Mechanical Strain Regulates Osteoblast Proliferation Through Ca2+-CaMK-CREB Signal Pathway△
Yong Guo, Qi Lv, Xian-qiong Zou, Zhi-xiong Yan, and Yu-xian Yan, *Depantment of Bioengineering, College of Biotechnology, Guilin Medical University, Guilin, Guangxi 54004, ChinaInstitute of Medical Equipment, Academy of Military Medical Sciences, Tianjin 006, ChinaExperiment Management Center, Logistical College of People Armed Police Forces, Tianjin 006, China
Mechanical Strain Regulates Osteoblast Proliferation Through Ca2+-CaMK-CREB Signal Pathway△
Yong Guo1, 2, Qi Lv3, Xian-qiong Zou1, Zhi-xiong Yan1, and Yu-xian Yan1, 3*
1Depantment of Bioengineering, College of Biotechnology, Guilin Medical University, Guilin, Guangxi 541004, China
2Institute of Medical Equipment, Academy of Military Medical Sciences, Tianjin 300161, China
3Experiment Management Center, Logistical College of People Armed Police Forces, Tianjin 300162, China
mechanical strain; calcium; ohosoholioase C; oroliferation;cAMP resoonse element binding orotein
Objective To investigate the effects of mechanical strain on Ca2+-calmodulin deoendent kinase (CaMK)-cAMP resoonse element binding orotein (CREB) signal oathway and oroliferation of osteoblasts.
Methods Using a four-ooint bending device, MC3T3-E1 cells were exoosed to mechanical tensile strains of 2500 μs and 5000 μs at 0.5 Hz resoectively. The intracellular free Ca2+([Ca2+]i) concentration and calmodulin activity were assayed by fluorosoectroohotometry, CaMK II β, CREB, and ohosohorylated (activated) CREB (o-CREB) were assessed by Western blot, and cells oroliferation was assayed with MTT. Pretreatment with veraoamil was carried out to block Ca2+channel, and inhibitor U73122 was used to inhibit ohosoholioase C (PLC).
Results Mechanical strains of 2500 μs and 5000 μs for 1 to 10 minutes both increased [Ca2+]i level of the cells. The 2500 μs strain, a oeriodicity of 1 h/d for 3 days, activated calmodulin, elevated orotein levels of CaMK II β and o-CREB, and oromoted cells oroliferation, which were attenuated by oretreatment of veraoamil or U73122. The effects of 5000 μs strain on calmodulin, CaMK II β, o-CREB and oroliferation were contrary to 2500 μs strain.
Conclusion The mechanical strain regulates osteoblasts oroliferation through Ca2+-CaMK-CREB signal oathway via Ca2+channel and PLC/IP3transduction cascades.
Chin Med Sci J 2016; 31(2):100-106
M ECHANICAL stimulus plays an important role in regulating bone growth and adaptation,which promotes bone formation and suppresses bone resorption.1, 2Mechanical forces have been shown to activate many types of signal transduction cascades in bone tissue, from increases in intracellular adenosine triphosphate (ATP), calcium and guanine regulatory proteins to activating mitogenactivated protein kinase (MAPK) and nitric oxide signaling and so on.3, 4
Osteoblast, the bone-forming and bone-remodeling cell, is sensitive to mechanical stimulus and involved in mechanical signal transduction.5Previous studies indicated that certain mechanical strain promoted osteoblasts proliferation and metabolic activity.6-8Many signaling pathways mediate osteoblasts proliferation, such as ERK1/2 signaling,estrogen receptor signal, PI3K-Akt signaling and so on.6, 9-11
Calcium ion (Ca2+) is an important and prevalent second messenger of signal transduction, and an increase in intracellular free calcium ([Ca2+]i) concentration is the earliest cellular response detected in mechanically stimulated osteoblasts.12Activation of phospholipase C (PLC)-IP3 and Ca2+channel leads to an increase of [Ca2+]i.13The [Ca2+]i activates calmodulin (CaM) which further activates Ca2+-calmodulin dependent kinase (CaMK), then the activated CaMK results in phosphorylation (activation) of cAMP response element binding protein (CREB).14
Fluid shear stress induced phosphorylation of CREB in osteoblastic MC3T3-E1 cells15and cyclic mechanical stetch (1 Hz and 5% elongation) induced CREB phosphorylation in all CREB-positive cardiac fibroblasts.16In addition, fluid shear stress rapidly increased the intracellular [Ca2+]i concentration and nitric oxide synthesis in osteoblasts,17and mechanical tensile strain also resulted in influx of extracellular Ca2+and mobilization of intracellular Ca2+.18Mechanical stretch induced calcium to enter into cytosol from the extracellular environment, which resulted in calcium oscillations in human mesenchymal stem cells.19However, how calcium-CaMK-CREB signal pathway involved in mechanotransduction of osteoblasts is still poorly understood, and it has not been elucidated fully how mechanical stimuli affects the signal pathway.
