对乙酰氨基酚诱导急性肝衰竭小鼠动物模型的建立
明雅南 李春敏 张静怡 刘晓琳 茅益民
·论著·
对乙酰氨基酚诱导急性肝衰竭小鼠动物模型的建立
明雅南李春敏张静怡刘晓琳茅益民
200001上海交通大学医学院附属仁济医院消化内科,上海市消化疾病研究所
【摘要】目的通过腹腔注射高剂量对乙酰氨基酚(APAP)构建稳定的用于研究药物导致急性肝衰竭的动物模型。方法本研究首先进行生存率实验,取60只C57BL/6小鼠随机分为4组,每组15只,分别腹腔注射0.9%氯化钠溶液及不同剂量(300 mg/kg、500 mg/kg 、750 mg/kg)APAP后,观察不同组别72 h内小鼠的精神、活动状态和生存率。根据生存率分析结果,另选取180只C57BL/6小鼠,随机分为3组,分别腹腔注射0.9%氯化钠溶液、低剂量(300 mg/kg)和高剂量(750 mg/kg)APAP,每组分别在 0、1、3、6、12 h等时间点随机选取12只小鼠,留取血清和肝组织,验证小鼠的生化和组织学是否符合急性肝衰竭的表现。结果生存率实验结果显示,0.9%氯化钠溶液组、300 mg/kg以及500 mg/kg组72 h内无小鼠死亡;750 mg/kg组72 h死亡率为100%,推测750 mg/kg组可能为急性肝衰竭导致的死亡。生化和组织学验证实验结果发现,对照组各时间点转氨酶均无明显升高;APAP处理的两组动物模型ALT 3 h时开始升高,6 h时低剂量组ALT升高达到峰值[(6766.5±2001.27) IU/L],而高剂量组ALT于12 h达到(11707.58±1882.45) U/L(P<0.01)。从HE病理染色来看,0.9%氯化钠溶液组各时间点肝脏形态结构正常。APAP处理的两组动物模型的肝组织学,主要表现为以中央静脉为中心的肝细胞变性坏死,并随时间延长,损伤范围逐渐扩大。低剂量组坏死周围界限清楚,汇管区肝细胞结构形态正常,12 h可见坏死区周围肝细胞增生表现。高剂量组表现为典型的急性大片状坏死特点,仅汇管区残存少量变性的肝细胞,细胞快速坏死后,留下空的网状纤维支架,肝窦淤积大量红细胞,未见肝细胞增生。HAI评分结果显示,高剂量组的HAI得分(7.33±1.5)显著高于低剂量组(5.25±2.26,P<0.05)。结论C57BL/6小鼠腹腔注射高剂量APAP(750 mg/kg)后,生化和组织学改变与急性肝衰竭相似,本研究构建的动物模型对于探索APAP导致的AHF的发病及进展机制研究具有潜在的应用价值。
【关键词】对乙酰氨基酚;肝衰竭;动物模型
药物因素是临床上引起急性肝衰竭(AHF)的重要原因之一,其中,对乙酰氨基酚(APAP)已成为欧美国家AHF的最主要病因,也是导致患者接受肝移植的第二大原因[1]。但目前对药物导致AHF发病机制的认知仍非常有限,缺乏合适的动物模型是主要原因。APAP诱导的肝损伤模型稳定且重复性好,尽管尚存在剂量和时间依赖性,曾有研究报道应用过量APAP诱导AHF,但是否真正建模成功却并不明确。有研究者利用兔[2]、狗[3, 4]、猫[5]以及大鼠等进行过量APAP诱导AHF,但上述动物模型体型大,成本高,且重复性差,因此应用较局限。而且,大鼠对于过量APAP反应敏感性较差,无法较好模拟人体的情况[6, 7]。
