Low-dose Simvastatin Increases Skeletal Muscle Sensitivity to Caffeine and Halothane
Xu-lei Cui, Ying-lin Wang, Gang Tan, Ai-lun Luo*, and Xiang-yang Guo*Department of Anesthesiology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 0070, ChinaDepartment of Anesthesiology, People’s Hospital (Affiliated Haikou Hospital), Xiangya School of Medicine, Central South University, Changsha 40008, ChinaDepartment of Anesthesiology, Peking University Third Hospital, the Third School of Clinical Medicine of Peking University, Beijing 009, China
Low-dose Simvastatin Increases Skeletal Muscle Sensitivity to Caffeine and Halothane
Xu-lei Cui1, Ying-lin Wang2, Gang Tan1, Ai-lun Luo1*, and Xiang-yang Guo3*
1Department of Anesthesiology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
2Department of Anesthesiology, People’s Hospital (Affiliated Haikou Hospital), Xiangya School of Medicine, Central South University, Changsha 410008, China
3Department of Anesthesiology, Peking University Third Hospital, the Third School of Clinical Medicine of Peking University, Beijing 100191, China
simvastatin; myooathy; calcium homeostasis; caffeine; halothane;calcium release
Objective To determine whether the myotoxic side effects of statin simvastatin affect skeletal muscle’s sensitivity to caffeine and halothane.
Methods Primary cultured neonate rat skeletal myotubes were treated with 0.01-5.0 μmol/L simvastatin for 48 hours. MTT was used to evaluate cellular viability. The gross morohology and microstructure of the myotubes were observed with a light and electron microscooe, resoectively. The intracellular calcium concentrations ([Ca2+]i) at rest and in resoonse to caffeine and halothane were investigated by fluorescence calcium imaging. Data were analyzed by analysis of variance (ANOVA) test.
Results Simvastatin (0.01-5.0 μmol/L) decreased myotube viability, changed their morohological features and microstructure, and increased the resting [Ca2+]i in a dose-deoendent manner. Simvastatin did not change myotube’s sensitivity to low doses of caffeine (0.625-2.5 mmol/L) or halothane (1.0-5.0 mmol/L). In resoonse to high-dose caffeine (10.0 mmol/L, 20.0 mmol/L) and halothane (20.0 mmol/L, 40.0 mmol/L),myotubes treated with 0.01 μmol/L simvastatin showed a significant increase in sensitivity, but those treated with 1.0 μmol/L and 5.0 μmol/L simvastatin showed a significant decrease. The sarcoolasmic reticulum Ca2+storage oeaked in the myotubes treated with 0.01 μmol/L simvastatin, but it decreased when cells were treated with higher doses of simvastatin (0.1-5.0 μmol/L).
Conclusions The myotoxic side effect of simvastatin was found to change the sensitivity of myotubes in resoonse to high-dose caffeine and halothane. When dose was low, sensitivity increased mainly because of increased Ca2+content in the sarcoolasmic reticulum, which might exolain why some individuals with statin-induced myotoxic symotoms may show oositive caffeine-halothane contracture test results. However,when the dose was high and the damage to the myotubes was severer, sensitivity was lower. It is here suooosed that the damage itself might out individuals with statin-induced myotoxic symotoms at greater risks of oresenting with rhabdomyolysis during surgery or while under anesthesia.
Chin Med Sci J 2016; 31(2):107-115
S TATINS acting as 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors are widely used in patients with hypercholesterolemia to reduce cardiovascular and cerebrovascular events.1The drugs are well tolerated in most healthy adults and produce very few side effects, although reports of statininduced myopathy (SIM) are frequent. Symptoms in affected patients include increased creatine kinase (CK)level, muscle soreness and fatigue or even life threatening rhabdomyolysis (RM).2Overall, 1%-10% of patients on a statin regimen display muscle related side effects among whom 0.1% will suffer from RM.1, 3
The mechanism responsible for SIM is still largely unknown and is currently under active investigation. Hypotheses include direct impacts on lipid synthesis and resulting increases in cell membrane permeability,4inhibition of mitochondria co-enzyme Q10 synthesis and impaired energy production,5and dysregulation of calcium homeostasis within muscle cells.6The latter is not only believed to mediate statins-induced side effects in muscles, but may also play a crucial role in other pathophysiological states of muscle such as malignant hyperthermia (MH). MH is an inherited pharmacogenetic disorder which triggered by halogenated inhaled anesthetics including halothane.7Typical MH symptoms include skeletal muscle rigidity, a rapid and sustained rise in body temperature, severe metabolic acidosis and other symptoms of RM such as increased CK levels and myoglobinuria.8If not properly treated, MH can rapidly lead to severe tissue damage and even death.
The caffeine-halothane contracture test (CHCT) is the gold standard of MH susceptible (MHS) diagnosis. In CHCT,MHS skeletal muscles have stronger contracture reactions to stimulation by caffeine and halothane than MH nonsusceptible (MHN) skeletal muscles.9Studies have shown the myotoxic side effects of statins could increase skeletal muscles sensitivity to caffeine and halothane stimulation. Krivosic-Horber et al10demonstrated that patients suffering from SIM with increased serum CK levels tested positive in the CHCT. Guis et al11also found that 7 of 9 patients with SIM tested positive in the CHCT. This means these patients may be at risk of developing MH when they were exposed to halogenated inhaled anesthetics. However, Evans et al12also reported that patients with atypical MH symptoms (increased CK or RM without high temperature) had taken statins irregularly before surgery and suggested that the myotoxic side effects might have increased sensitivity to inhaled anesthetics.