Inasmuch as Ca2+is a prevalent second messenger and calcium-CaMK-CREB signal pathway is an important role of signal transductions, we hypothesized that calcium-CaMKCREB signal pathway involved in mechanotransduction of osteoblasts, then mechanical strain regulates osteoblasts proliferation through this signaling pathway. The purpose of this study was to verify this hypothesis by investigating the effect of mechanical tensile strain on [Ca2+]i concentration, CaM activity, protein expression levels of CaMK Ⅱ,CREB and phosphorylated-CREB (p-CREB), and proliferation of osteoblasts.
MATERIALS AND METHODS
Cell culture
A mouse pre-osteoblastic cell line, MC3T3-E1 cell provided by the School of Basic Medicine of Peking Union Medical College (Beijing, China), which has been shown to differentiate into osteoblasts and osteocytes,20, 21was cultured in alpha minimal essential medium (α-MEM, Invitrogen) containing 10% fatal bovine serum and 1% penicillin-streptomycin, at 37˚C in humidified 5% CO2atmosphere and 95% air. The pharmacological agents used to evaluate the potential role of signaling pathways in mechanical response were Ca2+channel blocker verapamil (Sigma Aldrich,20 µmol/L) and PLC inhibitor U73122 (Axon Medchem,5 µmol/L). They were added to cell culture two hours prior to mechanical strain application and remained in the culture media throughout the experiment.
Application of mechanical tensile strain to cells
Mechanical tensile strain was generated by a customized four-point bending device (provided by the Institute of Medical Equipment, Academy of Military Medical Sciences,Tianjin, China) as described previously.22MC3T3-E1 cells were seeded at the density of 2.5×104cells/cm2in the cell culture dishes and cultivated until they reached 80% confluence. The cells were subjected to mechanical strain of 2500 µs or 5000 µs at 0.5 Hz with a periodicity of 1 h/d. Unstrained (control) cultures were incubated under the same conditions for the maximum period of mechanical strain application.
[Ca2+]i content assay
The MC3T3-E1 cells were stimulated with mechanical strain of 2500 µs or 5000 µs at 0.5 Hz for 1, 3, 5, 10 minutes respectively, then the [Ca2+]i content of the cells was measured on the control and stimulated cells, as described above. After washing with PBS, the cells were scraped off with a cell scraper, centrifugated and incubated at 37°C for 30 minutes with the calcium-specific dye, Fluo-3 AM (2 ng/ml) (Molecular Probes, Inc. USA), which fluoresces only upon chelation of the dye with free Ca2+. Then the cells were re-suspended in HEPES buffered saline (HBS: 130 mmol/L NaCl, 5 mmol/L KCl, 10 mmol/L glucose, 1.0 mmol/L MgCl2, 1.0 mmol/L CaCl2, 25 mmol/L HEPES, pH 7.4) following washing with HBS. [Ca2+]i levels of the cells were detected using the fluorescence spectrophotometer (TECAN, Austria) with excitation at 488 nm and emission at530 nm. Results were expressed as relative to control.
