Adsorption equilibrium for Z-ligustilide on C18-bonded silica from supercritical carbon dioxide
Huisheng Lü,Shuangyan Liu,Yonghui Li*,Fanmei Meng,Dawei Yao,Xufei Mo
Key Laboratory for Green Chemical Technology of Ministry of Education,Tianjin University R&D Center for Petrochemical Technology,Tianjin 300072,China
1.Introduction
Z-ligustilide is the main active ingredient in angelica oil,which is a kind of herbal medicine obtained from Radix Angelica[1–3].Z-ligustilide has exhibited various biological activities such as improving cardiovascular system,circulatory system and enhancing immune function[4,5].Also Z-ligustilide is regarded as an active ingredient for the quality evaluation of Angelica and other related herbs,such as the rhizome ofLigusticum chuanxiongand the root of Lovage[6].However,the established separation and purification techniques of Z-ligustilide such as column chromatography and thin layer chromatography technology,suffer from the disadvantages of the complex procedure,low recovery and massive solvent residue[7–9].Therefore,innovative separation technology is urged to provide purified Z-ligustilide economically and technologically.In recent years,supercritical fluid chromatographic(SFC)using supercritical dioxide(SC-CO2)as the mobile phase gains more and more attention in refining processes because of its great economic and ecological advantages.This technique has not only been applied for analytical separations,but has also been used at a preparative scale in a variety of industries,such as pharmaceuticals,foods,cosmetics,agrochemicals,petrochemical and natural product[10].The inherent speed,efficiency,and versatility of SFC have transformed the perceptions of the technology from novelty to integral tool for the modern labs,especially for those expecting to maximize throughput[11].Based on the advantages and wide applications of SFC,it is thought to be an attractive method for separation of Z-ligustilide from the angelica oil.
Adsorption isotherms and related mathematical models are necessary for the optimization and engineering design of the SFC separation.Especially in the simulated moving bed-supercritical fluid chromatography(SMB-SFC)plant,which combines simulated moving bed(SMB)and SFC in an apparatus with their unique features,the adsorption isotherms measured are needed for separations at higher feed concentrations.In spite of the increasing importance and necessity,the knowledge of adsorption behavior under high pressure is still scarce[12].As a problem of physical chemistry,adsorption of solute from supercritical fluids is of a unique nature in comparison with the adsorption from gas or common liquid solution,so it is hard to theoretically predict adsorption isotherms because of the complex nature of adsorbent–adsorbate interactions.Up to now,the adsorption of only a few solutes from SC-CO2has been reported in publications,such as EPA-EE,DHA-EE[13],toluene[14,15],tocopherol[16,17]and so on.Therefore,it is significant to measure the thermodynamic data and determine adsorption isotherm of Z-ligustilide for the operation and development of the SFC adsorption processes.
In this study,an enrichment method of Z-ligustilide was developed based on the preparative SFC(pre-SFC)for the firsttime.In ourprevious work[18],we studied the separation of Z-ligustilide from the angelica oil by pre-SFC,and found out the optimum operation conditions.As a continuation of our previous study,the present work is intended to determine the adsorption isotherms of Z-ligustilide from SC-CO2on C18-bonded silica.The Span and Wagner[19]method is used to predict the density of SC-CO2at different temperatures and pressures.The method of elution by characteristic points(ECP)is adopted to determine the adsorption isotherm of Z-ligustilide.The data were fitted into Langmuir and Freundlich isotherm models.Data from this work can be applied for the engineering design and the optimization of separation of Z-ligustilide with pre-SFC.
2.Experimental
2.1.Materials and experimental set-up
Carbon dioxide with purity higher than 99.9%in mass fraction was obtained from the Tianjin Liufang Ind.(Tianjin,China).Z-ligustilide standard was purchased from the Tianjin Marco Co.(Tianjin,China).Ethanol(analysis grade)was provided by the Tianjin Jiangtian Chemical Technology Co.ZorBax SB-C18 column(9.4 mm × 250 mm i.d,5 μm)was purchased from Agilent,USA.SFC-200(Thar Company,USA)was used for the measurements of adsorption isotherms under the specified experimental conditions(see Figs.1 and 2).

