Investigation on molar heat capacity,standard molar enthalpy of combustion for guaiacol and acetyl guaiacol ester
Changshuai Shen,Wenli Li,Cairong Zhou*
School of Chemical Engineering and Energy,Zhengzhou University,Zhengzhou 450001,China
1.Introduction
Guaiacol(C7H8O2,CAS 90-05-1),also known as o-methoxyphenol,o-hydroxy anisole and methylcatechol,is an important chemical intermediate that can be used for synthesis of guaiacol sulfonic acid potassium of anti-tussive and expectorant drug,and synthesis spicese.g.vanillin.It can also be used as an analytical reagent to test some materials such as copper,hydrocyanic acid and nitrite[1–3].It is also a raw material for synthesizing 5-nitroguaiacol sodium salt which belongs to a kind of cell activating agent and plant growth regulator.The 5-nitro guaiacolsodiumsaltcan quickly penetrate into plants,regulate and control the synthesis of nucleic acids,proteins and enzymes,and increase cell vitality to promote plant germination in different stages[4,5].The molar mass of guaiacol is 124.13.AGE(C9H10O3,CAS 613-70-7),is also known as o-methoxyphenyl acetate.An acetylate is synthesized by using guaiacol as a raw material,acetic anhydride as an acylating agent.It is the chemical intermediate that synthesized 5-nitroguaiacol sodium salt[6].The molar mass of AGE is 166.17.
Although guaiacol has been widely used,several important thermodynamic properties are still unknown,such as the heat capacity and the standard molar enthalpy of combustion.The study not only provides basic information about thermodynamic properties for guaiacol and AGE,but also is useful for exploiting the new synthesis method,engineering design and industry production.
2.Experimental
2.1.Materials
The chemical sample descriptions are given in Table 1.They were used without further purification.
2.2.Synthesis and puri fi cation of AGE
Acetic anhydride was added to guaiacol with stirring in the presence of an USY zeolite catalyst at room temperature.The reaction would become permanently when heated to 100°C for 3 h.Then the mixtures were cooled to room temperature,centrifuged and the catalyst decanted.Next excess acetic anhydride and acetic acid were separated with water as extraction agent by cross current multi-stage extraction.Finally,the vacuum distillation method was used to separate the guaiacol and AGE.The mass fraction purity of AGE was determined to be 0.995 with a gas chromatography.The acylation reaction equation is written by Eq.(1).

Table 1Provenance and mass fraction purity of the samples studied
2.3.Instrument and methods
The differential scanning calorimetry(type DSC-60)is provided by Shimadu Company in Japan.The SPN-500-type nitrogen generator,XRY-1C calorimeter,and WQF Fourier Transform Infrared Spectrometer are provided by Beijing BCHP Analytical Technology Institute,Changji Geological Instrument Co.,Ltd.,and Beijing Beifen-Ruili Analytical Instrument(Group)Co,Ltd.,respectively.The precision of the electronic balance(FA1004,Shanghai Precision&Scientific Instrument Co.,Ltd.)and microthermometer are 0.1 mg and±0.01°C,respectively.
The thermal analysis apparatus was calibrated by measuring a standard specimen before analysing the tested samples.α-Al2O3was used as the reference sample in the whole analysis process.The specific heat capacities of samples were determined by differential scanning calorimetry(type DSC-60).The heat capacity of the Oxygen Bomb Calorimeter can be obtained by the enthalpy of combustion of benzoic acid used as the standard substance.The reliability of the instrument was tested by using naphthalene as reference material.The standard molar enthalpies of combustion of samples were determined by XRY-1C calorimeter.
2.4.The specific heat measurement principle
The measuring methods of the specific heat capacity from DSC are described in the literature[7–14].The relationship between the specific heat capacity and heat flow rate at constant pressure can be expressed by Eq.(2)[15].

wheremis the mass of the tested sample,g;Cpis the specific heatcapacity,kJ·K-1·kg-1or J·K-1·g-1,subscript p indicates an isobaric process;Tis Kelvin temperature,K;Qis the quantity of heat.
The absolute value ofcannot be determined accurately because of the instrumentitselfand the operating conditions[16].So the indirect measurement method is used in the experiment.This indirect procedure can be done in two ways,that is the continuous-scanning method or the stepwise-scanning method[17].The continuous-scanning method was used for guaiacol and acetyl guaiacol ester,which is described in detail in ASTMnorm E1269-11[18].The specific heat capacity Cpcan be calculated by Eq.(3)[18].