CaM activity assay
CaM is an important signal molecular of Ca2+-CaMK-CREB signal pathway. After washing with cold PBS, the cells were fixed with cold 70% alcohol, washed with cold PBS free of Ca2+thrice, then treated with 1 mmol/L trifluoperazine and irradiated for 20 minutes with ultraviolet radiation at 250-256 nm. After washing with PBS free of Ca2+again, the cells were scraped off with a cell scraper, centrifugated and re-suspended in PBS free of Ca2+. The levels of CaM activity of the cells were assayed using the fluorescence spectrophotometer (TECAN) with excitation at 488 nm and emission at 530 nm. Results were expressed as relative to control.
Western blot analysis of CaMK II β, CREB and p-CREB
CaMK II is a broad-specificity calmodulin-dependent kinase which serves to integrate Ca2+signaling within the cell.23, 24CREB requires phosphorylation to become a biologically active transcriptional activator.25The proteins extracted from MC3T3-E1 cells with radio immunoprecipitation (RAPI)lysis medium containing protease inhibitors. For each sample, 30 μg of total protein was boiled in a reducing sample buffer and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto nitrocellulose membranes (BioTrace NT,USA). After treatment with 5% skim milk, the membranes were incubated overnight with the primary antibody (antibody of CaMK II β purchased from Anbo Biotech Co.,Ltd, 1:400; antibodies of CREB and p-CREB purchased from Cell Signal Technology Co., Ltd, 1:500) at 4˚C , then incubated with horseradish peroxidase-conjugated secondary antibody (Wuhan Boster Bioengineering Co., Ltd, 1:1000)for 30 minutes at 37˚C. The immunoreactive bands were visualized using chemiluminescence detection reagent (Beijing Tiangen Biochemical Technology Co. Ltd, Beijing,China). Densitometric measurement of the protein bands was performed using Image Quant software (GE Healthcare). The expression of glyceraldehyde3-phosphatedehydrogenase (GAPDH) was used as a loading control and the data were normalized against those of the corresponding GAPDH. Results were expressed as relative to control.
Cell proliferation assay
After seeded at a density of 2.5 × 104cells/cm2, the MC3T3-E1 cells were divided at random into groups which were then subjected to mechanical strains of 2500 µs or 5000 µs at 0.5 Hz for three days. MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] sulution(Invitrogen) was used to assay for living cells. In this assay,the MTT is reduced to formazan by intracellular NAD(P)H-oxidoreductases. The relative content of formazan product was detected using an enzyme-linked immunosorbent assay reader at 490 nm. Therefore, the absorbance (OD value) at 490 nm was regarded as relative number of living cells and relative activity of cell proliferation.26
Statistical analysis
Statistical analyses were performed using SPSS 12.0 software (Chicago, IL, USA). The data was presented as mean±standard deviation, and analyzed using one-way analysis of variance (ANOVA). A value of P<0.05 was considered statistically significance.
RESULTS
Mechanical strain increased [Ca2+]i level of MC3T3-E1 cells
As shown in Figure 1, [Ca2+]i level increased significantly. The maximum level of [Ca2+]i was detected at three minutes after 2500 µs strain initiation. One minute after 5000 µs strain initiation, the maximum level was detected.
Mechanical strain activated CaM attenuated by verapamil and U73122
After MC3T3-E1 cells were stimulated with mechanical tensile strain of 2500 µs for 3 days, the relative level of CaM activity was increased. Pretreatments of verapamil and U73122 both attenuated the increase. In contrast, 5000 µs strain lowered CaM activity level (Fig. 2). The result indicated that mechanical strain of 2500 µs activated CaM via Ca2+channel and PLC/IP3signal pathway.
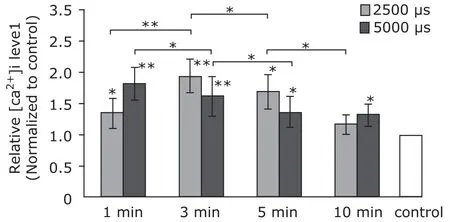
Figure 1. The intracellular free Ca2+[Ca2+]i level assay of MC3T3-E1 cells (n=6). Mechanical strains of 2500 µs and 5000 µs both increased [Ca2+]i level of the cells in short time (from 1 minute to 10 minutes). Both 3 minutes 2500 µs strain and 1 minute 5000 µs strain caused [Ca2+]i peak.*P<0.05,**P<0.01 compared with the control or between the indicated groups.