Fig.1.Experimental apparatus of SFC.

Fig.2.Experimental flow sheet of SFC.1—CO2 cylinder,2—stop valve,3—CO2 condenser,4— flow meter,5—CO2 pump,6—modifier,7—modifier pump,8—preheater,9—sample,10—sample pump,11—injection loop,12—heat exchanger,13—column,14—autopressure regulator,15—pressure regulator,16—collection CS1,CS2,CS3,CS4.
2.2.Theoretical fundamentals
2.2.1.Single-component isotherms by ECP method
For SFC,Martinet al.analyzed the adsorption isotherm data generated by the different methods and validated by comparing computer simulated elution profiles to find that the methods based on elution profiles,i.e.,elution by characteristic points(ECP),the inverse method(IM)and the retention time method(RTM),were able to accurately predict overloaded experimental elution profiles while the perturbation peak(PP)method,based on generating data from concentration plateaus,was not able to do so in these SFC experiments[20].
ECP method can be used for deriving single-component isotherms from the overloaded elution profile.This method is based on a simple equation using the rear diffusive part of an overloaded elution band.When a large amount of sample is injected into a chromatographic column packed with adsorbent,an unsymmetrical band with a steep front and a diffuse rear profile or a steep rear and a diffuse front profile is obtained by elution[10].The method uses the ideal model of chromatography which is equivalent to assuming that the column efficiency is infinite and that the competitive effect of all other components can be neglected.For a Langmuir model with a homogenous surface the number of theoretical plates should be at least 2000 to reduce the error to less than 3%and at least 5000 to reduce the error to less than 5%for a heterogeneous surface described by the bi-Langmuir model[21–23].
Assuming that the column efficiency is infinite and the instant adsorption equilibrium is reached between the adsorption phase and the mobile phase,the adsorption capacityqat the concentrationccan be expressed by the equation[22]:

In the equation,Vais the volume of adsorbentin the column,V is the retention volume of the characteristic point of the diffuse profile at concentrationc,andV0is the hold-up volume.
2.2.2.Adsorption isotherms
Adsorption is a process in which molecules from the mobile phase attach themselves on the surface of the stationary phase.During the process,adsorption equilibrium can be reached and the adsorption isotherm is always applied to describe this process[24].The most common theoretical models for modeling adsorption equilibrium data are the Langmuir and Freundlich isotherm models because of the relative simplicity and reasonable accuracy[25,26].Langmuir equation can be used to describe a monolayer adsorption,whereas the Freundlich equation can be used to describe a monolayer adsorption as well as a multilayer adsorption[27,28].
The Langmuir isotherm is based on a kinetic approach of sorption,assuming that all sorption sites are equivalent from an energetic standpoint.It is a two-parameter model,in whichqmrepresents the maximum adsorbed limit corresponding to a complete monolayer saturation,andKLis the affinity(or Langmuir)constant, figuring the attraction between the surface and the solute molecules.Langmuir equation is as follows:

The Freundlich isotherm is one of the first empirical equations used for correlating experimental data.In the model KFis the Freundlich constant that indicates the adsorption capacity,andnis an empirical constant related to the magnitude of the adsorption driving force.Freundlich equation is as follows:


Table 1SC-CO2 density at different temperatures and pressures
2.3.Experimental procedure
2.3.1.Calculation of the number of theoretical plates
According to the plate theory[23,29],a higher column efficiency is required while ECP method was used to determine adsorption isotherm,as compared to the Langmuir case whereN=2000(less than 3%error)is a minimum andN=5000(less than 2%error)is recommended with a good accuracy.The efficiency of the column should be high enough to meet the requirement of the accuracy for using the ECP method to determine the adsorption isotherms.Therefore,the number of theoretical plates under experimental conditions must be measured to ensure the accuracy of ECP method for the determination of adsorption isotherms for Z-ligustilide.The experiments were conducted at 313.15 K with different SC-CO2densities at 0.687 g·cm-3,0.750 g·cm-3,0.792 g·cm-3,0.816 g·cm-3.0.01 ml of Z-ligustilide ethanol solution with a concentration of 2.54 mg·ml-1was injected into the chromatographic system.According to the elution profiles,the calculation of the theoretical plate number was done by2.3.2.Calibration of c vs.S

The relationship ofS-t(S:the detector signal,t:the retention time)was required to be converted to that ofc-t(c:the mobile phase concentration,t:the retention time)before data processing.The calibration ofS-cwas carried out by the static method described in Tan's work[14].It was found that the detector signal(S)was linear with respect to the mobile phase concentration(c),i.e.,S=k×cin the range of the experimental concentration.The value ofkdepended on the temperature and density of the mobile phase.However,the value ofkcould be treated as a keeping constant when temperature and density of mobile phase changed in a small range,and fitted by the method of least squares.
