的间接指标,溶解曲线分析采用默认条件。结果以18S rRNA 进行校正,用ΔΔCt 法计算相对基因表达量。取PCR产物DNA进行琼脂糖凝胶电泳。胶浓度为2%,电压为100V,电泳时间45 min,紫外灯下观察并打印胶片。
Western blot检测处理后的细胞用预冷的PBS清洗,0.125%胰酶消化后加入蛋白裂解液,提取全细胞蛋白,BCA法蛋白定量(BCATMprotein assay kit)。取50 μg总蛋白进行SDS-聚丙烯酰胺凝胶电泳(胶浓度15%),转膜至0.2 μm的PVDF膜上。5%脱脂奶粉室温封闭1 h,孵育MIF抗体(1∶200稀释),4 ℃过夜。PBST洗膜5 min,3次,孵育相应二抗,室温避光1 h。PBST洗膜后Odyssey红外荧光扫描成像系统扫描成像并进行定量分析。内参GAPDH测定步骤相同。
免疫荧光法测定细胞内过氧化物酶体增殖物激活受体γ的含量及高内涵分析细胞接种于96孔培养板中,每孔1×104个细胞,过夜贴壁。给予药物处理后,进行免疫荧光染色,过氧化物酶体增殖物激活受体γ(peroxisome proliferator-activated receptor-γ,PPAR-γ)抗体(1∶50稀释)孵育,DAPI染细胞核后使用高内涵仪器配备的Thermo Scientific CellInsight personal cell imaging (PCI) 平台及Thermo Scientific Cellomics iDEV 软件对96孔板进行扫描成像。机器扫描时,每孔随机选取49个视野,且细胞数目不小于4000个。采用Cellomics Cell Health Profiling BioApplication 软件对荧光强度信息进行分析,定量测定各处理条件下J774A.1中PPAR-γ的含量。分析结束后,根据需要选择输出图像和数据统计结果。结果以细胞核内荧光强度/细胞质内荧光强度±标准误形式给出。
统计学处理采用SPSS 16.0统计软件,数据以均数±标准误表示,组间比较采用 student’st检验,P<0.05为差异有统计学意义。
结果
J774A.1在基因和蛋白水平均表达MIF细胞免疫荧光检测结果显示,MIF蛋白(红色荧光)在J774A.1中有表达,且与小鼠单核/巨噬细胞的表面标志物F4/80(绿色荧光)有共定位(图1)。引物设计PCR产物MIF片段的分子量为118bp,用琼脂糖凝胶电泳技术在相应位置检测到MIF的存在(图2)。
15 d-PGJ2下调J774A.1细胞炎症模型中MIF mRNA和蛋白表达水平用1 μg/ml LPS诱导J774A.1炎症反应,RT-qPCR方法检测到细胞中MIF mRNA表达上调到对照组的1.75倍(P=0.037)。给予细胞5 μmol/L 15 d-PGJ2预孵育1 h后再用LPS诱导炎症反应,细胞中MIF mRNA表达的上调被抑制,下调到LPS组的50%(P=0.026)。单独应用15 d-PGJ2处理组细胞中MIF mRNA表达水平与对照组相比差异无统计学意义(P=0.099)(图3A)。MIF蛋白水平变化情况与mRNA水平相一致(图3B)。
GW9662逆转15 d-PGJ2对MIF mRNA和蛋白表达的抑制功能用PPAR-γ拮抗剂GW9662预处理细胞,再孵育5 μmol/L 15 d-PGJ21 h后,用1 μg/ml LPS诱导细胞炎症反应,RT-qPCR检测结果显示,与Vehicle组相比,GW9662逆转了15 d-PGJ2对MIF mRNA表达的下调(P=0.016)。单独给予GW9662处理对MIF mRNA表达无影响(P=0.26)(图4A)。MIF蛋白水平变化情况与mRNA水平相一致(图4B)。