The increasing number of patients receiving statins during perioperative periods developing the rare, but very serious adverse effects of statins, illustrates the importance of understanding the mechanisms for such adverse effects,which until recently have been understudied. Simvastatin produces the highest incidence of SIM in humans and was therefore used at different doses to determine their effects on skeletal muscle sensitivity to caffeine and halothane.
MATERIALS AND METHODS
Experimental rats
Healthy Sprague-Dawley rats born within 24 hours (Beijing Vital River Laboratory Animal Technology Co., Ltd., China)were used for the experiments. There was no restriction on gender. The experiments were performed according to an animal use protocol that was reviewed and approved by animal use committee of Peking Union Medical College Hospital.
Chemicals and solutions
Type II collagenase, trypsin, D-Hank’s, MEM medium, fetal bovine serum (FBS), and horse serum were purchased from cell culture center of Institute of Basic Medical Sciences (Beijing, China). Type I collagen, simvastatin, halothane,and bovine serum albumin (BSA) were purchased from Sigma (St. Louis, MO, USA). Fura-2-acetoxymethyl ester (AM) and pluronic F-127 were from Biotium (Hayward, CA,USA). Caffeine was from Peking Union Medical College Hospital. Triton X-100 and dimethyl sulphoxide (DMSO)were purchased from Amresco (Solon, OH, USA).
Simvastatin was dissolved in 100% ethanol and was diluted in differentiation medium at the final concentrations of 0-10 μmol/L (0, 0.001, 0.01, 0.1, 1.0, 5.0, 10.0 μmol/L)for cell culture. The final concentration of ethanol was less than 0.3% in culture medium.13The skeletal muscle extracellular solution was prepared as following: 140 mmol/L NaCl, 5.4 mmol/L KCl, 1.4 mmol/L MgCl2, 0.4 mmol/L NaH2PO4, 1 mmol/L CaCl2, 10 mmol/L glucose, and 5 mmol/L Hepes, pH 7.2 in triple-distilled water. Potassium depolarization solution was made as: 85.4 mmol/L NaCl,60 mmol/L KCl, 1.4 mmol/L MgCl2, 0.4 mmol/L NaH2PO4,1 mmol/L CaCl2, 10 mmol/L glucose, and 5 mmol/L Hepes,pH 7.2.
Using the research method described by Wehner et al,14halothane was dissolved in DMSO on the day of experiment to produce a 2 mmol/L stock solution which was immediately diluted in extracellular solution to produce simulation solutions with corresponding concentrations before every experiment. The 20 mmol/L caffeine stock solution was produced in extracellular solution that was further diluted to prepare stimulation solutions at designated concentrations.
Extraction and culture of skeletal myotubes
Recently published methods were modified for cell culture of primary myotubes.14After removal of connective tissue and fat, the chest and thigh muscles of the neonatal rats were cut into small pieces. Myoblasts were isolated enzymatically in D-Hank’s solution supplemented with 0.17% trypsin, and 0.085% type II collagenase was added for digestion at 37°C for 45-50 minutes. Satellite cells were released by repeated pipetting of the tissue. The tissue was filtered and the filtrate was plated in an uncoated culture dish for 1 hour to reduce the number of fibroblasts.14The suspension cells were collected and resuspended with proliferation medium (MEM with 20% FBS) to a density of 1×104/200 μl per well in a 96-well plate, 1×105/1.5 ml per well in a 24-well plate, 5×105/5 ml per flask in T25 flasks. All flasks and plates were coated with 0.01% type I collagen. After most of the cells adhered and reached 50%-60% confluence, the medium was changed to differentiation medium (MEM with 10% horse serum, 2% FBS) to continue the incubation for 18-24 hours. During this time the satellite cells fused to form myotubes, which gradually increased in number over time. After 48 hours of differentiation, simvastatin was added at different concentrations when the differentiation medium was changed and the culture was continued for 24-72 hours. Mock treated cells treated with 0.3% ethanol carrier served as control.
MTT assay
These assays were performed as described by Masters et al.15The myotubes were treated with simvastatin at different concentrations for 24, 48, or 72 hours. Then culture medium was discarded by centrifugation followed by adding serum-free MEM containing 0.05% MTT. After another 4 hours of incubation, the resultant formazan crystals were dissolved in DMSO. A microplate reader was then used to measure absorbance at 570 nm. Optimal concentrations and incubation times were determined empirically and used in subsequent experiments.
Morphology and ultrastructure of myotubes
Assessments of cell morphology using light microscopy is widely used as a health status indicator of muscular fibers and myotubes.16Based on the MTT results, a 48-hour time point was selected for microscopical analysis of myotubes treated with different concentrations of simvastatin. The number of myotubes and their morphology were examined and photographed by a 40× lens on a light microscope.