Thus,at 313.15 K,14 MPa and 10 g·min-1of CO2flow rate,k was measured by injecting different concentrations(2.54 mg·ml-1,1.83 mg·ml-1,0.98 mg·ml-1)and different volumes(10 μl,20 μl,30 μl)of Z-ligustilide ethanol solution to get a series of elution profile.The wavelength of UV detection was set to 275 nm.As a result,k=243 was calculated with an R2of 0.9990,and thus the relationship ofS=243×ccould be obtained.
2.3.3.Measurement of adsorption capacity at different conditions
Experiments were carried out at temperatures of 305.15 K,313.15 K and 323.15 K with different outlet column pressures varying from 12 MPa to 18 MPa.A fixed flow rate of CO2was set to 10 g·min-1.0.01 ml of Z-ligustilide ethanol solution with a concentration of 2.54 mg·ml-1was injected into the chromatographic system.Based on the elution profiles adsorption capacityqwas then calculated according to Eq.(1).In this paper,the Span and Wagner method was adopted to calculate the density of SC-CO2mobile phase.The effect of temperature and density of the mobile phase on the adsorption capacity was investigated.Then,the adsorption equilibrium data were fitted by Langmuir and Freundlich isotherm models,respectively.
3.Results and Discussion
3.1.Adsorption tests by ECP method
Adsorption is a process that is related to the temperature and density of the mobile phase.Before data processing the density of the mobile phase was calculated by the Span and Wagner equation of state[19].The density of SC-CO2at different pressures and temperatures is summarized in Table 1.
Fig.3 shows the typical diffuse profiles of Z-ligustilide of the chromatogram at 313.15 K at four different densities.It can be seen from the figures that the front of the Z-ligustilide elution peaks is almost vertical to the abscissa,which is a typical result of a favorable adsorption isotherm under the instant equilibrium condition.According to Eq.(4),the number of the theoretical stages of the column was calculated,indicating that the theoretical stages' number for all experiments was higher than 3000,partly higher than 5000.The efficiency of the column was high enough to meet the requirement of the accuracy for using the ECP method to determine the adsorption isotherms of Z-ligustilide from SC-CO2.
By analyzing the chromatogram,the equilibrium adsorption capacityqat different densities of the mobile phase and the temperatures were calculated according to Eq.(1).All the experiments were repeated for three times,and the average value was taken with the error in 3%.Fig.4 illustrates the adsorption data with different densities at fixed temperatures.The figures showed that the adsorption amount of Z-ligustilide declines when the density of the mobile phase increases.With the density of SC-CO2increasing,there are more CO2molecules surrounding the molecules of Z-ligustilide.The distances between the molecules of CO2and Z-ligustilide are shortened,leading to the enhanced solubility of Z-ligustilide in SC-CO2.Therefore,the eluting power of the mobile phase is intensified,so the adsorption capacityqof Z-ligustilide decreases significantly with the increasing density.