whereCp(s)andCp(st)are the constant-pressure specific heat capacities of both a tested sample and a calibration material,α-Al2O3is used as the calibration material and the values ofCp(st)are extracted from ISO 11357-4[15];WsandWstare the mass of tested sample andα-Al2O3,respectively;Dsis the vertical displacement between the empty crucible and the test sample on DSC thermal curves at a given temperature;Dstis the same asDswhich is between the empty crucible and α-Al2O3.
2.5.Estimate the molar heat capacity by a group contribution model
A group contribution method is widely used in process design software to predict the physical and thermodynamic properties of chemical components.The basic assumption of group contribution method is that the properties of the component depended on the functional groups which make up its chemical structure,and that each group provides a fixed contribution towards the properties,irrespective of the species involved[19].The method for estimatingCpdepends on the concept that a liquid mixture may be considered a solution of the structural units from which the molecules are formed rather than a solution of the molecules themselves.These structural units are called“subgroups”.The advantage of group contribution method is that a relatively small number of subgroups combine to form a very large number of molecules.The Missenard and Rozicka-Domalski methods[20,21]are used to estimate the molar heat capacities of chemical components in the paper.
2.5.1.Missenard method
Missenard method is given by Eq.(4)onCpof liquid organic compounds at temperature-25,0,25,50,75,and 100°C[20].

whereirepresents theitype subgroup,niis the number of the subgroup,CpLiis the contribution value of all i subgroup.
2.5.2.Rozicka-Domalski method
Rozicka and Domalski have reported the relationship between some heat capacity and temperature on some liquid substances based on group contribution method,the model is expressed by Eq.(5)[20]

where R and T are the universal gas constant and Kelvin temperature;ai,
biandciare the contribution values of each subgroup.
2.6.The standard molar enthalpy of combustion
The heat capacity(ε)of the Oxygen Bomb Calorimeter is obtained based on the enthalpy of combustion of benzoic acid which is used as the standard reference material.The detailed procedure is described as follow:(1)Weigh the benzoic acid about 0.5–1.0 g,press the powder into tablet,make it touch with the ignition wire,then fill the oxygen at 3–4 MPa;(2)put the oxygen bomb into the bottle which is filled with water of 3200 ml.After the temperature is constant,the blender is turned on.The metal wire is ignited after 6 min.(3)The test sample starts combustion when temperature is quickly raised.Temperature readings are taken at 30 s intervals after the ignition.After temperature reach at the most height point and continue 10 min,the test could be stopped automatically.Other substances are proceeded the same as above.
According to the principle of thermodynamics,when the material is completely burned,the constant pressure heat of combustion can be obtained.Because Qp= ΔH,Qv= ΔU,the enthalpy change can be represented by Eq.(6).

Fig.1.FT-IR spectrum of guaiacol.
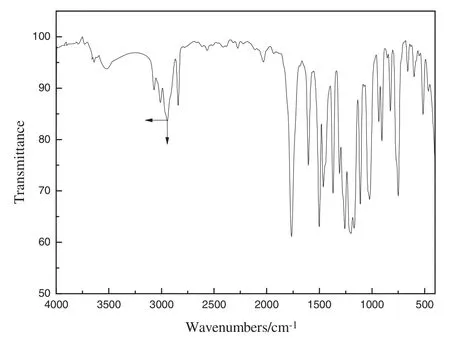
Fig.2.FT-IR spectrum of AGE.

where Δn,R and T are the change in number of moles between the gaseous reactants and products before and after the reaction,the universal gas constant and Kelvin temperature,respectively[22,23].
When the test samples are completely burned,the exothermic heat can make the temperature of calorimeter(bomb)itself and surrounding medium increased,which is water here.The heatQvof combustion reaction for the test material can be obtained at a constant volume by Eq.(7)after the heat capacity of Oxygen Bomb Calorimeter(c)is defined[24].