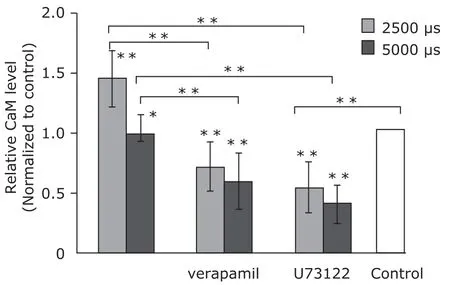
Figure 2. Calmodulin (CaM) activity assay of MC3T3-E1 cells (n=6). Mechanical strains of 2500 µs elevated CaM activity level, and the elevation was attenuated by pretreatments of verapamil and U73122. Application of 5000 µs strains caused lower CaM activity level than the control group, pretreatments of verapamil and U73122 resulted in lower CaM level in the group of 5000 µs strains.*P<0.05,**P<0.01 compared with the control or between the indicated groups.
Mechanical strain elevated protein levels of CaMK II β and p-CREB weaked by verapamil and U73122
After application of 2500 µs strain to MC3T3-E1 cells, the protein levels of CaMK II β and activated CREB (p-CREB)were elevated. Verapamil pretreatment weakened the elevation, so did U73122 (Figs. 3, 4). However, application of 5000 µs strain caused decrease of the protein levels (Figs. 3, 4). The result indicated that the mechanical strain of 2500 µs increased protein level of CaMK II β and activated CREB via Ca2+channel and PLC/IP3signal pathway.
Mechanical strain promoted proliferation attenuated by verapamil and U73122.
The proliferation assay showed that 2500 µs strain promoted proliferative activity of MC3T3-E1 cells, and the proliferation rate was reduced by pretreatments of verapamil and U73122, the effect of 5000 µs strain on cells proliferation was contrary to 2500 µs (Fig. 5).
DISCUSSION
Mechanical stimuli is a potent regulator of bone remodeling and maintenance of bone mass, and many signaling pathways involve in bone cells,responding to mechanical strain.27, 28However, the molecular events involved in mechanical signal transduction in osteoblasts are not fully understood.
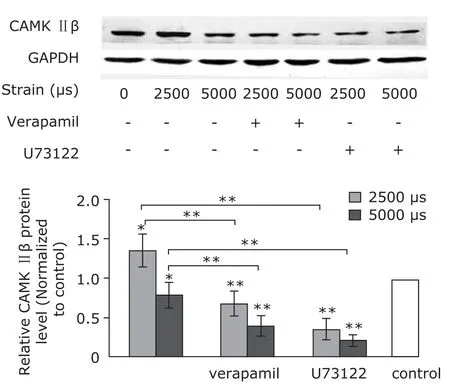
Figure 3. Western blotting analysis of Ca2+-calmodulin-dependent kinase (CaMK) II β (n=5). Mechanical strains of 2500 µs elevated protein level of CaMK II, and the elevation was attenuated by pretreatments of verapamil and U73122. Mechanical strains of 5000 µs decreased the protein levels.*P<0.05,**P<0.01 compared with the control or between the indicated groups.
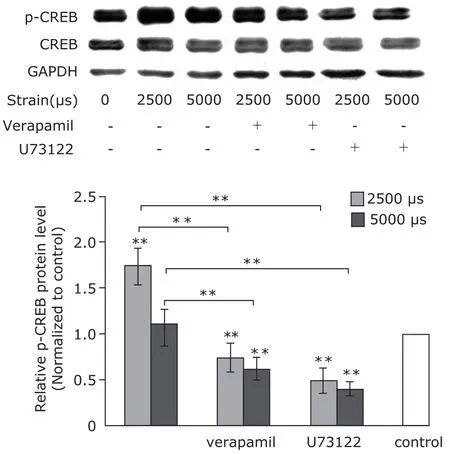
Figure 4. Western blotting analysis of cAMP response element binding protein (CREB) and phosphorylated-CREB (p-CREB) (n=5). In different groups, the protein levels of CERB were nearly the same. Mechanical strains of 2500 µs elevated protein levels of p-CERB, and the elevation was attenuated by pretreatments of verapamil and U73122. Strain of 5000 µs decreased the protein levels.*P<0.05,**P<0.01 compared with control or between the indicated groups.
