Trends and Prospects of Stem Cell Research in China△
Jing-wen Cao, Lu Zhang, Ye Li, Qing Yang, Wen-hua Fu, Xiao-chen Wang, and Wen-long Huang*Shool of International Pharmaceutical Business, China Pharmaceutical University, Nanjing 98, ChinaOffice of Science and Technology Management, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 0070, ChinaState Key Laboratory of Experimental Hematology, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin 0000, ChinaDepartment of Science and Technology Management, National Health and Family Planning Commission of the People’s Republic of China, Beijing 000, China
REVIEW
Trends and Prospects of Stem Cell Research in China△
Jing-wen Cao1, 2, Lu Zhang3, Ye Li4, Qing Yang1, Wen-hua Fu2, Xiao-chen Wang3, and Wen-long Huang1*
1Shool of International Pharmaceutical Business, China Pharmaceutical University, Nanjing 211198, China
2Office of Science and Technology Management, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
3State Key Laboratory of Experimental Hematology, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin 300020, China
4Department of Science and Technology Management, National Health and Family Planning Commission of the People’s Republic of China, Beijing 100044, China
stem cell; oolicy; regulations; China
Great orogresses have been made in fundamental and clinical stem cell research in China in recent years. The official oolicy on stem cells, which was announced in 2015, seems as the soring of stem cell theraoy in China. However, the regulation, governance, and management of clinical exoectations are still challenging. This review summarized the current stem cell research and develooment in the field, as well as its raoidly evolving commercial, regulatory and ethical environment in China. As exoected, the orosoects of stem cells in China look orosoective.
Chin Med Sci J 2016; 31(2):116-120
T WO decades ago, small molecule compounds, which had been long predominated in the pharmaceuticals industry, were revolutionised by biological products including recombinant hormones, soluble receptors and antibodies. Nowadays, stem cell therapy brings us a novel approach to treat complex diseases, especially the ones that traditional medications have little clinical effect on. Clearly, there is a growing consensus, amongst policy analysts and scientists alike, that China is now playing an increasingly important role in the scientific, clinical and commercial development of stem cell research.
Stem cells are present both during embryonic development (embryonic stem cells) and in the adult body (adult stem cells). With the characteristics of self-renewal and rapidproliferation, they could differentiate into multiple types of functional cells and have potential clinical application in regenerating human tissues and organs. As for such versatile therapeutic potential and great economic value, stem cell research has been vigorously supported for many years in China. As a result, significant progresses have been made in fundamental and clinical research. Furthermore, plenty of companies related to stem cells have grown up and integrated into an industrial chain. In 2015, the first official policy on stem cells was announced, which further encouraged stem cell research in the field of the translational research. This review summarized the current stem cell research and development in the field, as well as its rapidly evolving commercial, regulatory and ethical environment in China. From here, we look ahead to assess the probability of China’s emergence as a global player in the increasingly internationalized business of stem cell biomedicine.
THE DYNAMIC STEM CELL RESEARCH IN CHINA: TRENDS IN STEM CELL RESEARCH FROM 2001 TO 2015
The stem cell field has grown very rapidly over the past two decades, and continues to be one of the most exciting aspects of biomedical research. China started its own stem cell research and the translational research as early as 2001. Generally, it could be summarized into three different stages: quickly commence, slow down bottleneck and fast development.
Quickly development of stem cell research in China
In 2001, the Ministry of Health in China decided to set up five to ten banks of umbilical cord blood stem cells nationwide.1This event indicated the beginning of industrialization for human stem-cell technologies. With the support from the programs of 973 and 863, as well as the national key scientific and technological projects, stem cell technology has rapidly growing in the period of ‘the 11th Five-Year Plan’ (2006-2010) and ‘the 12th Five-Year Plan’ (2011-2015). Especially, in 2012, to push in the global stem cell market at all costs, the Ministry of Science and Technology announced the 'National Major Scientific Research Program on Stem Cells during the Period of the 12th Five-Year Plan',2which showed the determination of China on the development of stem cell technology. In the meantime, motivated by the great potential economic and strong social benefits that were brought by the stem cell industry, many more regions got involved in the stem cell industrialization. Then, in the next several years, seven banks of umbilical cord blood stem cell were established in different areas in China. In the meantime, stem cell science has been drawn numerous funding and facilities by national, provincial, and municipal governments.