Fig.5 demonstrates that the adsorption capacityqof Z-ligustilide decreases with the increasing temperature at similar densities(0.816 g·cm-3and 0.818 g·cm-3)of the mobile phase,which could be viewed as constant when studying the effect of temperature on adsorption capacity at 313.15 K and 305.15 K,respectively.It suggests that the adsorption of Z-ligustilide onto C18-bonded silica has an exothermic nature,which also conformed to the basic principle of thermodynamics that low temperature facilitates adsorption[10].In the process of Z-ligustilide adsorbed on the C18 column,there exist several kinds of chemical bonding between Z-ligustilide and C18 silica stationary phase such as the electron donor–acceptor interaction and the hydrogen bond interaction.When the temperature increases,the molecular motion is enhanced,leading to the decrease in the interaction of molecular groups.So the decline in adsorption capacity at constant density with increasing temperature is attributed to the weakening interaction of Z-ligustilide with the adsorbent at a higher temperature.Moreover,the rising temperature accelerates thermal motion of Z-ligustilide and CO2molecules,causing the enhanced eluting power of the mobile phase,which also lead to the decrease of the adsorption capacity of Z-ligustilide with increasing temperature at constant density of the mobile phase.

Fig.4.Adsorption data at temperatures of(a)305.15 K,(b)313.15 K,(c)323.15 K.
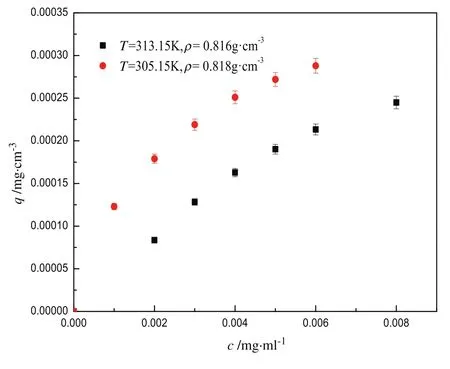
Fig.5.Adsorption data of Z-ligustilide at similar densities.
Both Figs.4 and 5 indicate that the adsorption capacityqincreases with the increasing concentration of Z-ligustilide.The increase is more at low concentration than at high one,which might be related to the fact that there are more available adsorption sites.The results obtained here can be used to control the dosage of Z-ligustilide on the carrier.
3.2.Modeling of the adsorption data
The adsorption data at 305.15 K,313.15 K and 323.15 K with different densities of SC-CO2are correlated by Langmuir and Freundlich isotherm models(Eqs.(2)and(3)),respectively.The adsorption isotherms are shown in Fig.6.The parameters of the fitted isotherm equation are summarized in Tables 2 and 3.The calculated correlation coefficient of both equations was higher than 0.996,which implied that both models could well illustrate the adsorption behavior.
From the parameters of Table 2,the range of monolayer adsorption capacity is from 3.0 × 10-4mg·cm-3to 5.5× 10-4mg·cm-3,the calculated averageqmis 4.0×10-4mg·cm-3,with a standard deviation of 0.70 × 10-4mg·cm-3.The theoretical maximum adsorption capacityqmof Z-ligustilide determined from the Langmuir equation is 5.5 × 10-4mg·cm-3under the condition of 323.15 K and 18 MPa.The parameterKL,an indicator of the stability of the combination between adsorbate and adsorbent surfaces,decreases when the temperature or the density of the mobile phase increased,which is consistent with the trend of adsorption capacityqin Figs.4 and 5.
In Freundlich equation,the adsorption takesplace easily when the 1/n value is between 0.1 and 0.5,and it does not take place easily if 1/nvalue is above 2.0[30].In Table 3,the 1/nvalue was between 0.41 and 0.59,which indicated that C18-bonded silica is favorable for the separation of Z-ligustilide.
治疗3、6、12个月恩替卡韦组HBeAg转阴率均明显高于阿德福韦酯组,差异有统计学意义(P<0.05)。见表3。
While in terms of the calculated correlation coefficients R2,the experimental data are in better agreement with the Langmuir isotherm model,in which the correlation coefficients are higher than 0.9992 under all conditions.The Langmuir isotherm can reasonably explain the adsorption process,suggesting the monolayer coverage of Z-ligustilide onto C18-bonded silica.