wherem,Cwater,ε,QfandQvare the mass of sample,the specific heat capacity of water(Cwater=4.18 J·K-1·g-1),the heat capacity of calorimeter,additional heat by air in the boom and the constant-volume energy of combustion of the pure substance,respectively.
3.Results and Discussion
3.1.FTIR analysis of guaiacol and AGE
The FTIR of guaiacolis shown in Fig.1.The matching degree with standard spectrum of guaiacol was 99.01%,so the guaiacol sample is reliable.
The FTIR of AGE is shown in Fig.2.From the IR spectrum,1764 cm-1is a stretching vibration peak of C=O,1605,1502,and 1442.5 cm-1are benzene skeleton vibration,1462.5,1370 cm-1are–CH3anti symmetric and symmetric deformation vibration,1258,1198 cm-1are the characteristic peaks of–OCH3connected with benzene ring,1308,1215,1170 cm-1are=C–O stretching vibration absorption peak,1022 cm-1are anti-symmetric stretching vibration of C–O–O,824–750 cm-1are AR-H out-of-plane bending vibration,which is in agreement with the molecular structure of AGE.Comparing the IR spectrum of the synthesis AGE with the standard IR spectrum in literature[6],we find that both of them are the same,which shows that the product synthesized is AGE.
3.2.The constant-pressure molar heat capacities of guaiacol and AGE
The measurement is carried out in the temperature range of 280–360 K according to the procedure described in the norm[18]:The heating rate was 5 K·min-1,but note that it has heat preservation period at the beginning and the end about 4 min;the flow rate of dynamic dry nitrogen gas was 20 ml·min-1,the mass of tested sample is 3 to 6 mg.Each sample was measured for five times at least.To determine the reliability of the instrument,the constant-pressure molar heat capacities of trans-1,2-Cyclohexanediol in the liquid phase andα-Al2O3in the solid phase as standard samples were measured.
The experimental results and the literature values[15]about α-Al2O3and trans-1,2-Cyclohexanediol are shown in Table 2.Therelative error between the measured and the literature values lie within 1%.The accuracy is in the ideal range proposed by Hugger[25].
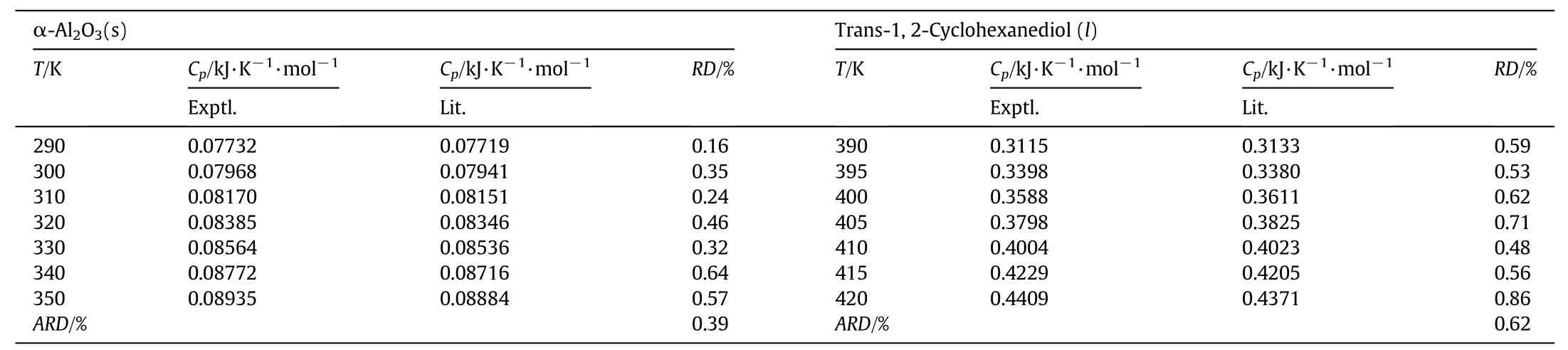
Table 2Experimental and literature values of molar heat capacities for α-Al2O3 and trans-1,2-Cyclohexanediol①
The relationship between the molar heat capacity and temperature for α-Al2O3can be represented by Eq.(8),the linear correlation coefficient(R2)of Eq.(8)is 0.9995.
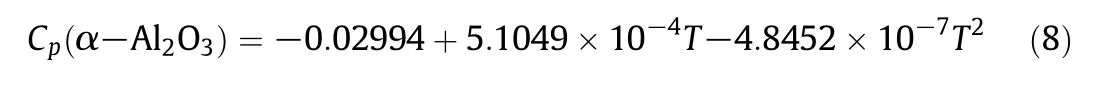
In order to verify further the reliability of the method,the molar heat capacity of trans-1,2-Cyclohexanediol in the liquid phase was measured by DSC.The results are summarized in Table 2 and Fig.3.The relationship between the molar heat capacity and temperature for trans-1,2-Cyclohexanediolcan be expressed by Eq.(9),the linear correlation coefficient(R2)is 0.9979.