本研究旨在通过近亲系C57BL/6小鼠腹腔注射高剂量APAP,探索既能够区别于APAP诱导的肝损伤动物模型,又能构建稳定模拟APAP导致AHF病变过程的动物模型,以期能够为APAP导致AHF的临床转化研究提供较为可靠、有效、稳定的动物模型。
材料和方法
一、 实验材料
240只C57BL/6小鼠购于上海斯莱克动物实验中心;新鲜APAP以及用于麻醉的戊巴比妥钠购于美国Sigma公司;全自动生化分析仪(SIEMEN SADVIA 1800,美国);全自动脱水机(ASP300)、石蜡包埋机(EG1150C)、脱蜡机(Auto taainer XL)等均为德国Leica公司产品。
二、生存率观察
先取小鼠60只,随机分为4组,每组15只。各组腹腔分别注射体积相近的0.9%氯化钠溶液和300 mg/kg、500 mg/kg、750 mg/kg APAP[8, 9]。观察各组小鼠72 h内的精神、活动状态和生存率。
三、 构建稳定的AHF动物模型
根据生存率实验结果,确定用于AHF造模剂量。验证实验采用0.9%氯化钠溶液以及300 mg/kg APAP作为对照组。验证实验时另外选取180只小鼠,随机分为3组,每组60只;分别腔注射体积相似的0.9%氯化钠溶液、低剂量APAP(300 mg/kg)、高剂量APAP(750 mg/kg),在0、1、3、6、12 h等时间点,每组随机抽出12只小鼠,处死、留取血浆和肝组织,验证小鼠的生化和组织学是否符合AHF的表现。
四、转氨酶检测
戊巴比妥钠麻醉小鼠,抗凝管眼球取血,立即3000×g,4 ℃离心,取上清液,稀释后,应用全自动生化仪检测ALT和AST。
五、肝组织HE染色和病理评分
处死小鼠后,打开腹腔,肉眼观察不同组别动物腹腔、肝脏。剪取动物模型同一部位肝脏,4%多聚甲醛固定48 h,修剪脱水、石蜡包埋,HE染色。HE切片分别由两位病理专家进行盲法HAI打分。
六、 统计学处理
统计分析应用SPSS 19.0,作图应用GraphPad Prime 6.01。计量资料采用均数±标准差表示,组间比较采用t检验,生存率分析采用Log-rank (Mantel-Cox)。P<0.05为差异有统计学意义。
结果
一、 生存率实验结果
72 h内0.9%氯化钠溶液组、APAP 300 mg/kg以及500 mg/kg组无小鼠死亡,750 mg/kg组小鼠全部死亡,与其他各组相比,差异有统计学意义(P=0.0082)。4组小鼠分别处理后,0.9%氯化钠溶液组、300 mg/kg组小鼠精神、活动状态正常;500 mg/kg组小鼠精神状态较以上两组差,活动度减少; 750 mg/kg组小鼠精神不振,活动量极度降低。 6 h后750 mg/kg组小鼠出现尿液变黄, 12 h可见腹腔内透明积液;500 mg/kg组小鼠偶见尿液变黄,无腹腔积液;其他两组小鼠未见尿液变黄和腹腔积液。因此,根据生存率实验,推测750 mg/kg组小鼠死亡的原因可能为AHF,750 mg/kg剂量将作为APAP诱导AHF建模的目标剂量,待后续的生化和组织学验证。