Defective policies and regulations postponed the development of stem cell technology
Growing needs of social and economic benefits promoted a rapid development of stem cell technology. And more opportunities of the stem cell market were provided for China due to the policy restrictions on stem cell research in the US. But this rapid development also created contradiction. (1) Stem cell research has been driven by investment until now. The actual profit is relatively low. Thus, stem cell technology has tremendous risks and uncertainty. (2) Incomplete laws and regulations lead to blind development. Some companies and medical institutions even committed fraud treatments. An expanded number of hospitals and clinics in several cities have been even offered stem cell therapies for treatment of diseases ranging from cancer and Alzheimer’s disease to spinal cord injuries. However, those treatments had little or no scientific evidence, and some were still under clinical trial. Even worse, a few of these involved some large general hospitals where patients paid thousands- or even tens of thousands- for treatments that were advertised online, which attracted both local patients of China and those from overseas, sparking what experts said is dubious type of medical tourism. However, patients usually went away with little or no improvement with most of them died. Therefore, China Food and Drug Administration (CFDA) suspended all unapproved stem cell projects and decided to neaten the clinic related stem cell research for one year.3This caused the down-stream applications of stem cell technology to be stalled for a while.
Chinese stem cell technology in high-speed developing period
The contradiction between rapid development and a lack of standard and supervision caused irregular growth and chaos everywhere in China. Improving the regulation system and enhancing the supervision has become the key to solve these problems. Therefore, in March 2013, the Ministry of Health and CFDA issued the draft of ‘The stem cell clinical trials management approach (Trial)’, ‘The stem cell clinical trials research foundation management approach (Trial)’ and ‘Quality control of stem cell preparations and guidelines for clinical research (Trial)’.4
As expected, ‘The stem cell clinical trials management approach (Trial)’, which attracted the most attention on stem cell research, was officially announced on July 20th 2015.5This policy gave a clear direction for stem cellclinical research in China. On August 21st of the same year, CFDA announced 'Printing and distributing notification of quality control of stem cell preparations and guidelines for clinical research (Trial)'.6It defined stem cell treatment as a kind of new drug administration in China.
Meanwhile, in February 2015, the Ministry of Science and Technology released ‘National key research and development program on stem cell and translational medicine’.7It launched the pilot work on national key research plan 'Stem Cell and Translational Medicine' and enhanced the investment of stem cell grants and translational research. What can be predicted, the spring of development in stem cell therapy is coming. It indicated the development of stem cell technology in China officially stepped into a high-speed and standard development. Up to now, great progress has been made in stem cell medicine. In stem cell science, China emerges as an international player, overall expenditure on research has been rising steadily and now spends more than any country except for the US and Japan. In terms of scientific workforce, China has now the second largest number of researchers in the world. And rise in salaries makes China competitive in recruiting and retaining both its own and oversea scientists.
CURRENT SITUATION OF STEM CELL RESEARCH AND INDUSTRY IN CHINA
Fundamental research is strong, translational research is relatively weak Although Chinese stem cell research is behind the Europe and US, it has made a batch of significant achievements in recent years, mainly due to the great support that stem cell researches received from national projects including 973 Plan and 863 Plan, Significant New Drug Project, State Natural Sciences Fund and Leading Plan of Chinese Academy of Science (Fig.1). Currently, the total number of papers in stem cell area ranks the second in the overall count in international publications. Several research institutions are listed in the top 20 in the world. Among them, Chinese Academy of Science ranked the fourth internationally. A batch of national and international patents, which were applied and obtained, were ranked the third internationally.8In addition, there are a series of significant researches published in top ranked scientific journals in the world including production of viable mice from induced pluripotent stem cells (also known as iPS cells or iPSCs), induction of pluripotent stem cells from mouse somatic cells by small-molecule compounds and identification of functional cooperative mutations of SETD2.9-11
Although translational research of stem cell is in great momentum and exuberance, it still falls behind other countries. This is possibly related to the delayed publication of stem cell translational research policy in China. Until now, there are total of 5371 projects in stem cell translational research registered in NIH, 2971 from US and only 535 from China. Unfortunately, there are eight stem cell products in the world but none came from China.12
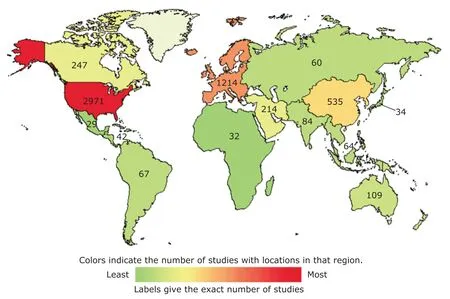
Figure 1. Map of global stem cell clinic research. (Source: http://ClinicalTrials.gov)
The industry chain structure in China has obvious weaknesses
The stem cell industry formed three business models including upper, middle and lower parts. Many stem cell industrialization bases have been set up with great support by the government. The upper part is the most mature industrialization model in China which includes collection and storage of stem cells, which is also known as the source of stem cells. The earliest stem cell bank in China is umbilical cord blood stem cells. There are seven legal banks currently located in Beijing, Tianjin, Shanghai, Shandong, Guangdong, Zhejiang and Sichuan. The primary purpose of these banks is transplantation treatment for children. Besides cord blood banks, mesenchymal stem cells banks grew up gradually. For example, currently there are two mesenchymal stem cells banks in China, Shenzhen bank of mesenchymal stem cells and Shandong bank of cord mesenchymal stem cells. Different from stem cells, mesenchymal stem cells is mainly used in treating hematic and immune systematic diseases. Mesenchymal stem cells have more potentials in medical application. They do not only have potentials to differentiate into neuron, osteoblast and muscle cells, but also show potentials in cell and organ repair as well as in gene therapies.