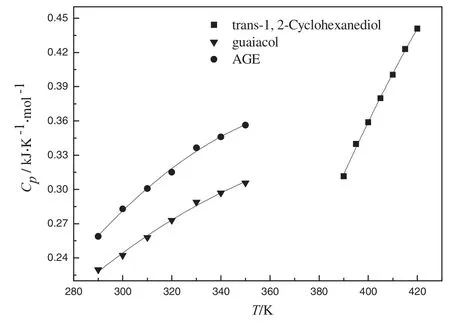
Fig.3.The molar heat capacities for trans-1,2-Cyclohexanediol,guaiacol and AGE.
Comparing the calculated values of Eqs.(8)and(9)with the experimental values,the relative deviationRDand average relative deviationARDare listed in Table 2.And average relative deviation is defined as

The results show ARD is respectively 0.39 and 0.62 for α-Al2O3and trans-1,2-Cyclohexanediol.
Figs.4 and 5 show the DSC thermograms about the baseline,α-Al2O3,guaiacol and AGE.The results of the constant-pressure molar heat capacities for guaiacol and AGE are shown in Fig.3 and Table 3.The relationships between the molar heat capacity and temperature for guaiacol and AGE can be represented by Eqs.(11)and(12),respectively.The linear correlation coefficients(R2)of them are 0.9936 and 0.9956.

TheARD%is 0.60±0.02 about the molar heat capacities of guaiacol and AGE.
Estimated values of the molar heat capacities for guaiacol and AGE by group contribution methods are summarized in Tables 4 and 5.

Fig.4.The heat flow dependence on time for baseline,а-Al2O3 and guaiacol.
Fig.5.The heat flow dependence on time for baseline,a-Al2O3 and AGE.
Comparing the estimated and experimental values in Table 6,it can be found that theARDis less than 8.0%,which is 6.97%and 2.65%for guaiacol by the Missenard and Rozicka–Domalski methods,respectively,and of another is 5.06%and 7.54%by those for AGE.Generally,the average relative deviation of all estimated heat capacity values is(5%–20%)by the group contribution methods[26].The results show that the estimated molar heat capacities and the experimental values are consistent.The group contribution method is widely used in engineering calculation to estimate the heat capacities of the materials,but the values cannot be used as the physical parameters of the materials because in most cases it is not precise.Two methods we used are not applicable in a wide temperature range,but the estimated results have important engineering significance in verifying the correctness of the results of the experiment.The average relative deviation of the experimental and estimated values limits in the value of 10%reflect the reliability of the experimental data from the side.
3.3.The standard molar enthalpies of combustion of guaiacol and AGE
3.3.1.The energy equivalent of the calorimeter
The enthalpy of combustion(Qv)of benzoic acid is-26460 J·g-1from the literature[27,28].The heat capacity(ε)of Oxygen Bomb Calorimeter can be obtained to be 1940 J·K-1by Eq.(7)based on the constant-volume heat of benzoic acid in Table 7.
The instrument reliability was tested by using naphthalene as reference material.We compared the molar enthalpy of combustion of naphthalene measured(-5162.1 kJ·mol-1)with its standard value(-5161.1 kJ·mol-1)in the literature[29].The result indicates that the absolute error and relative error are 1.0 kJ·mol-1and 0.02%,respectively.

Table 3Experimental and calculated values of molar heat capacities for guaiacol and AGE①

Table 4Estimated values of molar heat capacities for guaiacol and AGE by Missenard method