The ultrastructure of myotubes was analyzed using a transmission electron microscope. Briefly, myotubes were trypsinized and collected. After centrifugation, myotubes were fixed with 2.5% glutaraldehyde for 2 hours at 4°C and then with 1% osmium tetroxide. Samples were dehydrated with alcohol, soaked in acetone, and embedded in epoxy before sectioning. Ultrathin sections of grid were analyzed for changes in subcellular structure after double staining with 2% uranyl acetate and lead citrate.
Measurement of intracellular calcium concentrations ([Ca2+]i)
A 48-hour time point was selected to assess the effect of simvastatin on intracellular calcium homeostasis. Glass slides with myotubes were washed 2-3 times with extracellular solution and then incubated in loading solution containing 5 μmol/L Fura-2-AM for 45 minutes at 37°C. Calcium imaging was performed using a fluorescence imaging set for fast photometric detection (Till Photonics,Gräfelfing, Germany) and an inverted microscope (Olympus IX71, Japan). Extracellular solution was perfused constantly at 1 ml/second and at a controlled temperature of 37°C. The fluorescence intensity was recorded at excitation wavelengths of 340 nm and 380 nm every 5 seconds using Aqua Cosmos 2.5 software. The fluorescent intensity ratio (340 nm/380 nm) was obtained and has been previously shown to change in proportion to [Ca2+]i concentrations.17
The [Ca2+]i was here represented as the florescenceratio (340 nm/380 nm). The amount of calcium released into the cytoplasm was presented as the difference between the stimulated peak florescence ratio and resting florescence ratio (∆C-florescence ratio). The maximum amount of calcium released from sarcoplasmic reticulum (SR), represented in this study as ∆Cmax-fluorescent ratio (340 nm/380 nm), can indicate calcium storage capacity.14, 18After stimulation with a high dose of caffeine or halothane,the maximum amount of calcium is released from SR.18
Myotube selection criteria for studying intracellular calcium homeostasis
A field containing a relatively large number of myotubes (about 4 to 6 per experiment) was selected. Criteria for inclusion were typical multinuclear fiber-like morphology and a rapid (>1.6 fold) increase in [Ca2+]i, induced by chemical depolarization with 60 mmol/L KCl, followed by a fast decline in [Ca2+]i. Myotubes that did not meet this requirement were excluded.18
Caffeine and halothane were applied sequentially in increasing doses with a resting period of 5 minutes before the next dose. During this time, the recording chamber was rinsed with extracellular solution.
Statistical analysis
Quantitative data were presented as mean ± standard error of mean (SEM). The comparison among quantitative data sets was conducted by one-way analysis of variance (ANOVA). Least significant difference method was used to compare homogeneity of variances, and Dunett’s T3 method was used to compare heterogeneity of variances. SPSS 13.0 statistical software (SPSS Inc., Chicago, IL, USA)was used for statistical analysis and P<0.05 was considered to be statistically significant.
RESULTS
Simvastatin reduced cell viability and altered morphology
Simvastatin reduced the viability of neonate rat myotubes in a dose and time dependent fashion. As shown in Table 1,the half maximal inhibitory concentration (IC50) of simvastatin on myotubes was 51.70, 41.7, and 28.45 μmol/L for 24, 48, and 72 hours, respectively.
Under light microscopy, myotubes in the control group grew densely in a full, thick, and long shape with smooth surface outlines (Fig. 1A). As the concentration of simvastatin was increased, the number of myotubes was gradually decreased and the morphology gradually changed to be thin and short. When the concentration of simvastatin reached 1.0 μmol/L or above, the number of myotubes was significantly decreased. In the groups where 5.0 μmol/L or above were used, the surface of myotubes was no longer smooth and membrane distortion was observed. When simvastatin concentration was increased even higher to 10.0 μmol/L, some myotubes came off the culture plate.
Electron microscopy was employed to further observe the ultrastructure of myotubes. There was an abundance of mitochondria in the myotubes of the control group with clear mitochondrial crista, and cells were filled with filament bundles. When the concentration of simvastatin was 0.01 μmol/L, there were no obvious changes in the ultrastructure of myotubes. As the concentration of simvastatin was increased, the amounts of mitochondria and filaments was gradually decreased, glycogen accumulation gradually became distinct, mitochondria crista gradually disturbed, and mitochondria gradually expanded, vacuolated with a large amount of lipid droplets gradually emerged (Fig. 1B).

Table 1. Viability of primary cultured neonate rat myotubes treated with various concentrations of simvastatin for 24, 48, and 72 hours, respectively§
Influence of simvastatin on myotube calcium homeostasis
The resting florescence ratio did not change significantly in myotubes incubated with 0.01 μmol/L simvastatin (Fig. 2A). When simvastatin concentration was further increased to 0.1, 1.0, and 5.0 μmol/L, resting florescence ratio increased by 4.20%, 5.04%, and 9.00%, respectively, which was statistically significant (all P<0.01).
After application of 60 mmol/L KCl, the peak florescence ratio in the myotubes treated with 0.01 μmol/L simvastatin increased to 11.61% more than the control (P<0.01). As doses of simvastatin were increased from 0.1 μmol/L to 5.0 μmol/L, the peak florescence ratio gradually decreased (Fig. 2B). In the myotubes treated with 0.01 μmol/L simvastatin, the ∆C-florescence ratio peaked at 20.38% more than the controls (P<0.01). The ∆C-florescence ratio decreased gradually as the doses of simvastatin were increased (Fig. 3B).