二、 生化和组织学验证实验结果
(一)血浆转氨酶变化与0.9%氯化钠溶液组相比,APAP处理后1 h,高剂量组和低剂量组均可见AST明显升高;两组ALT/AST水平随时间延长,逐渐升高。低剂量组在6 h时,ALT/AST水平达到峰值;高剂量组12 h时,转氨酶仍继续升高。低剂量组和高剂量组ALT/AST在12 h之前变化趋势相似,12 h时两组之间差异有统计学意义(P<0.01),见图1。
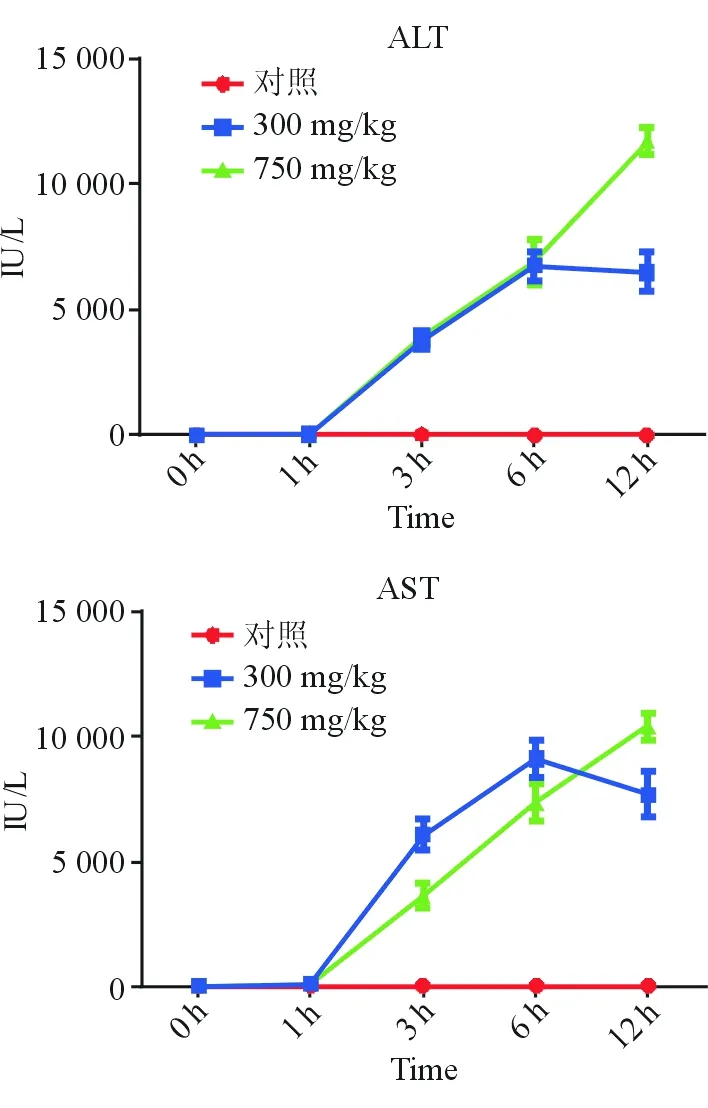
图1 各组不同时间点ALT、AST变化折线图
(二)各组动物模型的组织学改变肉眼观察,0.9%氯化钠溶液组各时间点小鼠肝脏形态正常,红色,质地软;低剂量组肝脏早期肉眼观察无明显改变,随着APAP作用时间延长,肉眼观察红色变浅,质地变硬;高剂量组肝脏早期也无显著改变,但6 h及12 h时肉眼可见显著的肝脏淤血,肝脏暗红色,质地显著变硬。
显微镜下可见0.9%氯化钠溶液组肝组织结构完整,肝板排列整齐,肝细胞形态结构正常。1 h时,低剂量组和高剂量组镜下均可见围绕中央静脉周围肝细胞微泡性改变。低剂量组随时间延长,在6 h和12 h时可见典型的3区肝细胞变性坏死,汇管区周围肝细胞形态结构正常,坏死区和正常肝细胞界限清晰,12 h时交界处可见肝细胞增生。高剂量组6 h时3区肝细胞坏死严重,肝窦扩张,红细胞淤积,坏死区边界不清晰;12 h时,高剂量组可见大片坏死,仅汇管区残存少量变性肝细胞,肝细胞快速坏死后,留下空的网状纤维支架,肝窦显著扩张,填充大量红细胞,高剂量组未见明显的肝细胞增生(见图2)。上述表现提示高剂量组的组织学表现符合急性肝衰竭时典型的急性大块性肝细胞坏死[10]。
对两组动物模型的肝损伤情况进行HAI评分,结果显示,随时间延长,HAI评分逐渐增高,12 h时AILI组和AILF组之间差异显著,高剂量组HAI评分(7.33±1.5)显著高于低剂量组(5.25±2.26,P<0.05),见表1,提示高剂量组肝损伤严重。
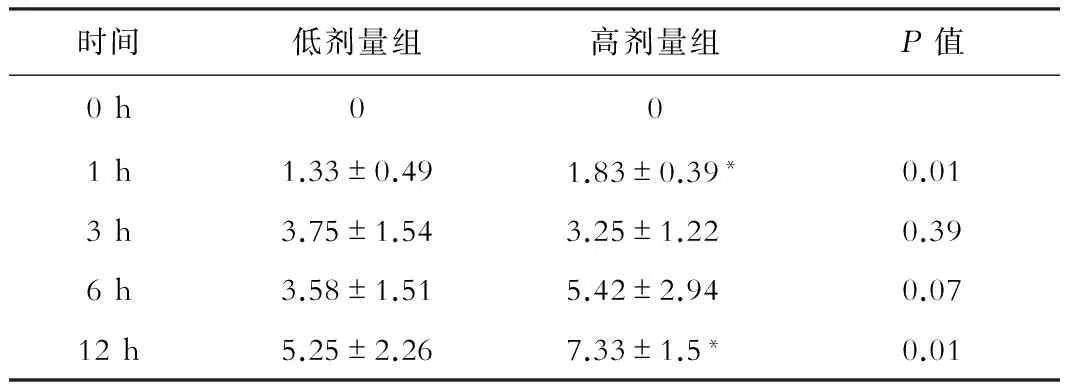
表1 低剂量组和高剂量组HAI评分
注:*P<0.05
讨论
本研究采用高剂量APAP(750 mg/kg)诱导AHF动物模型,与其他AHF模型相似:死亡率高,进展快,转氨酶明显升高,肝脏组织学可见大块和亚大块坏死;且根据生存率、生化以及组织学结果,能够与经典的DILI动物模型[10-12]相区别。
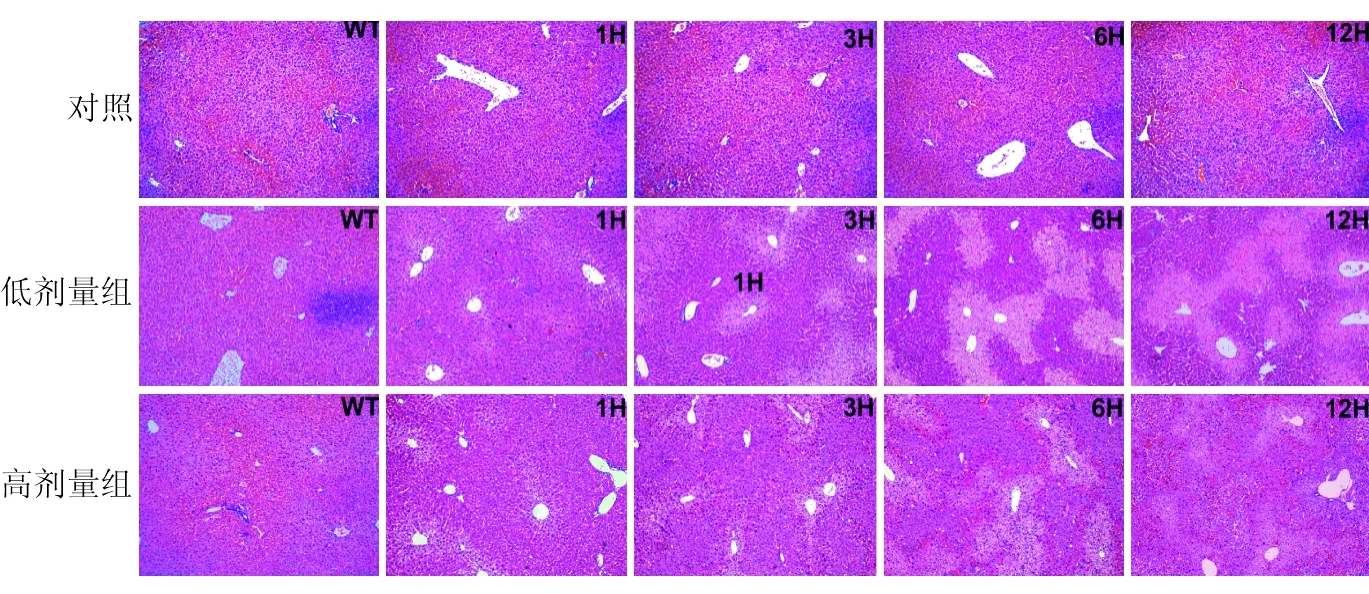
图2 低剂量组和高剂量组病理变化(HE×200)
APAP致小鼠肝损伤的主要机制是肝脏P450酶代谢APAP产生NAPQI[13],其病变过程与人体相近,因此,APAP小鼠DILI模型[14-16]成为目前应用较多的用于研究固有型DILI发病机制的动物模型[17]。而对于APAP诱导AHF的动物模型,很多研究也曾尝试利用,但研究中采用的动物多为狗、猫等体型较大的动物,其应用受到限制;除此之外,由于不同种类动物对APAP的敏感性存在差异,如过量APAP仅能导致大鼠产生轻微的肝损伤,且模型的重复性也差,因此,APAP诱导AHF尚缺乏合适的动物模型供研究。本研究采用的是近亲系的C57BL/6,组内不同动物之间的相似性高达90%,从研究的生存率、生化和组织学改变情况来看,可以成功制备APAP诱导AHF动物模型,且重复性和一致性均较好。