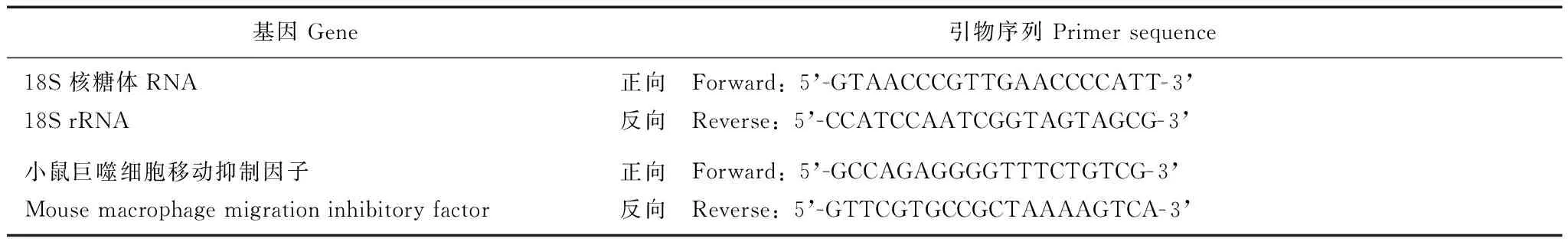
表 1 PCR引物序列
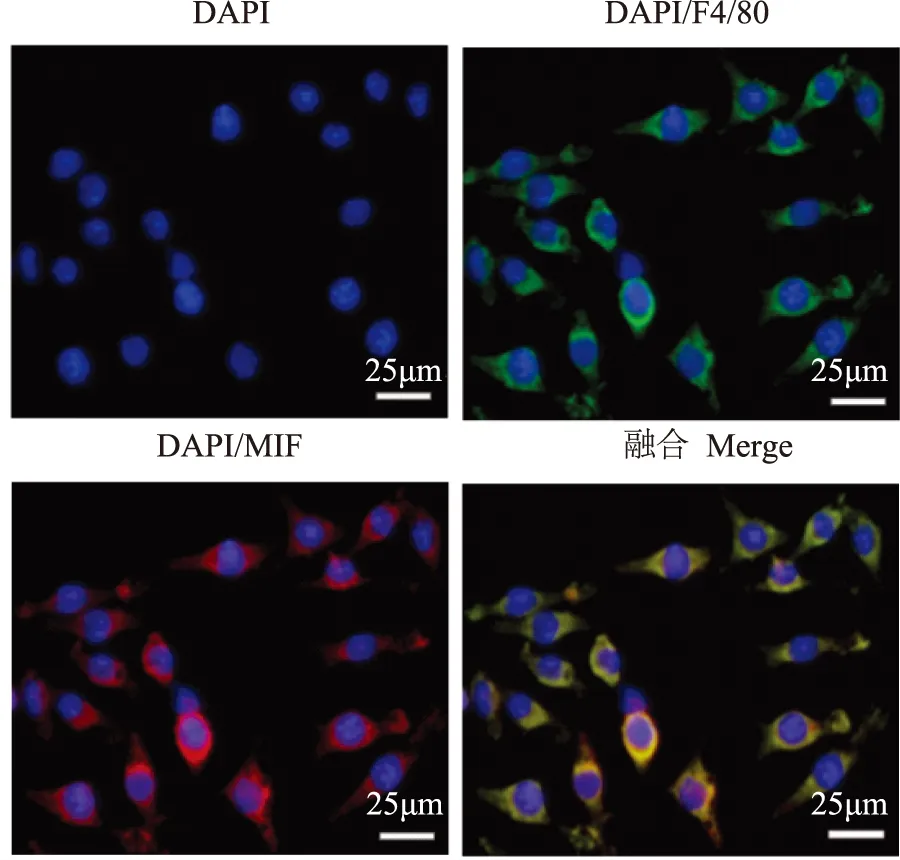
MIF:巨噬细胞移动抑制因子;绿色代表F4/80,红色代表MIF,蓝色代表细胞核,细胞核染料为DAPI
MIF:macrophage migration inhibitory factor;cells were stained for F4/80 (green) and MIF (red),DAPI was used to visualize nuclei (blue)
图 1小鼠单核/巨噬细胞系J774A.1表达MIF蛋白
Fig 1Protein expression of MIF in mouse monocyte/macrophage cell line J774A.1
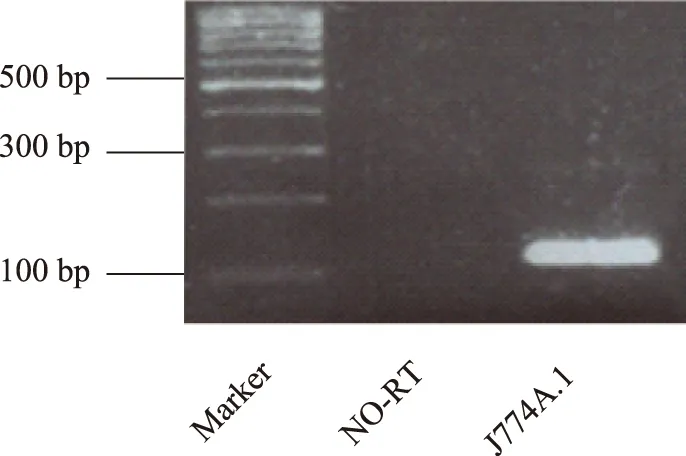
NO-RT:在逆转录时不加逆转录酶;提取J774A.1全细胞RNA进行RT-qPCR反应,产物经2%琼脂糖凝胶电泳在118 bp位置检测到MIF条带
NO-RT:do not add reverse transcriptase in reverse transcription;RT-qPCR analysis of MIF in J774A.1 using total cellular RNA, the products of PCR were size-fractionated in a 2% agarose gel, cDNA corresponding to expected length of MIF was detected
图 2MIF在小鼠单核/巨噬细胞中基因水平的表达
Fig 2Gene expression of MIF in mouse monocyte/macrophage cell line J774A.1
15 d-PGJ2调节炎症反应时J774A.1中PPAR-γ的核转位细胞饥饿12 h后,用1 μg/ml LPS 诱导细胞炎症反应,给予或不给予5 μmol/L 15 d-PGJ2处理,1 h后终止。用免疫荧光技术特异性显示PPAR-γ后发现,与对照组相比,LPS组细胞内PPAR-γ的核质比没有明显变化(P=0.161),15 d-PGJ2处理组细胞的胞核中PPAR-γ含量明显上调。高内涵自动成像系统分析结果显示,15 d-PGJ2处理组细胞中PPAR-γ的核质比上调至LPS组的1.39倍(P=0.003)(图5)。

15 d-PGJ2:15-脱氧-前列腺素J2;LPS:脂多糖;与对照组比较,aP=0.037;与LPS组比较,bP=0.026;细胞经或不经过5 μmol/L 15 d-PGJ2预处理1 h后,给予1 μg/ml LPS孵育1 h,分别检测细胞中MIF mRNA(内参为18S 核糖体RNA)和蛋白(内参为GAPDH)的表达情况,实验结果以均数±标准误形式给出,实验重复3次
15 d-PGJ2:15-deoxy-Δ12,14-prostaglandin J2;LPS:lipopolysaccharide;aP=0.037 compared with controls;bP=0.026 compared with LPS group; cells were exposed to LPS at 1 μg/ml for 1 h with or without 5 μmol/L 15 d-PGJ2pretreatment; results were normalized relative to 18S rRNA or GAPDH expression; data are presented as the mean±SEM; results were confirmed in three independent experiments