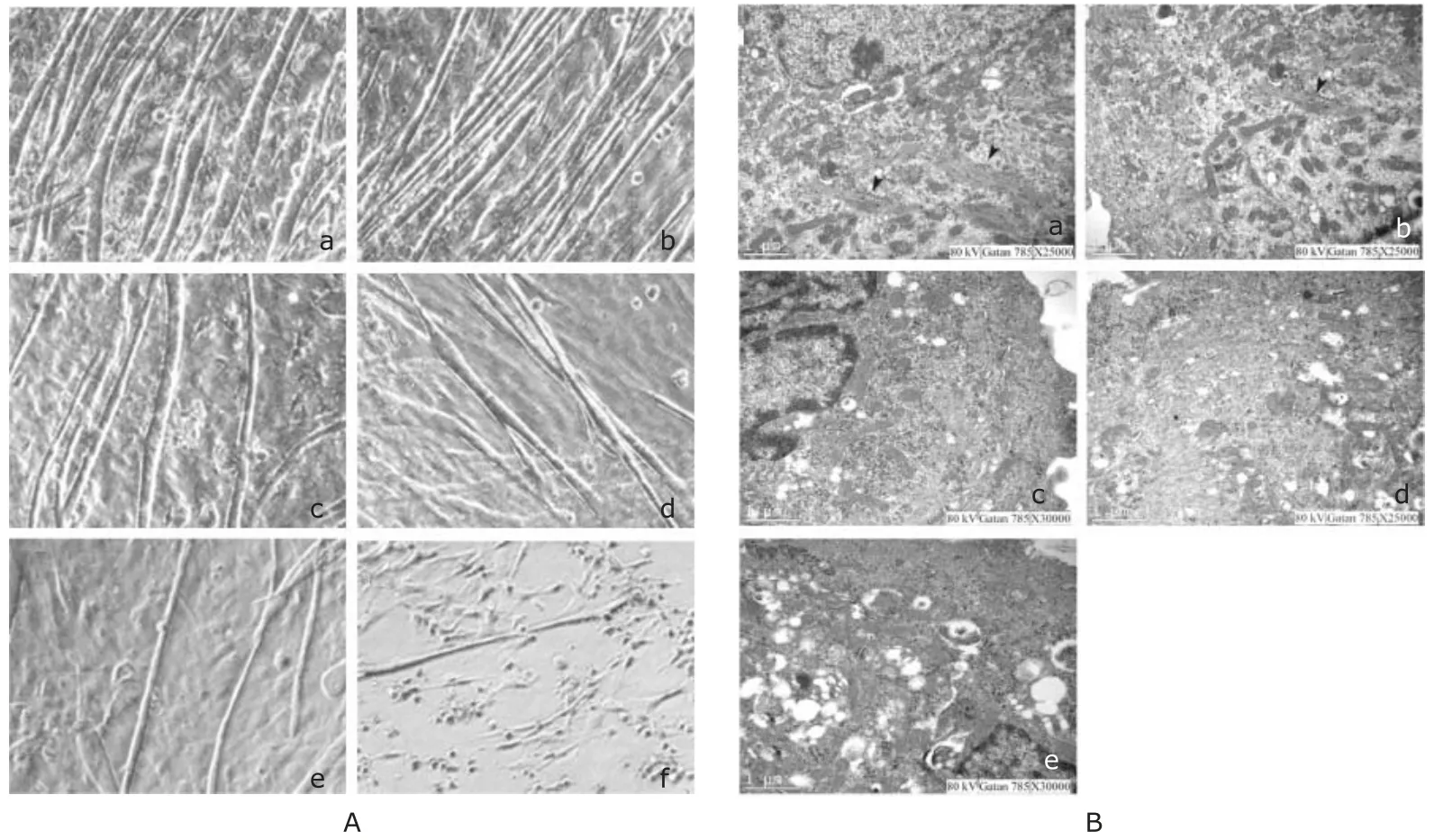
Figure 1. Simvastatin alters myotube morphology. A. Light micrograph of cultured primary neonatal rat myotubes treated for 48 hours with (A-a) 0, (A-b) 0.01, (A-c) 0.1,(A-d) 1.0, (A-e) 5.0 or (A-f) 10.0 μmol/L simvastatin. B. Electron micrograph of primary cultured neonate rat myotubes treated with (B-a) 0, (B-b) 0.01, (B-c) 0.1, (B-d) 1.0,or (B-e) 5.0 μmol/L simvastatin for 48 hours. Arrowheads indicate myosin.