目前研究用于ALF动物模型有很多种,如研究肝脏再生以及评估人工肝灌注疗效的外科手术肝衰竭模型;药物诱导AHF动物模型如D-氨基半乳糖、四氯化碳、硫代乙醇胺、脂多糖以及刀豆蛋白A等,D-氨基半乳糖能够干扰RNA的代谢,脂多糖是经典的内毒素休克模型,刀豆蛋白A主要用于研究AHF的免疫反应以及自身免疫反应导致的AHF的病变过程。尽管如此,但无论从机制还是病变过程来看,与APAP导致ALF均存在较大差别[18],上述模型用于APAP导致的AHF发病机制的研究并不合适。
尽管毒性产物NAPQI引起线粒体功能损伤机制[19]、肝细胞坏死[20]是APAP导致DILI的重要发病机制,但仍有很多问题尚未完全阐明,尤其是APAP诱导的AHF。本研究构建的稳定的AHF模型,对于研究APAP导致AHF的发病机制,具有潜在的应用价值。
DILI发病机制复杂,涉及肝损伤的药物可能是单一药物,也可能是多种药物,临床表型也几乎包括所有肝损伤类型,因此DILI研究困难重重。本研究提供的动物模型只涉及APAP一种单一药物,仅适用于APAP导致AHF的研究,而对于其他药物引起,尤其是特异质型肝损伤,其应用可能有限。因此构建稳定且应用范围广的药物诱导AHF的动物模型仍面临巨大的挑战。
参考文献
[ 1 ]Larsen FS, Wendon J. Understanding paracetamol-induced liver failure. Intensive Care Med, 2014, 40:888-890.
[ 2 ]Rahman TM, Selden AC, Hodgson HJ. A novel model of acetaminophen-induced acute hepatic failure in rabbits. J Surg Res, 2002, 106:264-272.
[ 3 ]Kelly JH, Koussayer T, He DE, et al. An improved model of acetaminophen-induced fulminant hepatic failure in dogs. Hepatology, 1992, 15:329-335.
[ 4 ]Francavilla A, Makowka L, Polimeno L, et al. A dog model for acetaminophen-induced fulminant hepatic failure. Gastroenterology, 1989, 96:470-478.