Table 5Estimated values of molar heat capacities for guaiacol and AGE by the Rozicka–Domalski method
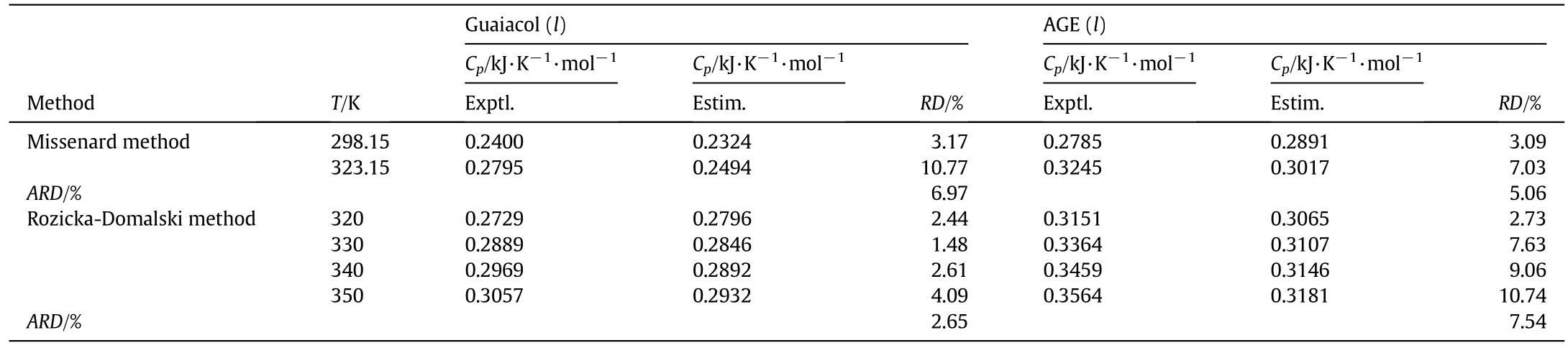
Table 6Experimental and estimated values of molar heat capacities for guaiacol and AGE①
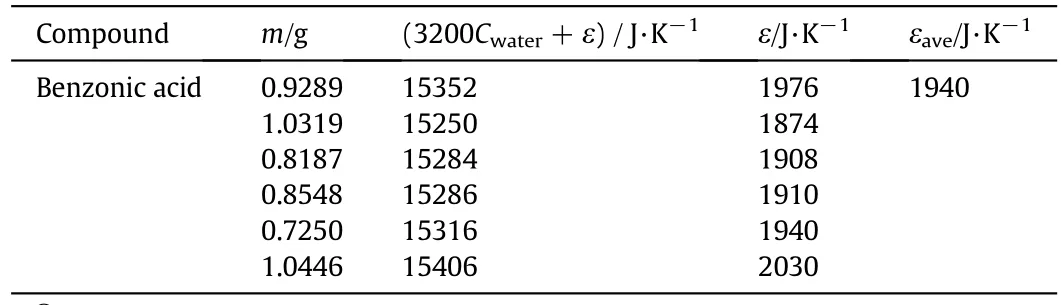
Table 7Heat capacity of the Oxygen Bomb Calorimeter①
3.3.2.Determination of standard molar enthalpy of combustion ΔcHmθ
The combustion reactions of guaiacol and AGE can be written as following Eqs.(13)and(14)
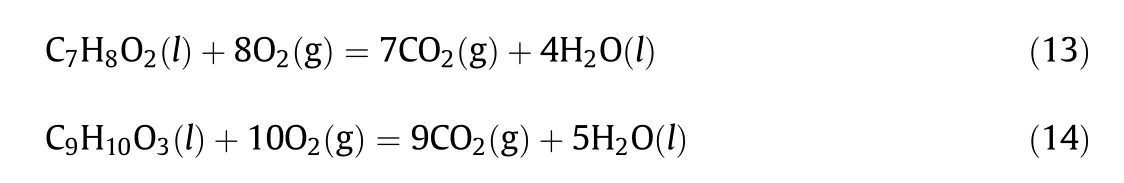
Based on Eq.(6),the constant pressure enthalpies of combustion of both guaiacol and AGE are expressed by Eq.(15)

