Cytomegalovirus and Helicobacter Pylori Co-infection in an Adult with Ménétrier’s Disease: A Case Report
Xiang-yao Wang and Hao Liang*Department of Gastroenterology, Chinese People’s Liberation Army General Hospital, Beijing 100853, China
Cytomegalovirus and Helicobacter Pylori Co-infection in an Adult with Ménétrier’s Disease: A Case Report
Xiang-yao Wang and Hao Liang*
Department of Gastroenterology, Chinese People’s Liberation Army General Hospital, Beijing 100853, China
Ménétrier’s disease; cytomegalovirus; helicobacter oylori; theraoy
Chin Med Sci J 2016; 31(2):129-133
M ÉNÉTRIER'S disease (MD) is a rare disease characterized by hypertrophy of gastric folds, and nausea, emesis, abdominal pain and peripheral oedema are the main manifestations. Hypochlorhydria and hypoalbuminemia are also common.1MD is always misdiagnosed due to its rarity. Especially, the symptoms caused by hypoalbuminemia, for example, pleural effusions or ascites,make the correct diagnosis more difficult. Since the disease was first reported by Ménétrier in 1888, there have not been large scale studies due to its rarity. The pathogenesis of MD is still unknown. For adult MD,some scholars think helicobacter pylori (Hp) is a risky factor,2but some claim it is a premalignant disease.3While more data are required to confirm for the both viewpoints. For child MD, cytomegalovirus (CMV) is considered to be an important cause,4but not all the patients have the infection. Moreover, Hp infection has been reported to be associated with child MD.2We report an adult MD patient with CMV and Hp coinfection, and briefly discuss the etiology, diagnosis,and therapy of MD.
CASE DESCRIPTION
A 26-year-old man was admitted to our department due to complaints of early satiety, mild abdominal distention, and peripheral oedema. He began to vomit about 3 weeks ago without known triggers; there were no bile, blood, or coffee-ground materials present. He then gradually developed early satiety, mild abdominal distention,and peripheral oedema. Nonsteroid anti-inflammatory drugs were not used recently. There was no history of smoking or alcohol, or illicit drugs. Family history was negative for renal disease, liver disease, and celiac disease.
Physical exam revealed a well-developed and wellnourished man. Mild epigastric tenderness without rebound or abdominal rigidity was noted. Marked bilateral lower limb oedema was also found. There were no other remarkable findings. Laboratory tests showed an elevated white cell count (4.31×109g/L) with 62% lymphocytes, 8% monocytes, and 23% neutrophils. The serum total protein was 34.2 (normal range, 60 to 80) g/L, albumin 19.8 (normal range, 35 to 50) g/L, alanine aminotransferase 105.9 (normal range, 0 to 40) U/L, and aspartate aminotransferase 86 (normal range, 0 to 35) U/L. Viral serologic test was positive for IgM and IgG antibodies to CMV. The titer of CMV-IgG was 80.1 (>14 was considered positive),with that of CMV-IgM being 70.4 (>22 was deemed positive). Antibodies to Epstein-Barr virus, human immunodeficiency virus and hepatitis C virus as well as hepatitis B virus surface antigen and total antibodies to hepatitis B virus core antigen were negative.13C urea breath test showed positive result.
Abdominal computed tomographic scans (Fig. 1A)showed the wall of gastric fundus and corpus was markedly thickened with density increase. In addition, mild perihepatic and perisplenic ascites and small pleural effusions were revealed. The liver and spleen were not enlarged.
Gastric endoscopy (Fig.1 B) showed enlarged gastric folds throughout the stomach body and fundus without the antrum. Diffuse hyperemia and edema of the gastric mucosa with partial erosion and large amount of secretions were found. Endoscopic ultrasonography (Fig. 1C) revealed a thickened lesion in mucosa layer with the size of about 6 mm, and submucosa layer and muscularis propria layer were clear and integrated. Narrow-band imaging (NBI) and magnifying endoscopy (Fig. 1D) demonstrated the structure of mucosal adenous ducts was existent, but they were disorganized and variable in size, with circuitous microvesseles seen among them. A deep snare biopsy of corpus lesions was performed, and the pathologic results after hematoxylineosin-eosin staining (Fig. 1G) showed active and chronic gastritis and marked epithelial hyperplasia with tortuous and cystically dilated foveolar glands. Specific immunohistochemical staining for CMV showed positive result (Fig. 1H).