[ 5 ]Rumbeiha WK, Lin YS, Oehme FW. Comparison of N-acetylcysteine and methylene blue, alone or in combination, for treatment of acetaminophen toxicosis in cats. Am J Vet Res, 1995, 56:1529-1533.
[ 6 ]Liu P, McGuire GM, Fisher MA, et al. Activation of Kupffer cells and neutrophils for reactive oxygen formation is responsible for endotoxin-enhanced liver injury after hepatic ischemia. Shock, 1995, 3:56-62.
[ 7 ]Laskin DL, Gardner CR, Price VF, et al. Modulation of macrophage functioning abrogates the acute hepatotoxicity of acetaminophen. Hepatology, 1995, 21:1045-1050.
[ 8 ]Kofman AV, Morgan G, Kirschenbaum A, et al. Dose- and time-dependent oval cell reaction in acetaminophen-induced murine liver injury. Hepatology, 2005, 41:1252-1261.
[ 9 ]Ramachandran A, McGill MR, Xie Y, et al. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology, 2013, 58:2099-2108.
[10]李继强. 急性肝衰竭的病理表现. 肝脏, 1997, 2:93-94.
[11]Numata K, Kubo M, Watanabe H, et al. Overexpression of suppressor of cytokine signaling-3 in T cells exacerbates acetaminophen-induced hepatotoxicity. J Immunol, 2007, 178:3777-3785.
[12]Yang R, Zhang S, Cotoia A, et al. High mobility group B1 impairs hepatocyte regeneration in acetaminophen hepatotoxicity. BMC Gastroenterol, 2012, 12:45.
[13]Zaher H, Buters JT, Ward JM, et al. Protection against acetaminophen toxicity in CYP1A2 and CYP2E1 double-null mice. Toxicol Appl Pharmacol, 1998, 152:193-199.