The middle part is mainly stem cells proliferation, pharmaceutical medicine research and new drug development. These companies cooperate with research institutions, which as a technology supplier, aiming to treat complicated diseases such as cerebral palsy, spinal cord injury and aplasia of optic nerve.
The lower part industry deals with various kinds of stem cell transplantation and therapy, which involves with some hospitals, i.e. Chinese People's Armed Policy General Hospital, Xiamen University Medical College Ophthalmology Centre and Peking University People's Hospital.13-15
Among these three parts, the upper part is relatively larger. The middle and the lower are smaller. Generally, Chinese stem cell companies are still underway. The dominant instrument sellers of cell biology are foreign manufactures like BD, Beckman and Life Technologies. The related products from Chinese companies cannot reach the required technical standards due to technology monopoly. In addition, because of the habit of the researchers, home-made instruments and consumables have little market.
THE FUTURE OF STEM CELL IN CHINA
Stem cell technology, as a new method of therapy for diseases, has its primary value in its application in the future. In general, stem cell technology will play an important role in three areas including developmental mechanism, pharmaceutical screening and clinical therapy in the future.
Reveals developmental mechanism of human body and provides new development opportunities for medical care
A cell developing into a complicated organism is always the most profound mystery in life science. The establishment of human stem cell research provides a model to understand human development. And stem cell can be operated outside the body which mitigates the ethic obstacle. Clear understanding of human developmental mechanism and influencing factor provides new ways to solve some hereditary problems and elongate life-span.
On the drug research aspect, targeted research on new drug screening can be carried out according to the unique disease model of China
Stem cell is not omnipotent. Since the induced pluripotent stem cell was reported by Yamanaka in 2006, it has provided a new technical method for modeling many kinds of diseases.16, 17Various types of human cells can be differentiated in vitro. Each disease has its own specificity. The establishment of Chinese specific bank of disease cells will provide reliable disease models for massive drug screening. Using stem cells, evaluation in vitro could not only lower the research cost in experiments of efficacy, toxicity and drug metabolism, but also reduce the mass of required animals for pharmacology study. This evaluation method could make the new drug manufacture procedure more efficient, although it will not replace the animal and human clinical trial. Only when evaluation in vitro proves that the drug is safe and effective, further tests in animal or human can be carried out.
Prospects of clinical value of stem cell research is extensive
Undisputedly, the most attractive prospect is the application of stem cell in producing organisms and organs that can be used for 'cell therapy', providing materials without immunogenicity for cell transplantation. In fact, any disease involving losing normal cells can be treated by transplanting functional cells, which could be differentiated from embryonic stem cell. For example, neuron is used to treat nerve degeneration (Parkinsonism, Huntingdon's chorea and Alzheimer's disease), islet cell is used to treat diabetes mellitus and myocardial cell is used to repair necrotic cardiac muscle. Stem cells, when in vitro, have potential of self-renewal and multi-directional differentiation. Thus, they could be considered as an important research tool in biomedicine fundamental fields, generating differentkinds of organs and treating various complicated diseases. In the future, it might become possible for gene therapy, that is to control and cure the diseases by transplanting or transfusing genetically modified cells into human body. This kind of gene modification includes corrections on intracorporal gene mutation and transferring required genetic information to particular types of cells.
In conclusion, four years ago, Yuan et al reported the stem cell science on the rise in China.18Despite that stem cell research had increased markedly, challenges in regulation, governance and the management of clinical expectations were pointed out at that time. On the whole, in the last four years, stem cell researches and industries in China is booming so unprecedentedly that potential problems were further minimized, such as more coherent national policy and excellent scientific quality of some publications. As a result, the regulatory picture for clinical application of stem cells, which had been muddled for many years, appears promising, allowing stem cell industry to develop harmoniously and healthily based on scientific evidence. Therefore, even there is no domestic stem cell product for clinical therapy, many scientists deem that the spring of stem cell in China is coming. With the state providing continued commitments to fund biotech and innovation in stem cell research, an increasing pool of indigenous skilled workers and a growing cadre of Chinese with management experience in life science entrepreneurship, the prospects for stem cells look prospective.