According to the results above, the diagnosis of MD was made. Given the patient’s acute course and physical condition, symptomatic and supportive treatment was given, which included dieresis (torasemide), acid suppression (omeprazole sodium), liver protection (reduced glutathione),and infusion of albumin. The patient’s condition was gradually improved after the treatment. Gastric endoscopy (Fig. 1E) was performed again after the treatment for 3 weeks, which showed the fundus became normal, the thickening of the corpus and the hyperemia and edema of mucosa were markedly alleviated. A few mucosal eminent lesions with a small amount of secretion were seen in the corpus near the antrum, and diameter of the lesions was about 0.5-0.6 cm. The patient was discharged 3 weeks later, without any complaints. B ultrasound showed no ascites or pleural effusions. The abnormalities of laboratory test restored to normal values except CMV-IgG (the titer was 113.0) and CMV-IgM (the titer was 69.4). The patient had no complaint or symptom at the follow-up of 3 months after the patient’s discharge. Gastric endoscopy (Fig. 1F)showed a normal gastric mucosa.
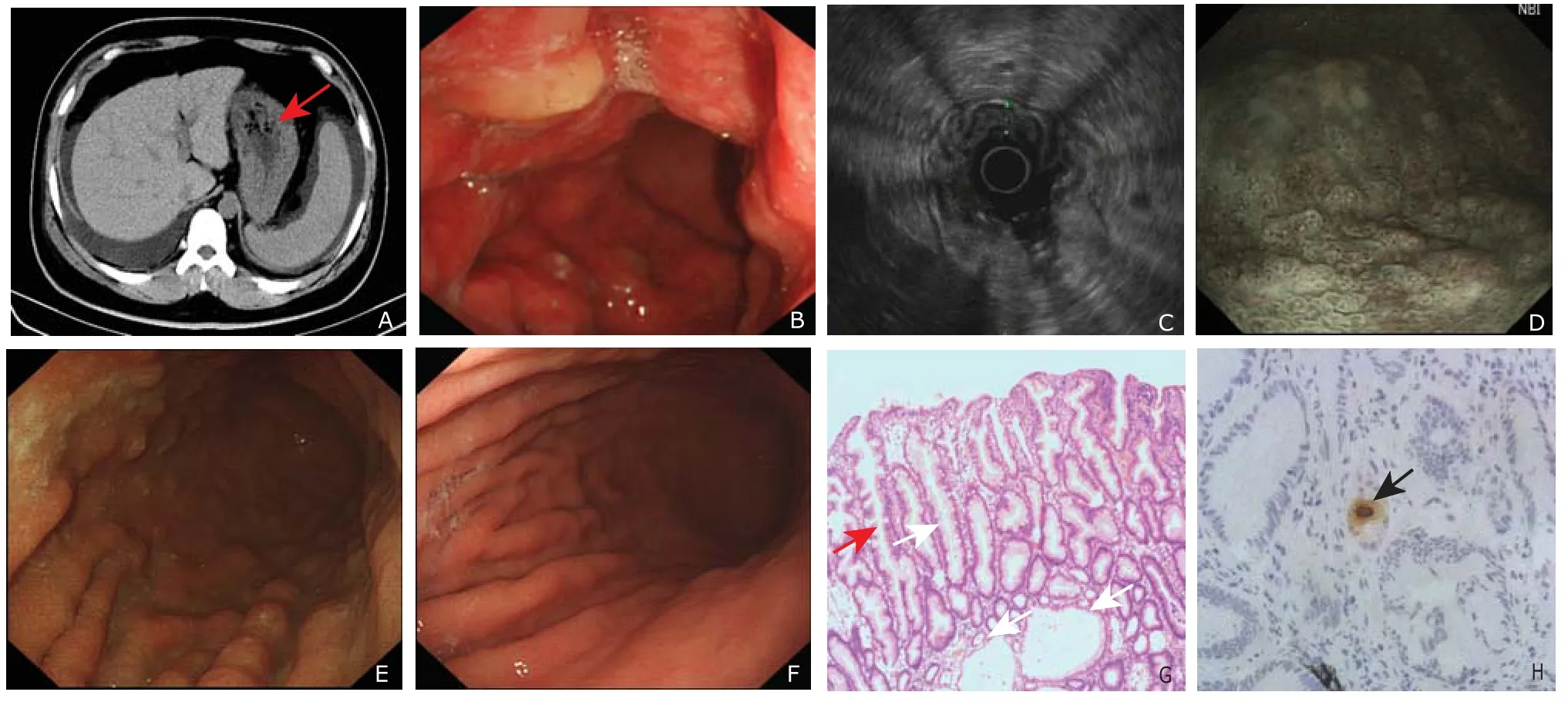
Figure 1. A 26-year-old Ménétrier's disease man with cytomegalovirus and helicobacter pylori co-infection. A. abdominal computed tomography on admission. The red arrow shows the wall of gastric fundus and corpus was markedly thickened with density increase; B. gastric endoscopy after admission; C. endoscopic ultrasonography; D. narrow-band imaging; E. gastric endoscopy after 3 weeks’ treatment; F. follow-up gastric endoscopy 3 months after discharge; G. pathologic examination of hematoxylineosin-eosin staining after admission (×100). Active and chronic gastritis and marked epithelial hyperplasia with tortuous (black arrows) and cystically (white arrows) dilated foveolar glands are visible. H. immunohistochemical staining with anti-cytomegalovirus early nuclear antigen. The brown staining indicated positive reaction against anti-cytomegalovirus antibodies (arrow) (×400).
DISCUSSION
MD is a type of disease with unknown pathogenesis,which is more common among men than women. Individuals aged 40-50 years are more affected.5As the case number of MD increases, more and more studies begin to focus on etiology and pathogenesis of the disease. The present evidences have showed MD is associated with Hp and CMV infection. Two retrospective studies have demonstrated that 30%-90% adult MD is associated with Hp infection.6, 7Although the reported adult MD cases associated with CMV infection are