A. RT-qPCR检测J774A.1中MIF mRNA表达水平;B. Western blot 检测J774A.1细胞中MIF蛋白表达水平
A.RT-qPCR analysis was performed to determine MIF mRNA levels in J774A.1;B. Western blot analysis was performed to determine MIF protein levels in J774A.1
图 315 d-PGJ2下调炎症时J774A.1中MIF mRNA和蛋白的表达水平
Fig 3MIF mRNA and protein expressions were down-regulated by 15 d-PGJ2in J774A.1 upon LPS challenge
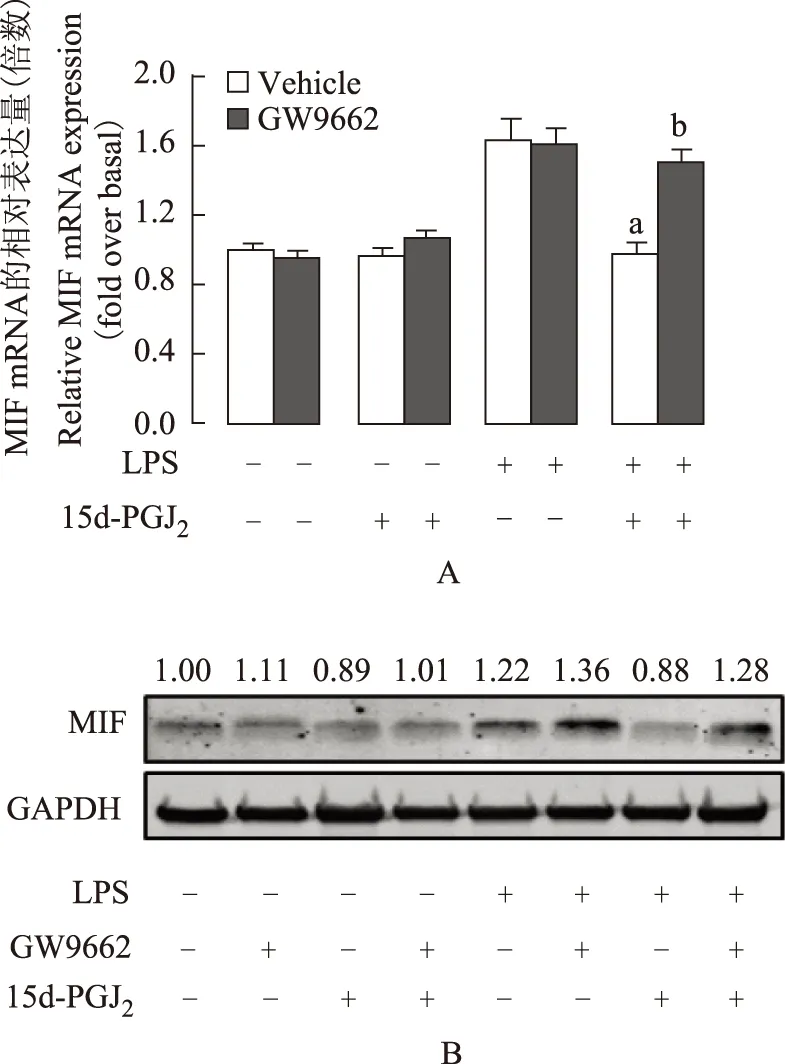
与LPS组比较,aP=0.012;与LPS+15 d-PGJ2组比较,bP=0.016;细胞分别给予10 μmol/L GW9662或其溶剂DMSO预处理1 h,后给予5 μmol/L 15 d-PGJ2孵育1 h,再用1 μg/ml LPS处理1 h,分别检测其MIF mRNA(内参为18S 核糖体RNA)和蛋白(内参为GAPDH)水平,实验结果以均数±标准误形式给出,实验重复3次aP=0.012 compared with LPS group;bP=0.016 compared with LPS+ 15 d-PGJ2group;cells were pretreated with or without 10 μmol/L GW9662,then incubated with 5 μmol/L 15 d-PGJ2for 1 h,followed by 1 μg/ml LPS, after quantification of the signals,the results were normalized relative to 18S rRNA or GAPDH expression, data are presented as the mean±SEM, results were confirmed in three independent experiments
A.RT-qPCR检测MIF mRNA表达水平;B. Western blot 检测MIF蛋白表达水平
A. MIF mRNA levels were detected by RT-qPCR;B. Western blot analysis was performed to determine MIF protein levels in J774A.1
图 4GW9662逆转15 d-PGJ2对MIF mRNA和蛋白表达的抑制功能
Fig 4Effect of 15 d-PGJ2on expression of MIF mRNA was inhibited by GW9662
讨论
炎症反应是组织器官受到损伤因素刺激后的一种免疫防御反应,是多种慢性疾病的主要诱发因素。作为免疫系统的第1道防线,单核巨噬细胞在接受LPS等刺激后可释放大量炎症介质和促炎因子,如MIF等,介导炎症反应的发生发展以及组织损伤。