After Qvobtained by Eq.(7)and ΔH by Eq.(15),which was listed in Table 8,the standard molar enthalpies of combustion Δc
of guaiacol and AGE can be calculated.Since the experimental temperature was not the same with standard temperature,it is necessary that the enthalpies of combustion at the experiment temperature are corrected.On the basis of Hess's law,the standard molar enthalpy of combustion(at 298.15 K)can be calculated by Eq.(16)where ΔcHmθand ΔcHmare the standard molar enthalpy of combustion(298.15 K)and the enthalpy of combustion at the experiment temperature.ΔHrand ΔHfare the enthalpies of the reactants and products.
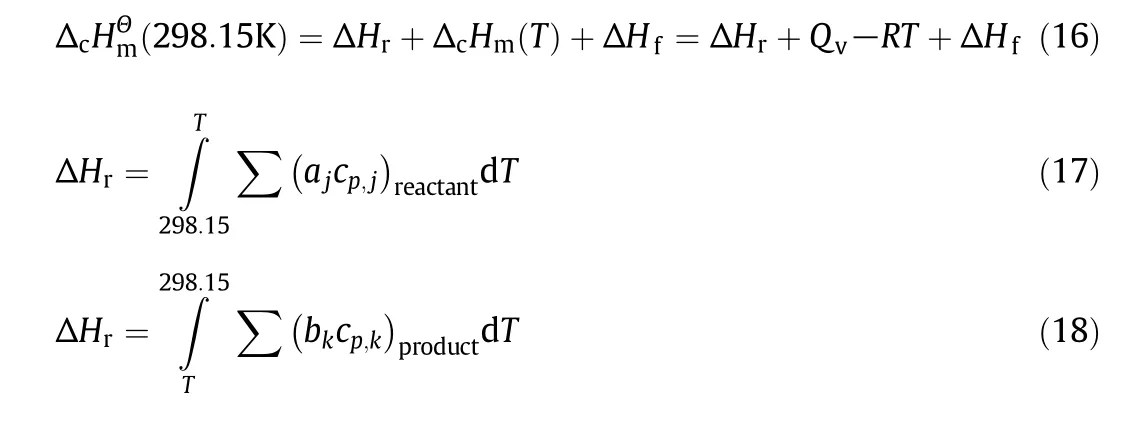
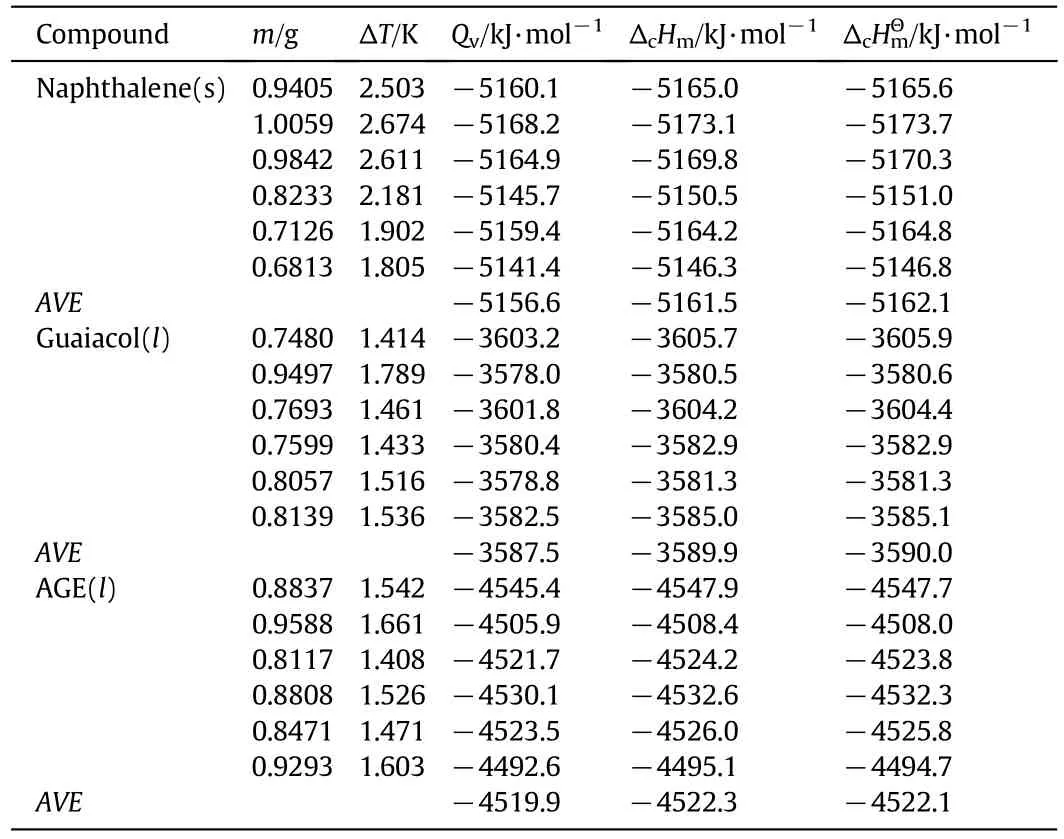
Table 8The standard molar enthalpies of combustion of naphthalene,guaiacol and AGE①
According to thermodynamic data manual[30],the constant pressure molar heat capacity of O2,CO2,H2O,and the test results of molar heat capacities for naphthalene,guaiacol,and AGE at the experiment temperature were listed in Table 9.From Eq.(16),the standard molar enthalpies of combustion of both guaiacol and AGE were determined to be-3590.0 kJ·mol-1and-4522.1 kJ·mol-1(in Table 8),respectively.
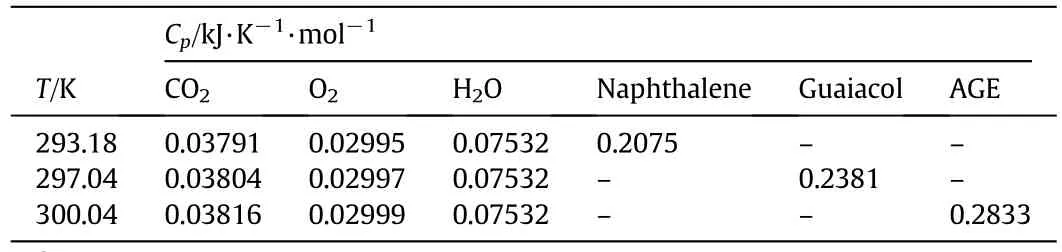
Table 9The constant-pressure molar heat capacity of compounds at the temperature of the experiment①
3.3.3.Determination of standard molar enthalpy of formation ΔcHmΘ
The complete combustion of organic compounds in oxygen atmosphere is easy,but it is difficult to achieve organic compounds through elemental direct synthesis.Therefore,a standard molar enthalpy of formation can be calculated with material's standard molar enthalpy of combustion.
The formation reaction of guaiacol and AGE can be written as following Eqs.(19)and(20)