Fig.6.Experimental data and fitted by Langmuir isotherms of(a)305.15 K,(b)313.15 K,(c)323.15 K;and Freundlich isotherms of(d)305.15 K,(e)313.15 K,(f)323.15 K at different densities.

Table 2Parameters of Langmuir model at different pressures and temperatures
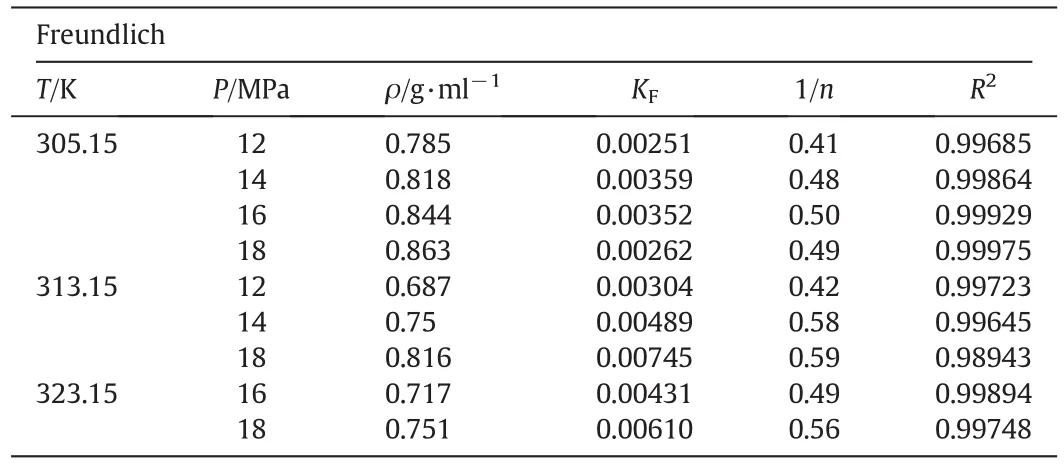
Table 3Parameters of Freundlich model at different pressures and temperatures
4.Conclusions
The adsorption of Z-ligustilide on the C18-bonded silica from supercritical CO2was studied experimentally.Adsorption capacityqdecreased with the increasing density of the mobile phase,which was related to the enhanced eluting power of the mobile phase.In addition,adsorption capacityqalso decreased with the increasing temperature,which conformed to the basic principle of thermodynamics that low temperature was favorable for adsorption.The equilibrium data were fitted by the Langmuir and Freundlich isotherm models.The Freundlich isotherm indicated that C18-bonded silica is favorable for the separation of Z-ligustilide.The Langmuir isotherm performed better for describing the whole adsorption process on the column,indicating that the adsorption of Z-ligustilide belonged to the monolayer adsorption.The monolayer saturation adsorption capacity of Z-ligustilide is in the range of 3.0 × 10-4mg·cm-3to 5.5×10-4mg·cm-3with an average value of 4.0 ×10-4mg·cm-3.The adsorption isotherms can be used to control the dosage of Z-ligustilide on the carrier.The obtained model can be applied for the simulation of chromatographic processes to ease the choices of suitable process parameter as well for engineering design and optimization of recovery and purification of Z-ligustilide with pre-SFC.
Nomenclature
c the sample concentration,mg·ml-1
KFthe numerical coefficient
KLthe numerical coefficient
Nthe number of theoretical plates
nthe numerical coefficient
Q the flow rate of CO2flow rate,ml·min-1
q(c) the equilibrium capacity when the fluid concentration isc,mg·cm-3
qmthe monolayer saturation adsorption capacity of the adsorbent,mg·cm-3
Sthe detector signal
tRthe retention time,s
Vthe retention volume of the characteristic point of diffuse profile at concentrationc,cm3
V0the hold-up volume of the column,cm3
Vathe volume of adsorbent in the column,cm3
Y1/2the peak width at half-height,s
[1]M.Kobayashi,M.Fujita,H.Mitsuhashi,Studies on the constituents of Umbelliferae plants.XV.Constituents ofCnidium officinale:Occurrence of pregnenolone,coniferylferulate and hydroxyphthalides(organic,chemical),Chem.Pharm.Bull.35(4)(1987)1427–1433.