MIF是一种作用广泛的前炎症细胞因子,可通过自分泌和旁分泌的方式参与炎症反应,能够促进其他细胞因子(如肿瘤坏死因子α等)的产生、活化巨噬细胞、抑制其游走移动等[1,5- 6]。本研究明确了小鼠单核巨噬细胞系J774A.1表达MIF,且LPS诱导的J774A.1炎症反应模型中其表达上调,并发现该上调可被15 d-PGJ2抑制,提示MIF可能是15 d-PGJ2发挥抗炎功能的重要作用靶点。

PPAR-γ:过氧化物酶体增殖物激活受体γ;与LPS组比较,aP=0.003;细胞经1 μg/ml LPS处理1 h后,给予5 μmol/L 15 d-PGJ2处理1 h,用免疫荧光的方法分别标记PPAR-γ(绿色)和细胞核(蓝色),用高内涵分析系统分析细胞中PPAR-γ的核质比,实验结果以均数±标准误形式给出,实验重复3次
PPAR-γ:peroxisome proliferator-activated receptor-γ;aP=0.003 compared with LPS group;cells were treated with 1 μg/ml LPS for 1 h followed by 5 μmol/L 15 d-PGJ2for 1 h or not and stained for PPAR-γ (green), DAPI was used to visualize nuclei (blue), the nuclear/cytoplasmic ratios for PPAR-γ were measured with high content analysis, data are presented as the mean±SEM, all results were confirmed in three independent experiments
图 515 d-PGJ2对炎症反应时J774A.1中PPAR-γ核转位的影响
Fig 5Effect of 15 d-PGJ2on PPAR-γ nuclear translocation in J774A.1 upon LPS challenge
15 d-PGJ2是近年来被各界学者广泛关注的具有多种生物学功能的内源性分子,可参与组织损伤、氧化应激以及炎症反应等[7- 9]。15 d-PGJ2是PPAR-γ的天然配体,能够将其活化而发生核转位,进而调控靶基因的表达。PPAR-γ是一种由配体激活的核转录因子,属于Ⅱ型核受体超家族成员中的一个亚型,可通过竞争抑制炎症信号通路[包括酪氨酸激酶-信号转导子和转录激活子、核因子-κB(nuclear factor-κB,NF-κB)等]或炎性介质的生成发挥抗炎功能,表达于多种组织细胞中。文献报道,巨噬细胞中PPAR-γ主要发挥抗炎功能[10]。本研究结果显示,J774A.1中15 d-PGJ2对MIF表达的抑制作用可被PPAR-γ的药理学拮抗剂GW9662逆转,且15 d-PGJ2能够促进炎症反应时PPAR-γ活化入核,说明15 d-PGJ2对MIF表达的调节依赖于PPAR-γ。有研究显示,PPAR-γ能够抑制NF-κB的合成和转录。在静息细胞中,NF-κB与抑制蛋白单体IκBα和IκBβ 以无活性的结合形式存于细胞质中,炎症反应时发生解离而诱导炎症介质基因表达[11- 12]。因此,推测本研究中15 d-PGJ2对MIF的基础表达影响很小的原因可能是炎症反应时MIF在 NF-κB的调控下表达量增加,而15 d-PGJ2预孵育细胞后激活PPAR-γ,后者竞争抑制NF-κB而下调MIF的表达。但此推论仍需进一步研究证实。此外,本研究结果也提示,MIF可能是15 d-PGJ2和PPAR-γ发挥抗炎功能的一个靶基因。
参考文献