[14]Mitchell JR, Jollow DJ, Potter WZ, et al. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther, 1973, 187:211-217.
[15]Davis DC, Potter WZ, Jollow DJ, et al. Species differences in hepatic glutathione depletion, covalent binding and hepatic necrosis after acetaminophen. Life Sci, 1974, 14:2099-2109.
[16]McGill MR, Williams CD, Xie Y, et al. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol Appl Pharmacol, 2012, 264:387-394.
[17]McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res, 2013, 30:2174-2187.
[18]Saracyn M, Zdanowski R, Brytan M, et al. D-Galactosamine Intoxication in Experimental Animals: Is it Only an Experimental Model of Acute Liver Failure? Med Sci Monit, 2015, 21:1469-1477.
[19]Hanawa N, Shinohara M, Saberi B, et al. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem, 2008, 283:13565-13577.
[20]Kon K, Kim JS, Jaeschke H, et al. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology, 2004, 40:1170-1179.
(本文编辑:钱燕)
基金项目:十二五科技重大专项(2012ZX09303-001,2012ZX09401004)
通信作者:茅益民,Emai: maoym11968@163.com
Corresponding author:MAO Yi-min, Email: maoym11968@163.com
(收稿日期:2016-04-13)
Establishment of acetaminophen-induced acute hepatic failure model in mice
MINGYa-nan,LIChun-min,ZHANGJing-yi,LIUXiao-lin,MAOYi-min.
DivisionofGastroenterologyandHepatology.RenjiHospital.SchoolofMedicine,ShanghaiJiaoTongUniversity,ShanghaiInstituteofDigestiveDisease,Shanghai200001,China
【Abstract】ObjectiveTo establish a stable animal model of drug-induced acute hepatic failure (AHF) with different doses of acetaminophen (APAP) by intraperitoneal injection. MethodsSixty mice, which were randomly divided into four groups (n=15), were intraperitoneally injected with saline and different doses of APAP (300 mg/kg, 500 mg/kg and 750 mg/kg), respectively. Mental status, activity and survival rates in different groups were observed within 72 hours. According to the analysis of survival rates, another 180 mice were divided into three groups randomly (n=60) with injection of saline, low (300 mg/kg) and high dose (750 mg/kg) of APAP, respectively. To detect the biochemical and pathological changes of AHF, 12 mice randomly selected from each group were sacrificed for serum and liver tissues collection at 0 h, 1 h, 3 h, 6 h and 12 h after injection, respectively. ResultsNo mice died within 72 h in the control group, APAP (300 mg/kg and 500 mg/kg group) , while the mortality of APAP 750 mg/kg group was 100%. In control group, aminotransferase (ALT) level showed no significant increase at all time points. However, ALT levels in two APAP groups (300 mg/kg and 750 mg/kg) began to increase at 3 h, and reached to peak at 6 h (6766.5±2001.27 IU/L) or 12 h (11707.58±1882.45 IU/L) in low-dose or high-dose APAP group, respectively. Additionally, ALT level in high-dose APAP group was significantly higher than that in low-dose APAP group at 12 h (P<0.01). In view of haematoxylin-eosin (HE) staining, control group displayed normal liver structure. In APAP group, degeneration and necrosis of hepatocytes mainly occurred around central vein, and damage extent gradually expanded over time. In low-dose group, boundaries of necrotic zones were clear with normal liver cell morphology in portal areas, and visible hepatocytes proliferation around the boundaries was observed at 12 h. In high-dose group, typical acute massive hepatic necrosis was found and few of degenerated hepatocytes stayed alive at portal areas. After rapid necrosis of hepatocytes, empty fiber mesh stent remained with large red blood cells deposited in sinusoids and no proliferation of hepatocytes. At 12 h, histological activity index (HAI) score of high-dose group (7.33±1.5) was higher than that of low-dose group (5.25±2.26), which showed statistically significant differences (P<0.05). ConclusionC57BL/6 mice injected with high dose of APAP (750 mg/kg) have similar biochemical and pathological changes with AHF, which might be a reliable AHF model for investigating the role of APAP in pathogenesis and development of liver failure.
【Key words】Acetaminophen; Liver failure; Animal model