[2]M.J.M.Gijbels,J.J.C.Scheffer,A.B.Svendsen,Analysis of phthalides from umbelliferae by combined liquid–solid and gas–liquid chromatography,Chromatographia14(8)(1981)452–454.
[3]Z.B.Feng,Y.P.Lu,X.M.Wu,P.Zhao,J.J.Li,B.Peng,Z.J.Qian,L.Zhu,Ligustilide alleviates brain damage and improves cognitive function in rats of chronic cerebral hypoperfusion,J.Ethnopharmacol.144(2)(2012)313–321.
[4]X.Kuang,Y.Yao,J.R.Du,Y.X.Liu,C.Y.Wang,Z.M.Qian,Neuroprotective role of Z-ligustilide against forebrain ischemic injury in ICR mice,Brain Res.1102(1)(2006)145–153.
[5]H.Y.Peng,J.R.Du,G.Y.Zhang,X.Kuang,Y.X.Liu,Z.M.Qian,C.Y.Wang,Neuroprotective effect of Z-ligustilide against permanent focal ischemic damage in rats,Biol.Pharm.Bull.30(2)(2007)309–312.
[6]A.Kemzūraitė,P.R.Venskutonis,R.Baranauskienė,D.Navikienė,Optimization of supercritical CO2extraction of different anatomical parts of lovage(Levisticum officinaleKoch.)using response surface methodology and evaluation of extracts composition,J.Supercrit.Fluids87(1)(2014)93–103.
[7]H.Bohrmann,E.Stahl,H.Mitsuhashi,Studies of the constituents of Umbelliferae plants.XIII.Chromatographic studies on the constituents ofCnidium officinaleMAKINO,Chem.Pharm.Bull.15(10)(1967)1606–1608.
[8]Y.Luo,J.Pan,K.Ding,Anticonvulsive constituents in the essential oil of Chaxiong(Ligusticum sinenseOliv cv.Chaxiong),Chin.Tradit.Herb.Drugs8(1996)456–457.
[9]M.Qian,L.Shi,L.Gao,J.Hu,Isolation of ligustilide from the essential oil ofLigusticum chuanxiong,Pharm.Care Res.8(2008)355–357.
[10]H.S.Lü,G.M.Wang,M.H.Zhang,Z.F.Geng,M.Yang,Y.P.Sun,Adsorption equilibrium of citric acid from supercritical carbon dioxide/ethanol on cyano column,Chin.J.Chem.Eng.23(6)(2015)905–911.
[11]W.P.Farrell,C.M.Aurigemma,D.F.Masters-Moore,Advances in high throughput supercritical fluid chromatography,J.Liq.Chromatogr.Relat.Technol.32(11)(2009)1689–1710.
[12]M.H.Chuang,M.Johannsen,Solubilities and adsorption equilibria of β-carotene in supercritical and near-critical fluids,J.Chem.Eng.Data56(5)(2011)1770–1777.
[13]B.G.Su,H.B.Xing,Y.S.Han,Y.W.Yang,Q.L.Ren,P.D.Wu,Adsorption equilibria of cis-5,8,11,14,17-eicosapentaenoic acid ethyl ester andcis-4,7,10,13,16,19-docosahexaenoic acid ethyl ester on C18-bonded silica from supercritical carbon dioxide,J.Chem.Eng.Data54(10)(2009)2906–2913.
[14]C.S.Tan,D.C.Liou,Adsorption equilibrium of toluene from supercritical carbon dioxide on activated carbon,Ind.Eng.Chem.Res.29(7(7))(1990)1412–1415.
[15]C.S.Tan,D.C.Liou,Desorption of ethyl acetate from activated carbon by supercritical carbon dioxide,Ind.Eng.Chem.Res.27(6)(1998)988–991.
[16]D.Bolten,M.Johannsen,Influence of 2-propanol on adsorption equilibria of alpha and sigma-tocopherol from supercritical carbon dioxide on silica gel,J.Chem.Eng.Data51(2006)2132–2137.