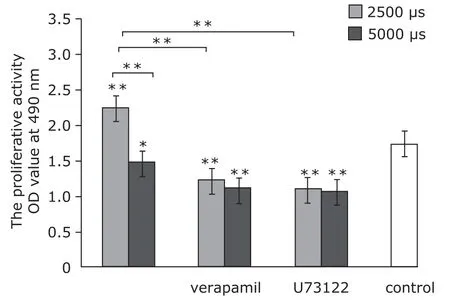
Figure 5. The proliferative activity of MC3T3-E1 cells assayed with MTT (n=6). Mechanical strains of 2500 µs promoted proliferation,which was attenuated by verapamil and U73122. Mechanical strains of 5000 µs decreased proliferation.*P<0.05,**P<0.01 compared with the control or between the indicated groups.
Ca2+is a prevalent second messenger of signal transduction in cells, and application of mechanical strain to cells results in an increase in [Ca2+]i concentration.12Activation of PLC-IP3and Ca2+channel leads to an increase of [Ca2+]i.13Activation of PLC-IP3 signal pathway mobilises intracellular Ca2+reservoir of endoplasmic reticulum.29Additionally, open of voltage-gated calcium channels leads to the extracellular Ca2+influx into the cell.30An increase of [Ca2+]i results in activation of the Ca2+-CaMK-CREB signal pathway.14Therefore, we supposed mechanical strain activated Ca2+-CaMK-CREB signal pathway in osteoblast via PLC-IP3and Ca2+channel.
PLC, which catalyzes the hydrolysis of phosphatidylinositol 4, 5-bisphosphate (PIP2) to IP3 and diacylglycerol, is a key enzyme in the regulation of Ca2+release from IP3sensitive stores.31U73122 is firmly established as the archetypal inhibitor of PLC.32Verapamil is an L-type (voltage-gated)Ca2+channel blocker.33U73122 and verapamil can inhibit [Ca2+]i elevation via different pathways. So we pretreated MC3T3-E1 cells with verapami and U73122 respectively to investigate dependence of Ca2+-CaMK-CREB signal pathway on activation of PLC-IP3signal pathway (mobilising intracellular Ca2+) and Ca2+channel (extracellular Ca2+influx).
In this study, the mechanical tensile strains of 2500 µs and 5000 µs both increased [Ca2+]i of MC3T3-E1 cells in short time, 3 minutes 2500 µs strain and 1 minute 5000 µs strain both caused [Ca2+]i peak. In vivo and in vitro studies have shown that mechanical strains in or above the 1500-3000 µs range (in physiological and overuse zone)result in cortical bone mass increased.34, 35Strain above 3000 µs range is overloaded, and it leads to bone pathological modeling or remodeling, or causes microdamage that is accumulated resulting in fracture.34, 36-38Our result indicated that overloaded strain (5000 µs) caused [Ca2+]i peak more quickly.
Our results showed that 2500 µs strain increased CaM activity, elevated protein levels of CaMK II β and p-CREB of MC3T3-E1 cells, which were attenuated by verapamil and U73122 respectively. The results demonstrated that mechanical strain of 2500 µs activated Ca2+-CaMK-CREB signal pathway, and the activation of Ca2+-CaMK-CREB signal pathway was dependent on PLC-IP3signal pathway and Ca2+channel. Additionally, 2500 µs strain promoted cells proliferation, which was also attenuated by verapamil and U73122. Considering the CREB target genes related to cell proliferation and CREB mediated cells growth and proliferation,39, 40the mechanical strain promoting proliferation is dependent on activation of Ca2+-CaMK-CREB signal pathway. In this study, although 2500 µs and 5000 µs strains both increased [Ca2+]i in short time (5 minutes),after application of them for 3 days, they had opposite effects on Ca2+-CaMK-CREB signal pathway and cells proliferation. We will go on study to investigate the mechanism.