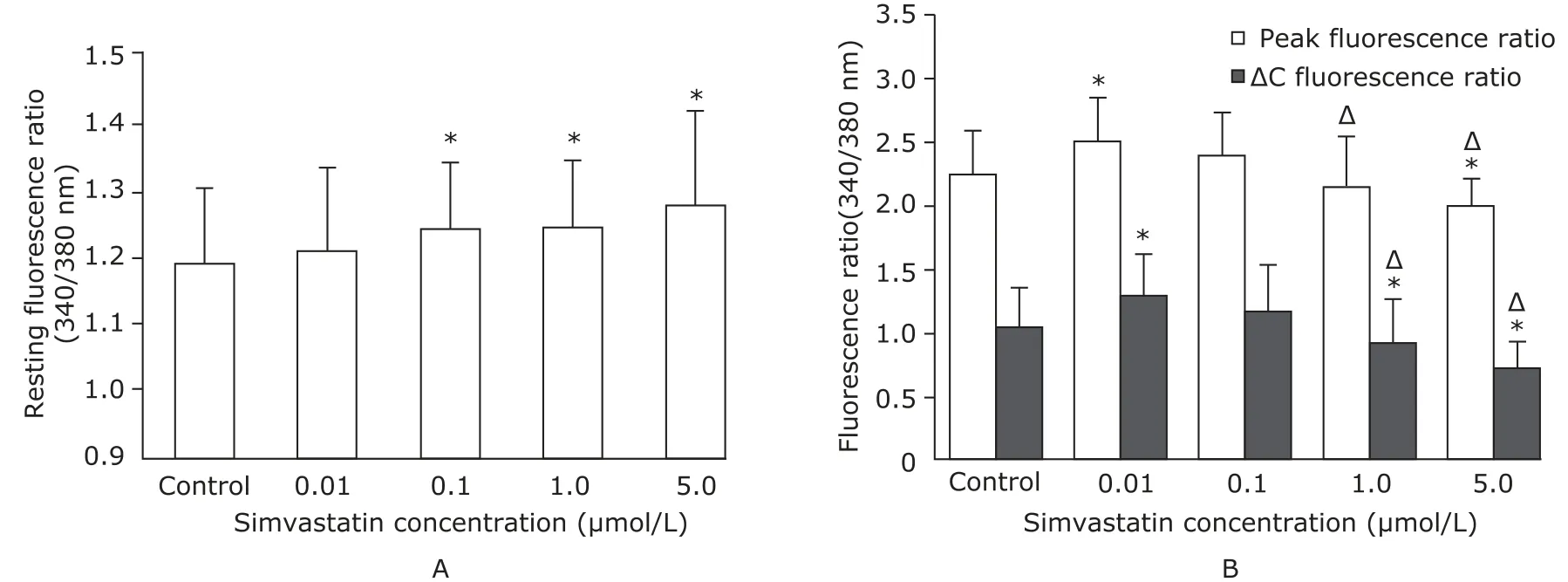
Figure 2. Simvastatin regulation of myotube intracellular calcium concentrations ([Ca2+]i). A. Resting [Ca2+]i levels were measured in the cultured primary neonatal rat myotubes after 48 hours in differentiation medium containing different concentrations of simvastatin.*P<0.01 compared with the control. B. [Ca2+]i and calcium release (ΔC) in the myotubes depolarized by 60 mmol/L KCl and cultured in different doses of simvastatin.*P<0.01 compared with the control.ΔP<0.01 compared with the myotubes treated with 0.01 μmol/L simvastatin.
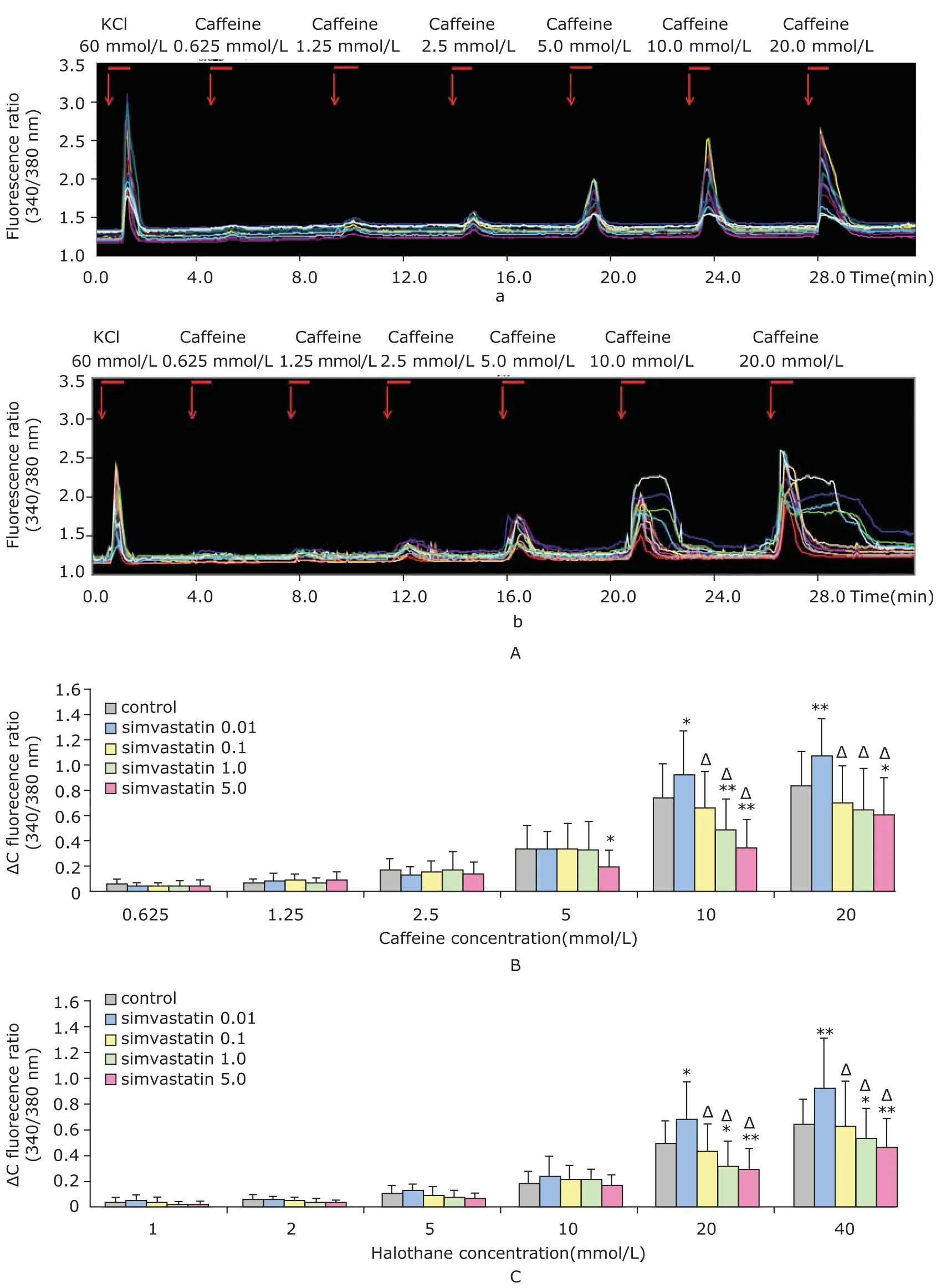
Figure 3. Influence of simvastatin on different doses of caffeine- and halothane-induced calcium release. A. Typical course of calcium release in response to caffeine induction. The effect of sequentially applied caffeine on calcium release in the myotubes treated without simvastatin (A-a) or with 5.0 μmol/L simvastatin (A-b) for 48 hours. Each line represents the averaged fluorescence ratio (340/380 nm) in one myotube. Red arrows and red bars indicate the application of caffeine. B. Effect of simvastatin on calcium release induced by increasing doses of caffeine. C. Effect of simvastatin on calcium release induced by increasing doses of halothane.*P<0.05,**P<0.01 compared with the control;ΔP<0.01 compared with the myotubes treated with 0.01 μmol/L simvastatin.
Impact of simvastatin on the response of myotubes to caffeine or halothane induction
Fig. 3 A-a represents a typical course of fluorescent ratio (340 nm/380 nm) in response to the stimulation of grading caffeine doses. Maximum calcium release was induced with 10.0 mmol/L or 20.0 mmol/L caffeine or halothane at concentrations of 20.0 mmol/L or 40.0 mmol/L. Higher concentrations of caffeine did not increase the florescence ratio, but caused myotoxicity as evidenced by morphological changes in myotubes (image not shown). As shown in Fig. 3A-b, when caffeine was used at 10.0 mmol/L or 20.0 mmol/L, the myotubes received 5.0 μmol/L simvastatin treatment showed delayed descending branches compared with that of the control group.