[17]M.Lubbert,G.Brunner,M.Johannsen,Adsorption equilibria of alpha-and deltatocopherol from supercritical mixtures of carbon dioxide and 2-propanol onto silica by means of perturbation chromatography,J.Supercrit.Fluids42(2007)180–188.
[18]X.F.Mo,H.S.Lü,M.H.Zhang,M.Yang,G.Q.Wang,The study on purification of Z-ligustilide by supercritical fluid chromatography,J.Chem.Eng.Chin.Univ.27(2013)737–742.
[19]R.Span,W.Wagner,A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa,J.Phys.Chem.Ref.Data25(1996)1509–1596.
[20]M.Enmark,J.Samuelsson,E.Forss,P.Forssén,T.Fornstedt,Investigation of plateau methods for adsorption isotherm determination in supercritical fluid chromatography,J.Chromatogr.A1354(2014)129–138.
[21]H.Dave,W.Thomas,S.Stephan,Expanding the elution by characteristic point method to columns with a finite number of theoretical plates,J.Chromatogr.A1413(2015)207–216.
[22]L.Ravald,T.Fornstedt,Theoretical study of the accuracy of the elution by characteristic points method for bi-Langmuirisotherms,J.Chromatogr.A908(2001)111–130.
[23]G.Hong,B.J.Stanley,G.Guiochon,Theoretical study of the accuracy and precision of the measurement of single-component isotherms by the elution by characteristic point method,J.Chromatogr.A659(1)(1994)27–41.
[24]T.Zhu,M.Tian,K.H.Row,Comparison of adsorption equilibrium of glycyrrhizic acid and liquiritin on C 18 column,J.Ind.Eng.Chem.16(6)(2010)929–934.
[25]X.M.Zhan,X.Zhao,A.Miyazaki,Y.Nakano,Lead removal from aqueous solutions using novel gel adsorbent synthesized from natural condensed tannin,Chin.J.Chem.Eng.4(2003)426–430.
[26]C.Ma,J.Tang,H.Wang,G.Tao,X.Gu,L.Hu,Preparative purification of salidroside fromRhodiola roseaby two-step adsorption chromatography on resins,J.Sep.Sci.32(2)(2009)185–191.
[27]X.L.Ren,L.Yang,M.Liu,Kinetic and thermodynamic studies of acid scarlet 3R adsorption onto low-cost adsorbent developed from sludge and straw,Chin.J.Chem.Eng.22(2)(2014)208–213.
[28]R.Wang,X.G.Peng,L.M.Wang,B.B.Tan,J.Y.Liu,Y.L.Feng,S.L.Yang,Preparative purification of peoniflorin and albiflorin from peony rhizome using macroporous resin and medium-pressure liquid chromatography,J.Sep.Sci.35(15)(2012)1985–1992.
[29]A.J.P.Martin,R.L.M.Synge,A new form of chromatogram employing two liquid phases:1.A theory of chromatography 2.Application to the micro-determination of the higher monoamino-acids in proteins,Biochem.J.2(N245)(1977).
[30]L.H.Yin,Y.W.Xu,Y.Qi,X.Han,L.Xu,J.Y.Peng,C.K.Sun,A green and efficient protocol for industrial-scale preparation of dioscin from Dioscorea nipponica Makino by two-step macroporous resin column chromatography,Chem.Eng.J.165(1)(2010)281–289.
 Chinese Journal of Chemical Engineering2016年12期
Chinese Journal of Chemical Engineering2016年12期
- Chinese Journal of Chemical Engineering的其它文章
- Hemicellulose in corn straw:Extracted fromalkali solution and produced 5-hydroxymethyl furfural in HCOOH/HCOONa buffer solution☆
- Molar volume of eutectic solvents as a function of molar composition and temperature☆
- Investigation on molar heat capacity,standard molar enthalpy of combustion for guaiacol and acetyl guaiacol ester
- Statistical mechanics and artificial intelligence to model the thermodynamic properties of pure and mixture of ionic liquids☆
- A comprehensive fractal char combustion model☆
- Development of a bifurcation analysis approach based on gPROMS platform☆