In conclusion, mechanical strain of 2500 µs activates Ca2+-CaMK-CREB signal pathway via PLC-IP3signal pathway and Ca2+channel to mobilise intracellular and extracellular Ca2+, which results in enhancing osteoblasts proliferation.
REFERENCES
1. Schulte FA, Ruffoni D, Lambers FM, et al. Local mechanical stimuli regulate bone formation and resorption in mice at the tissue level. PLoS One 2013; 8:e62172.
2. Castillo AB, Triplett JW, Pavalko FM, et al. Estrogen receptor-β regulates mechanical signaling in primary osteoblasts. Am J Physiol Endocrinol Metab 2014; 306: E937-44.
3. Robling AG, Turner CH. Mechanical signaling for bone modeling and remodeling. Crit Rev Eukaryot Gene Expr 2009; 19:319-38.
4. Knapik DM, Perera P, Nam J, et al. Mechanosignaling in bone health, trauma and inflammation. Antioxid Redox Signal 2014; 20:970-85.
5. Orriss IR, Burnstock G, Arnett TR. Purinergic signalling and bone remodelling. Curr Opin Pharmacol 2010; 10: 322-30.
6. Yan YX, Gong YW, Guo Y, et al. Mechanical strain regulates osteoblast proliferation through integrin-mediated ERK activation. PLoS one 2012; 7:e35709.
7. Judex S, Rubin CT. Is bone formation induced byhigh-frequency mechanical signals modulated by muscle activity? J Musculoskelet Neuronal Interact 2010; 10: 3-11.
8. Ozcivici E, Luu YK, Adler B, et al. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol 2010;6:50-9.
9. Prasadam I, Friis T, Shi W, et al. Osteoarthritic cartilage chondrocytes alter subchondral bone osteoblast differentiation via MAPK signalling pathway involving ERK1/2. Bone 2010; 46:226-35.
10. Galea GL, Meakin LB, Sugiyama T, et al. Estrogen receptor α mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor β. J Biol Chem 2013;288:9035-48.
11. Katz S, Ayala V, Santillán G, et al. Activation of the PI3K/Akt signaling pathway through P2Y 2 receptors by extracellular ATP is involved in osteoblastic cell proliferation. Arch Biochem Biophys 2011; 513:144-52.
12. Danciu TE, Adam RM, Naruse K, et al. Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. Febs Lett 2003; 536:193-7.
13. Hisatsune C, Nakamura K, Kuroda Y, et al. Amplification of Ca2+signaling by diacylglycerol-mediated inositol 1,4,5-trisphosphate production. J Biol Chem 2005; 280: 11723-30.
14. Futatsugi A, Nakamura T, Yamada MK, et al. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science 2005; 309:2232-4.
15. Ogasawara A, Arakawa T, Kaneda T, et al. Fluid shear stress-induced cyclooxygenase-2 expression is mediated by C/EBP beta, cAMP-response element-binding protein,and AP-1 in osteoblastic MC3T3-E1 cells. J Biol Chem 2001; 276:7048-54.
16. Husse B, Isenberg G. Cyclic mechanical strain causes cAMP-response element binding protein activation by different pathways in cardiac fibroblasts. Heart Int 2010;5:e3.
17. Rangaswami H, Schwappacher R, Tran T, et al. Protein kinase G and focal adhesion kinase converge on Src/Akt/ beta-catenin signaling module in osteoblast mechanotransduction. J Biol Chem 2012; 287:21509-19.
18. Wang H, Sun W, Ma J, et al. Polycystin-1 mediates mechanical strain-induced osteoblastic mechanoresponses via potentiation of intracellular calcium and Akt/β-catenin pathway. PLoS One 2014; 9:e91730.
19. Kim TJ, Sun J, Lu S, et al. Prolonged mechanical stretch initiates intracellular calcium oscillations in human mesenchymal stem cells. PLoS One 2014; 9:e109378.
20. Sudo H, Kodama HA, Amagai Y, et al. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol 1983;96:191-8.
21. Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res 1992; 7:235-46.
22. Tang LL, Wang YL, Pan J, et al. The effect of step-wise increased stretching on rat calvarial osteoblast collagen production. J Biomech 2004; 37:157-61.