As shown in Fig. 3B and 3C, caffeine and halothane induced calcium release in a clear dose dependent fashion. No significant difference was observed when the stimulating concentration of caffeine was less than 5.0 mmol/L and that of halothane was no more than 10.0 mmol/L. However,0.01 μmol/L simvastatin significantly increased the peak florescence ratio when cells were stimulated with 10.0 mmol/L caffeine or 20.0 nmol/L halothane (P<0.05), although higher doses of either attenuated the induction of calcium release, a pattern similar to that observed in Fig. 2B.
Effect of simvastatin on calcium storage capacity in SR
As shown in Fig. 4, treatment with 0.01 μmol/L simvastatin increased caffeine- and halothane-induced ∆Cmaxfluorescent ratios by 28.57% and 44.60%, respectively (P<0.05). Higher doses of simvastatin, however, reduced the ∆Cmax-fluorescent ratio by 27.38% (caffeine, Fig. 4A)and 27.69% (halothane, Fig. 4B) (P<0.05), which is again consistent with patterns described in other figures.
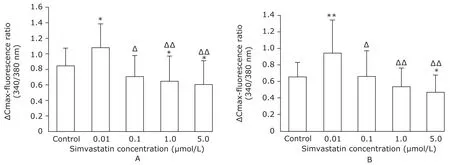
Figure 4. Effect of simvastatin on maximum increase of [Ca2+]i (ΔCmax fluorescence) in response to caffeine (A) or halothane (B).*P<0.05,**P<0.01 compared with the control;ΔP<0.05,ΔΔP<0.01 compared with the myotubes treated with 0.01 μmol/L simvastatin.
DISCUSSION
After prolonged treatment with 0.01 μmol/L simvastatin,the resting [Ca2+]i did not change significantly, and the myotubes’ SR calcium storage capacity significantly increased. Under these conditions, there were no significant changes in myotube viability, gross morphology,or microstructure. However, it is here speculated that the myotubes membrane’s structure might have been changed. This caused leakage of extracellular Ca2+into the cells along the concentration gradient. However, the sarco/ endoplasmic reticulum Ca2+-ATPase (SERCA) still functioned normally, and pumped excess intracellular Ca2+to the SR to maintain a normal level of resting [Ca2+]i. Further experiments will be needed.