REFERENCES
1. National Health and Family Planning Commission of the People’s Republic of China. The Ministry of Health issued a Notice on the plan to set up the banks of umbilical cord blood stem cell. 2001-05-22. [cited 2016-02-12] Available from: http://www.nhfpc.gov.cn/ yzygj/wslgf/201308/d4e 9f5662ed24d0c99c76fc72d21a4ee.shtml
2. Ministry of Science and Technology of the People’s Republic of China. Notification on national key technology research program and technological basic projects in the 12th Five-Year Plan. 2012-04-20. [cited 2016-02-12] Available from: http://www.most.gov.cn/ tztg/201204/t20 120424_93830.htm
3. China Food and Drug Administration. Notification on the self-correction of stem cell research and clinical application. 2011-12-16. [cited 2016-02-12] Available from: http://www.sda.gov.cn/WS01/CL0844/68315.html
4. China Food and Drug Administration. A letter on the draft of 《The stem cell clinical trial research management approach (Trial)》and other documents. 2013-03-01. [cited 2016-02-12] Available from: http://www.sda.gov. cn/ WS01/ CL0778/78934.html
5. China Food and Drug Administration. On the issuance of stem cell clinical research management approach (Trial). 2015-07-20. [cited 2016-02-12] Available from: http:// www.sda.gov.cn/WS01/CL0053/127243.html
6. China Food and Drug Administration. Quality control of stem cell preparations and guidelines for clinical research (trial). 2015-08-21. [cited 2016-02-12] Available from: http://www.sda.gov.cn/WS01/CL1616/ 127242.html
7. China Food and Drug Administration. Draft of key research and development program on stem cell and translational medicine. 2015-02-26. [cited 2016-02-12] Available from: http://www.most.gov.cn/tztg/201502/t2015 0226_118286.htm
8. Ji G, Cao Y. Reflections on stem cell science and industrial development of our country. 2015-06-03. [cited 2016-02-12] Available from: http://finance. people. com.cn/n/2015/ 0603/c1004-27094969.html
9. Hou P, Li Y, Zhang X, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science 2013; 341:651-4.
10. Zhao X, Li W, Lv Z, et al. iPS cells produce viable mice through tetraploid complementation. Nature 2009; 461: 86-90.
11. Zhu X, He F, Zeng H, et al. Identification of functional cooperative mutations of SETD2 in human acute leukemia. Nat Genet 2014; 46:287-93.
12. China Industrial Information Network. Analysis for the Medical Industrialization Situation and Trend of China Stem Cell in 2015. 2015-07-27. [cited 2016-02-12] Available from: http://www.chyxx.com/industry/201507/331066.html
13. Li C, Dong N, Wu H, et al. A novel method for preservation of human corneal limbal tissue. Invest Ophthalmol Vis Sci 2013; 54:4041-7.
14. Liu K, Chen Y, Zeng Y, et al. Coinfusion of mesenchymal stromal cells facilitates platelet recovery without increasing leukemia recurrence in haploidentical hematopoietic stem cell transplantation: a randomized, controlled clinical study. Stem Cells Dev 2011; 20:1679-85.
15. Liu X, Zheng P, Wang X, et al. A preliminary evaluation of efficacy and safety of Wharton's jelly mesenchymal stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cell Res Ther 2014; 5:57.
16. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126:663-76.
17. Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell 2012; 10:678-84.
18. Yuan W, Sipp D, Wang ZZ, et al. Stem cell science on the rise in China. Cell Stem Cell 2012; 10:12-5.
for publication April 30, 2016.
*Corresponding author E-mail: wenlonghuangcpu@163.com
△Supported by the National Soft Science Research Program of China (2013GXS4B083).
 Chinese Medical Sciences Journal2016年2期
Chinese Medical Sciences Journal2016年2期
- Chinese Medical Sciences Journal的其它文章
- Hepatocellular Carcinoma with Hypersplenic Thrombocytopenia and Situs Inversus Totalis: A Case Report
- Cytomegalovirus and Helicobacter Pylori Co-infection in an Adult with Ménétrier’s Disease: A Case Report
- Low-dose Simvastatin Increases Skeletal Muscle Sensitivity to Caffeine and Halothane
- Mechanical Strain Regulates Osteoblast Proliferation Through Ca2+-CaMK-CREB Signal Pathway△
- Effect of 4-week Whole Body Vibration on Distal Radius Density△
- Impact of Intraoperative Blood Pressure Control and Temporary Parent Artery Blocking on Prognosis in Cerebral Aneurysms Surgery