few, the number of the cases reported is increasing. Moreover, either immunocompetent or immunocompromised adults with MD may be related to CMV infection,8and the reported cases are increasing. But Hp and CMV co-infection is uncommon for the adult MD patients. For child with MD, CMV is considered to be an important cause. Cieslak et al4reported 70% of child MD patients are associated with CMV infection, but another report claimed that about half of the child MD patients are associated with CMV infection.9Meanwhile, the cases associated with Hp infection are increasing. Fretzayas et al2found 8 child MD patients associated with Hp infection by reviewing the literatures, and they assumed child MD patients should undergo Hp test. In general, the number of immunocompetent adult MD patients associated with CMV infection is small. Hp infection is commoner in adult MD patients than that in child MD patients. Hp and CMV co-infection is rare for either adult MD or child MD.
The patient in our report was infected with both Hp and CMV, and the greatest feature was that this patient was an adult while the previously reported MD cases with Hp and CMV co-infection were all children.10-13
There are no specific diagnostic criteria for MD, and the diagnosis is based on clinical and pathological examinations. The possible manifestations of MD include abdominal pain,nausea, emesis, diarrhea and peripheral oedema caused by hypoalbuminemia, etc. Among these manifestations,hypoalbuminemia is present in 20% to 100% of the patients, and this feature sometimes helps distinguish MD from its mimics. The endoscopic findings often show hypertrophy in the gastric body and fundus, with spare of the antrum. The histologic results are characterized by foveolar hyperplasia, tortuous and cystic dilatation, and common glandular atrophy. Full-thickness mucosal biopsy is essential for the diagnosis. Rich et al5proposed a diagnostic process based on the study of multiple cases,and they thought MD was most accurately diagnosed by clinicohistopathological analysis including oesophagogastroduodenoscopy with gastric pH, appropriate laboratory tests (complete blood count, serum albumin, serum gastrin,Hp and CMV serology), and full-thickness mucosal biopsy of the involved gastric mucosa. The histopathological features mainly include foveolar hyperplasia, glandular tortuosity and dilation, and a marked reduction in parietal cell number, and lamina propria smooth muscle hyperplasia and oedema are also common. Among the mimics, the most common diagnoses may be gastric polyps or polyposis syndromes.