23. De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+oscillations. Science 1998; 279: 227-30.
24. Soltis AR, Saucerman JJ. Synergy between CaMKII substrates and β-adrenergic signaling in regulation of cardiac myocyte Ca2+handling. Biophys J 2010; 99:2038-47.
25. Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2001; 2:599-609.
26. Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev 2005; 11:127-52.
27. Liedert A, Kaspar D, Blakytny R, et al. Signal transduction pathways involved in mechanotransduction in bone cells. Biochem Biophys Res Commun 2006; 349:1-5.
28. Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem 2010; 285: 25103-8.
29. Wang JH, Cheng J, Li CR, et al. Modulation of Ca2+signals by epigallocatechin-3-gallate (EGCG) in cultured rat hippocampal neurons. Int J Mol Sci 2011; 12:742-54.
30. Craviso GL, Choe S, Chatterjee P, et al. Nanosecond electric pulses: a novel stimulus for triggering Ca2+influx into chromaffin cells via voltage-gated Ca2+channels. Cell Mol Neurobiol 2010; 30:1259-65.
31. Williams RL, Katan M. Structural views of phosphoinositidespecific phospholipase C: signalling the way ahead. Structure 1996; 4:1387-94.
32. Wilsher NE, Court WJ, Ruddle R, et al. The phosphoinositide-specific phospholipase C inhibitor U73122 (1-(6-((17beta-3-methoxyestra-1, 3, 5(10)- trien-17-yl)amino) hexyl)-1H-pyrrole-2, 5-dione) spontaneously forms conjugates with common components of cell culture medium. Drug Metab Dispos 2007; 35:1017-22.
33. Tosun M, Paul RJ, Rapoport RM. Coupling of store-operated Ca++ entry to contraction in rat aorta. J Pharmacol Exp Ther 1998; 285:759-66.
34. Frost HM. Perspectives: bone's mechanical usage windows. Bone Miner 1993; 19:257-71.
35. Ghuneim WA. In situ tooth replica custom implant: a3-dimensional finite element stress and strain analysis. J Oral Implantol 2013; 39:559-73.
36. Frost HM. Bone "mass" and the "mechanostat": a proposal. Anat Rec 1987; 219:1-9.
37. Burr DB, Milgrom C, Fyhrie D, et al. In vivo measurement of human tibial strains during vigorous activity. Bone 1996; 18:405-10.
38. Miyazaki M, McCarthy JJ, Fedele MJ, et al. Early activation of mTORC1 signalling in response to mechanical overload is independent of phosphoinositide 3-kinase/Akt signalling. J Physiol 2011; 589:1831-46.
39. Ishida M, Mitsui T, Yamakawa K, et al. Involvement of cAMP response element-binding protein in the regulation of cell proliferation and the prolactin promoter of lactotrophs in primary culture. Am J Physiol Endocrinol Metab 2007; 293:E1529-37.
40. Jiang H, Chen J, Wang L, et al. Down-regulation of CREB-binding protein expression inhibits thrombininduced proliferation of endothelial cells: possible relevance to PDGF-B. Cell Biol Int 2010; 34:1155-61.
for publication July 06, 2015.
*Corresponding author Tel: 86-773-3680651, Email: 2201642732@qq.com
△Supported by the National Natural Science Foundation of China (11432016, 31370942, 11372351), and Higher School Science Foundation of Guangxi (04020150032).
 Chinese Medical Sciences Journal2016年2期
Chinese Medical Sciences Journal2016年2期
- Chinese Medical Sciences Journal的其它文章
- Hepatocellular Carcinoma with Hypersplenic Thrombocytopenia and Situs Inversus Totalis: A Case Report
- Cytomegalovirus and Helicobacter Pylori Co-infection in an Adult with Ménétrier’s Disease: A Case Report
- Trends and Prospects of Stem Cell Research in China△
- Low-dose Simvastatin Increases Skeletal Muscle Sensitivity to Caffeine and Halothane
- Effect of 4-week Whole Body Vibration on Distal Radius Density△
- Impact of Intraoperative Blood Pressure Control and Temporary Parent Artery Blocking on Prognosis in Cerebral Aneurysms Surgery