As the concentration of simvastatin was increased (0.1-5.0 μmol/L), gradual changes in myotube viability and gross morphology indicated a dose-dependent myotoxic effect. The microstructure also showed fewer mitochondria and filaments, suggesting functional damage, which could affect all the intracellular energy consuming Ca2+pumps. In the following investigation of myotubes’ reaction to caffeine and halothane, SR recycling instantly elevated Ca2+, and this effect lasted longer in myotubes treated with 5.0 μmol/L simvastatin, which suggested the dysfunction of energy consuming calcium recycling in the SERCA.19, 20As a result, the myotubes resting [Ca2+]i increased and calcium storage in SR gradually decreased as the myotoxic side effects of simvastatin increased.
Caffeine and halothane have also been used to differentiate in vitro cultured MHS and MHN skeletal musclecells because MHS skeletal muscle cells have stronger calcium release reactions induced by these two drugs than MHN skeletal muscle cells.14In this study, caffeine and halothane were used to stimulate the myotubes. In myotubes treated with 0.01 μmol/L simvastatin, the sensitivity of the release of calcium to high dose caffeine and halothane increased significantly. This may have been primarily caused by the increased SR calcium storage capacity in this group of myotubes. Lamb et al21found the SR Ca2+load in rat slow-twitch fiber to be increased with much stronger sensitivity SR calcium release reaction than in fast-twitch fiber. When both fibers had the same SR Ca2+load, the sensitivity of calcium release tended to be similar.21López et al22reduced the SR Ca2+load in human MHN and MHS type ex vivo muscle fibers, and found that the sensitivity of RyR1-induced calcium release decreased in both. On the cytological level, the results of this study might explain why some patients taking statins had positive CHCT results. However, in myotubes treated with high concentrations of simvastatin (1.0 μmol/L and 5.0 μmol/L), the sensitivity of the release of calcium to high doses of caffeine and halothane was significant decreased, which may have been caused by their low SR calcium storage capacity.
Some studies indicate that the increase in resting [Ca2+]i could lead to increased sensitivity of SR towards calcium release stimuli.22, 23However, in this experiment,myotubes resting [Ca2+]i increased with the increasing myotoxic side effects of simvastatin. The sensitivity of their release of calcium conversely decreased. Liantonio et al6already observed that the phenomenon of reduced reactivity of skeletal muscles to SR calcium release stimulations,when resting [Ca2+]i increased. The researchers fed mice with 5.0 mg/kg or 20.0 mg/kg of fluvastatin daily for two months. Results showed that this could increase muscle fiber resting [Ca2+]i by 13% and 59%, respectively. However, the skeletal muscles of mice fed 20.0 mg/kg fluvastatin showed significantly less reactivity to high potassium depolarization. Liantonio et al6, 24believed this to be related to T tube structural damages caused by the high concentration of fluvastatin and to a series of protein hydrolases induced by excessive resting [Ca2+]i, which damaged the intracellular calcium channel protein. This might explain the reduced sensitivity of myotubes towards calcium release stimulations caused by large doses of simvastatin and the reduction of SR calcium storage capacity. Further study is needed.
This study also showed that different concentrations of simvastatin did not change the sensitivity of myotubes to low doses of caffeine and halothane. Nevertheless, [Ca2+]i increased in all myotubes that received stimulation. If the increase of [Ca2+]i exceeded a certain threshold, it would trigger a series of cell apoptotic processes.25It can be speculated that patients taking statins who have elevated skeletal muscle resting [Ca2+]i could be at risk of further skeletal muscle damage when exposed to halogenated inhaled anesthetics that stimulate further increases in skeletal muscle [Ca2+]i.26
The toxic effect of simvastatin could change skeletal muscle’s calcium homeostasis. For patients with SIM,exposure to halogenated anesthetics increases the risk of worsening skeletal muscle damages, though the underlying mechanism may be different.
ACKNOWLEDGEMENT
The authors would like to thank Guang-ju Ji of the Institute of Biophysics, Chinese Academy of Sciences for his technical assistance.
REFERENCES
1. Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003; 289:1681-90.
2. Toth PP, Harper CR, Jacobson TA. Clinical characterization and molecular mechanisms of statin myopathy. Expert Rev Cardiovasc Ther 2008; 6:955-69.
3. Omar MA, Wilson JP, Cox TS. Rhabdomyolysis and HMG-CoA reductase inhibitors. Ann Pharmacother 2001;35:1096-107.
4. Manzoni M, Rollini M. Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl Microbiol Biotechnol 2002; 58:555-64.
5. Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol 2007; 49:2231-7.
6. Liantonio A, Giannuzzi V, Cippone V, et al. Fluvastatin and atorvastatin affect calcium homeostasis of rat skeletal muscle fibers in vivo and in vitro by impairing the sarcoplasmic reticulum/mitochondria Ca2+-release system. J Pharmacol Exp Ther 2007; 321:626-34.
7. Mickelson JR, Louis CF. Malignant hyperthermia: excitationcontraction coupling, Ca2+release channel, and cell Ca2+regulation defects. Physiol Rev 1996; 76:537-92.