[1]Barnes MA,McMullen MR,Roychowdhury S,et al. Macrophage migration inhibitory factor contributes to ethanol-induced liver injury by mediating cell injury,steatohepatitis,and steatosis[J]. Hepatology,2013,57(5):1980- 1991.
[2]Gregory JL,Morand EF,McKeown SJ,et al. Macrophage migration inhibitory factor induces macrophage recruitment via CC chemokine ligand 2[J]. J Immunol,2006,177(11):8072- 8079.
[3]Han Z,Zhu T,Liu X,et al. 15-deoxy-Delta12,14-prostaglandin J2reduces recruitment of bone marrow-derived monocyte/macrophages in chronic liver injury in mice[J]. Hepatology,2012,56(1):350- 360.
[4]Liu X,Yu H,Yang L,et al. 15-Deoxy-Delta(12,14)-prostaglandin J(2) attenuates the biological activities of monocyte/macrophage cell lines[J]. Eur J Cell Biol,2012,91(8):654- 661.
[5]Xu L,Li Y,Sun H,et al. Current developments of macrophage migration inhibitory factor (MIF) inhibitors[J]. Drug Discov Today,2013,18(11- 12):592- 600.
[6]Sánchez-Zamora YI,Rodriguez-Sosa M. The role of MIF in type 1 and type 2 diabetes mellitus[J]. J Diabetes Res,2014,2014:804519. doi:10.1155/2014/804519.
[7]Maier NK,Leppla SH,Moayeri M. The cyclopentenone prostaglandin 15 d-PGJ2 inhibits the NLRP1 and NLRP3 inflammasomes[J]. J Immunol,2015,194(6):2776- 2785.
[8]Kang ES,Hwang JS,Ham SA,et al. 15-Deoxy-Delta(12,14)-prostaglandin J2 prevents oxidative injury by upregulating the expression of aldose reductase in vascular smooth muscle cells[J]. Free Radic Res,2014,48(2):218- 229.
[9]Chen S,Liu C,Wang X,et al. 15-Deoxy-Delta(12,14)-prostaglandin J2 (15 d-PGJ2) promotes apoptosis of HBx-positive liver cells[J]. Chem Biol Interact,2014,214:26- 32. doi:10.1016/j.cbi.2014.02.009.