The determination of Hp or CMV infection depends on the laboratory and histopathological examinations. There are several methods to test Hp infection, among which breath test and serology are the commonest, but histopathology is more precise. For the detection of CMV infection, serological test is a convenient choice, and follow-up of the serum anti-CMV IgM titer is useful to assess the course of the infection to some extent, but it cannot make a definite diagnosis. The diagnosis may be established by immunohistochemical detection of CMV early nuclear antigen in gastric biopsy. Lachman et al14and Leonidas et al15reported the finding of CMV inclusion body in the gastric mucosa of a child MD patient. Detection of inclusion bodies further supports the diagnosis, but routine histological examination may fail to reveal their presence. Detection of CMV DNA in a gastric biopsy sample by PCR is a more sensitive assay than antigenemia and serology tests, but it is expensive and not available for some hospital. Accordingly, Megged et al11suggest that serology tests are appropriate for routine screening, and gastric biopsy samples should be examined by immunohistochemical studies and PCR for the final diagnosis. Therefore, no matter adult MD or child MD, the underlying causes should be considered,16such as Hp and CMV infection. Moreover,there are also other possible causes, such as physicochemical irritation, diet, congenital factors, and other unknown factors. The cases associated with trichobezoar of stomach support the hypothesis, to confirm which more data are still needed.
The present patient underwent laboratory test,radiology examination, gastric endoscopy and histopathology as well as NBI and endoscopic ultrasonography, which are important for the terminal and differential diagnosis. The use of NBI and magnifying endoscopy in the diagnosis of MD is seldom reported, which may be a promising tool. Hp infection of the patient was confirmed by13C-urea breath test. CMV infection was suggested by serology test, and immunohistochemistry staining was then performed to confirm the infection.
There is no criterion for the treatment of MD, and themain therapies include symptomatic and supportive treatment, drug treatment (such as antiviral, glucocorticosteroid, octreotide, cetuximab, etc.), surgery, etc. But surgery is the definitely effective therapy. Most of the child MD patients associated with CMV infection always present as a self-limited course,4and symptomatic and supportive treatment is given as the main treatment. But for severe and chronic cases, antivirus treatment or other treatments should be considered. It is reported that a case with refractory pediatric MD is successfully treated with octreotide, which achieves long acting release.17The eradication therapy is recommended in cases associated with Hp infection,2but more data are still needed to support the efficacy. The treatment of adult MD patients associated with CMV infection is similar to that of child MD patients. Actually, CMV-associated MD differs from classic MD in acute self-limited course and more focal involvement of the gastric fundus or antrum or both. Although most patients resolve spontaneously with supportive treatment only, a reported case of an immunocompetent adult MD patient is dead despite antiviral treatment.18Eradication therapy is suggested for the cases with Hp infection, and other treatment may be considered if it fails. For the cases with Hp or CMV infection, if no sign of improvement is shown after symptomatic and supportive treatment, other treatment should be considered. Burdick et al19reported the use of cetuximab (a monoclonal antibody that blocks epidermal growth factor receptor signaling) in the treatment of MD. Fiske et al20reported the significant efficacy of cetuximab in 9 individuals with clinically and histologically documented severe MD. Of the 7 patients who completed 1-month treatment, all showed statistically significant improvement, and 4 subsequently showed near-complete histological remission. However, the case number in this study is small, and more data are needed to further confirm the role of this medication. If the patients have gastric cancer or conservative treatment has failed, surgical operation will be needed.
The therapy of this patient was symptomatic and supportive treatment. After the treatment, the symptoms were relieved, and the laboratory indicators returned to normal levels. Gastric endoscopy showed marked remission of the hypertrophy of gastric mucosa. The eradication of Hp was not performed due to the patient’s refusal. Although the patient was co-infected with Hp and CMV, the exact cause was hardly known and follow-up was still required. The specific treatment such as Hp eradication therapy and antiviral treatment should be considered if the patient relapses in the future.
REFERENCES
1. Lambrecht NW. Ménétrier’s disease of the stomach: a clinical challenge. Curr Gastroenterol Rep 2011;13:513-7.
2. Fretzayas A, Moustaki M, Alexopoulou E, et al. Menetrier's disease associated with helicobacter pylori: three cases with sonographic findings and a literature review. Ann Trop Paediatr 2011; 31:141-7.
3. Pryczynicz A, Bandurski R, Guzińska-Ustymowicz K, et al. Ménétrier's disease, a premalignant condition, with coexisting advanced gastric cancer: a case report and review of the literature. Oncol Lett 2014; 8:441-5.