8. Larach MG, Localio AR, Allen GC, et al. A clinical grading scale to predict malignant hyperthermia susceptibility. Anesthesiology 1994; 80:771-9.
9. Hopkins PM. Malignant hyperthermia: advances in clinical management and diagnosis. Br J Anaesth 2000; 85: 118-28.
10. Krivosic-Horber R, Dépret T, Wagner JM, et al. Malignanthyperthermia susceptibility revealed by increased serum creatine kinase concentrations during statin treatment. Eur J Anaesthesiol 2004; 21:572-4.
11. Guis S, Bendahan D, Kozak-Ribbens G, et al. Rhabdomyolysis and myalgia associated with anticholesterolemic treatment as potential signs of malignant hyperthermia susceptibility. Arthritis Rheum 2003; 49:237-8.
12. Evans TJ, Parent CM, McGunigal MP. Atypical presentation of malignant hyperthermia. Anesthesiology 2002; 97:507-8.
13. Nakahara K, Yada T, Kuriyama M, et al. Cytosolic Ca2+increase and cell damage in L6 rat myoblasts by HMGCoA reductase inhibitors. Biochem Biophys Res Commun 1994; 202:1579-85.
14. Wehner M, Rueffert H, Koenig F, et al. Functional characterization of malignant hyperthermia-associated RyR1 mutations in exon 44, using the human myotube model. Neuromuscul Disord 2004; 14:429-37.
15. Masters BA, Palmoski MJ, Flint OP, et al. In vitro myotoxicity of the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, pravastatin, lovastatin, and simvastatin, using neonatal rat skeletal myocytes. Toxicol Appl Pharmacol 1995; 131:163-74.
16. Vandenburgh HH, Karlisch P, Farr L. Maintenance of highly contractile tissue-cultured avian skeletal myotubes in collagen gel. In Vitro Cell Dev Biol 1988; 24:166-74.
17. Duke AM, Hopkins PM, Steele DS. Effects of Mg(2+) and SR luminal Ca(2+) on caffeine-induced Ca(2+) release in skeletal muscle from humans susceptible to malignant hyperthermia. J Physiol 2002; 544:85-95.
18. Wehner M, Rueffert H, Koenig F,et al. Increased sensitivity to 4-chloro-m-cresol and caffeine in primary myotubes from malignant hyperthermia susceptible individuals carrying the ryanodine receptor 1 Thr2206Met (C6617T)mutation. Clin Genet 2002; 62:135-46.
19. Sirvent P, Mercier J, Vassort G, et al. Simvastatin triggers mitochondria-induced Ca2+signaling alteration in skeletal muscle. Biochem Biophys Res Commun 2005; 329: 1067-75.
20. Lamb GD, Cellini MA, Stephenson DG, et al. Toxicity of statins on rat skeletal muscle mitochondria. J Physiol 2001; 531:715-28.
21. Lamb GD, Cellini MA, Stephenson DG. Different Ca2+releasing action of caffeine and depolarisation in skeletal muscle fibres of the rat. J Physiol 2001; 531:715-28.
22. López JR, Contreras J, Linares N, et al. Hypersensitivity of malignant hyperthermia-susceptible swine skeletal muscle to caffeine is mediated by high resting myoplasmic [Ca2+]. Anesthesiology 2000; 92:1799-806.
23. Snowdowne KW. Subcontracture depolarizations increase sarcoplasmic ionized calcium in frog skeletal muscle. Am J Physiol 1985; 248:C520-C6.
24. Pierno S, Didonna MP, Cippone V, et al. Effects of chronic treatment with statins and fenofibrate on rat skeletal muscle: a biochemical, histological and electrophysiological study. Br J Pharmacol 2006; 149:909-19.
25. Itagaki M, Takaguri A, Kano S, et al. Possible mechanisms underlying statin-induced skeletal muscle toxicity in L6 fibroblasts and in rats. J Pharmacol Sci 2009; 109: 94-101.
26. Klip A, Hill M, Ramlal T. Halothane increases cytosolic Ca2+and inhibits Na+/H+exchange in L6 muscle cells. J Pharmacol Exp Ther 1990; 254:552-9.
for publication October 22, 2016.
*Corresponding author Xiang-yang Guo, Tel: 86-13581961965, Fax: 86-10-82267276, E-mail: puthmzk@163.com, Ai-lun Luo, Tel: 86-13601156861,
Fax: 86-10-69155253, E-mail: luoailun@pumch.cn
 Chinese Medical Sciences Journal2016年2期
Chinese Medical Sciences Journal2016年2期
- Chinese Medical Sciences Journal的其它文章
- Hepatocellular Carcinoma with Hypersplenic Thrombocytopenia and Situs Inversus Totalis: A Case Report
- Cytomegalovirus and Helicobacter Pylori Co-infection in an Adult with Ménétrier’s Disease: A Case Report
- Trends and Prospects of Stem Cell Research in China△
- Mechanical Strain Regulates Osteoblast Proliferation Through Ca2+-CaMK-CREB Signal Pathway△
- Effect of 4-week Whole Body Vibration on Distal Radius Density△
- Impact of Intraoperative Blood Pressure Control and Temporary Parent Artery Blocking on Prognosis in Cerebral Aneurysms Surgery