[10]Chinetti G,Fruchart JC,Staels B. Peroxisome proliferator-activated receptors:new targets for the pharmacological modulation of macrophage gene expression and function[J]. Curr Opin Lipidol,2003,14(5):459- 468.
[11]Sánchez-Hidalgo M,Martin AR,Villegas I,et al. Rosiglitazone,an agonist of peroxisome proliferator-activated receptor gamma,reduces chronic colonic inflammation in rats[J]. Biochem Pharmacol,2005,69(12):1733- 1744.
[12]Li M,Damania B,Alvarez X,et al. Inhibition of p300 histone acetyltransferase by viral interferon regulatory factor[J]. Mol Cell Biol,2000,20(21):8254- 8263.
基金项目:国家自然科学基金(81300335)和北京市高等学校创新团队建设与教师职业发展计划项目(IDHT20150502)Supported by the National Natural Sciences Foundation of China (81300335) and the Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges Under Beijing Municipality (IDHT20150502)
通信作者:李丽英电话/传真:010- 83950101,电子邮件:liliying@ccmu.edu.cn
中图分类号:R392.12
文献标志码:A
文章编号:1000- 503X(2016)03- 0247- 06
DOI:10.3881/j.issn.1000- 503X.2016.03.001
Corresponding author:LI Li-yingTel/Fax:010- 83950101,E-mail:liliying@ccmu.edu.cn
(收稿日期:2015- 06- 08)
Effect of 15-Deoxy-Δ12,14-prostaglandin J2on Expression of Macrophage Migration Inhibitory Factor in Mouse Monocyte/macrophage Cell Line J774A.1
LI Wei-yang,SHI Yu-meng,LIU Xin,YANG Lin,LI Li-ying
Municipal Laboratory for Liver Protection and Regulation of Regeneration,Department of Cell Biology,Capital Medical University,Beijing 100069,China
ABSTRACT:ObjectiveTo investigate the effect of 15-Deoxy-Δ12,14-prostaglandin J2 (15 d-PGJ2) on the expression of macrophage migration inhibitory factor (MIF) and its underlying mechanism in J774A.1. MethodsThe murine monocyte/macrophage cell line J774A.1 were divided into six groups:lipopolysaccharide (LPS) group,incubated with 1 μg/ml LPS for 1 h;normal control group,incubated with PBS for 1 h;negative control group,incubated with 5 μmol/L 15 d-PGJ2 for 1 h;15 d-PGJ2 group,incubated with 5 μmol/L 15 d-PGJ2 for 1 h followed by 1 μg/ml LPS for 1 h;GW9662 group,incubated with 5 μmol/L 15 d-PGJ2 for 1 h following GW9662 10 μmol/L for 1 h,and then incubated with 1 μg/ml LPS for 1 h;and Vehicle group,control of GW9662,GW9662 was replaced by its solvent DMSO. The expression of MIF was detected via immunofluorescence and agarose gel electrophoresis. RT-qPCR and Western blotting were used to test whether 15 d-PGJ2 could regulate mRNA and protein expression of MIF in J774A.1 upon LPS challenge. The effect of peroxisome proliferator-activated receptor-γ (PPAR-γ) antagonist GW9662 on the regulation of MIF by 15 d-PGJ2was observed. The effects of 15 d-PGJ2 on the nuclear translocation of PPAR-γ upon LPS challenge were detected via high content screening analysis. ResultsMIF DNA and protein expressions were detected in J774A.1. MIF mRNA expression was up-regulated (1.75±0.09,P=0.037) when challenged with LPS and 15 d-PGJ2 inhibited its upregulation (0.84±0.08,P=0.026) in J774A.1. The protein level was consistent with the mRNA level. PPAR-γ antagonist GW9662 reversed the effect of 15 d-PGJ2 (mRNA,1.48±0.06,P=0.016;protein,1.28). Furthermore,nuclear translocation of PPAR-γ was regulated by 15 d-PGJ2 in J774A.1 upon LPS challenge(1.39±0.02 vs. 1.01±0.03,P=0.003). Conclusion15 d-PGJ2 may down-regulate the MIF expression in J774A.1 in a PPAR-γ-dependent manner.
Key words:15-Deoxy-Δ12,14-prostaglandin J2;macrophage migration inhibitory factor;peroxisome proliferator-activated receptor-γ