4. Cieslak TJ, Mullett CT, Puntel RA, et al. Menetrier’s disease associated with cytomegalovirus infection in children: report of two cases and review of the literature. Pediatr Infect Dis J 1993; 12:340-3.
5. Rich A, Toro TZ, Tanksley J, et al. Distinguishing Ménétrier’s disease from its mimics. Gut 2010;59:1617-24.
6. Bayerdörffer E, Ritter MM, Hatz R, et al. Ménétrier’s disease and Helicobacter pylori. N Engl J Med 1993;329:60.
7. Bayerdorffer E, Ritter MM, Hatz R, et al. Healing of protein losing hypertrophic gastropathy by eradication of helicobacter pylori: is helicobacter pylori a pathogenic factor in Menetrier’s disease? Gut 1994; 35: 701-4.
8. Suter WR, Neuweiler J, Bovicka J, et al. Cytomegalovirus-induced transient proteinlosing hypertrophic gastropathy in an immunocompetent adult. Digestion 2000; 62:276-9.
9. Xiao SY, Hart J. Marked gastric foveolar hyperplasia associated with active cytomegalovirus infection. Am J Gastroenterol 2001; 96:223-6.
10. Tokuhara D, Okano Y, Asou K, et al. Cytomegalovirus and helicobacter pylori co-infection in a child with Ménétrier disease. Eur J Pediatr 2007; 166:63-5.
11. Megged O, Schlesinger Y. Cytomegalovirus-associated protein-losing gastropathy in childhood. Eur J Pediatr 2008; 167:1217-20.
12. Iwama I, Kagimoto S, Takano T, et al. Case of pediatric Ménétrier disease with cytomegalovirus and helicobacter pylori co-infection. Pediatr Int 2010; 52:e200-3.
13. Yoo Y, Lee Y, Lee YM, et al. Co-infection with cytomegalovirus and helicobacter pylori in a child with Ménétrier's disease. Pediatr Gastroenterol Hepatol Nutr 2013; 16:123-6.
14. Lachman RS, Martin DJ, Vawter GF. Thick gastric folds in childhood. Am J Roentgenol Radiat Ther Nucl Med 1971; 112:83-92.
15. Leonidas JC, Beatty EC, Wenner HA. Ménétrier’s disease and cytomegalovirus infection in childhood. Am J Dis Child 1973; 126:806.
16. Lalazar G, Doviner V, Ben-Chetrit E. Clinical problemsolving. Unfolding the diagnosis. N Engl J Med 2014;370:1344-8.
17. Di Nardo G, Oliva S, Aloi M, et al. A pediatric nonprotein losing Menetrier's disease successfully treated with octreotide long acting release. World J Gastroenterol 2012; 18:2727-9.
18. Eddleston M, Peacock S, Juniper M, et al. Severe cytomegalovirus infection in immunocompetent patients. Clin Infect Dis 1997; 24:52-6.
19. Burdick JS, Chung E, Tanner G, et al. Treatment of Menetrier’s disease with a monoclonal antibody against the epidermal growth factor receptor. N Engl J Med 2000; 343:1697-701.
20. Fiske WH, Tanksley J, Nam KT, et al. Efficacy of cetuximab in the treatment of Menetrier’s disease. Sci Transl Med 2009; 1:8ra18.
for publication January 12, 2016.
*Corresponding author Tel: 86-10-55499008, E-mail: lianghao301@ 163.com
 Chinese Medical Sciences Journal2016年2期
Chinese Medical Sciences Journal2016年2期
- Chinese Medical Sciences Journal的其它文章
- Hepatocellular Carcinoma with Hypersplenic Thrombocytopenia and Situs Inversus Totalis: A Case Report
- Trends and Prospects of Stem Cell Research in China△
- Low-dose Simvastatin Increases Skeletal Muscle Sensitivity to Caffeine and Halothane
- Mechanical Strain Regulates Osteoblast Proliferation Through Ca2+-CaMK-CREB Signal Pathway△
- Effect of 4-week Whole Body Vibration on Distal Radius Density△
- Impact of Intraoperative Blood Pressure Control and Temporary Parent Artery Blocking on Prognosis in Cerebral Aneurysms Surgery