The products of the combustion reaction of guaiacol are 7CO2(g)and 4H2O(l),the standard molar enthalpy of formation for guaiacol can be calculated by Eqs.(21)or(22)

The products of the combustion reaction of AGE are 9CO2(g)and 5H2O(l),the standard molar enthalpy of formation for AGE can be calculated by Eqs.(23)or(24)

Δc(C,graphite)and Δc(H2,g)are equal to Δf(CO2,g)and Δf(H2O,l),respectively. Δf(CO2,g)and Δf(H2O,l)are-393.514 kJ·mol-1,-285.838 kJ·mol-1[31],successively.The standard molar enthalpies of formation for guaiacol and AGE were calculated to be-307.95 kJ·mol-1and-448.72 kJ·mol-1,respectively.
4.Conclusions
The molar heat capacities of guaiacol and AGE were determined using differential scanning calorimetry.The relationships between the molar heat capacity of the samples and temperature were obtained from 290 K to 350 K.Two kinds of group contribution models were used to estimate the heat capacities,the average relative deviation is limited within 10%.Using XRY-1C calorimeter,the standard molar enthalpies of combustion for guaiacol and AGE were determined to be-3590.0 kJ·mol-1and-4522.1 kJ·mol-1,respectively.The standard molar enthalpies of formation for guaiacol and AGE were determined to be-307.95 kJ·mol-1and-448.72 kJ·mol-1,respectively.
Nomenclature
a,b,cgroup contribution parameters
Cthe molar heat capacity of sample,J·K-1·mol-1
D The signal value of DSC for sample,mW
Henthalpy,kJ·mol-1
mmass of the tested sample,mg
nithe quantity of the subgroup
Qquantity of heat,J·mol-1or kJ·mol-1
Rgas constant,8.314 J·K-1·mol-1
Tabsolute temperature,K
U energy of thermodynamics,kJ·mol-1
ε the heat capacity of calorimeter,J·K-1
Δ variable quantity
Superscript
Θ standard state
Subscripts
c combustion
f formation
iserial number or theitype subgroup
m material
p constant pressure
pL liquid compounds at constant pressure
s sample or solid state
st standard sample
v constant volume
[1]W.G.Ji,L.Xin,The synthesis of guaiacol by methylation of catechol,Chem.Res.13(2002)24–27.
[2]P.Cao,P.Li,Y.Yang,J.X.Ma,Progress in synthesis of guaiacol,Chem.World46(2005)122–125.
[3]G.M.Li,Y.Z.Liu,Q.L.Zhang,L.H.Zhou,Research progress of preparation method of guaiacol,Chem.Intermed.6(2010)20–25.
[4]M.J.Cheng,X.Q.Wang,C.R.Zhou,Review on the synthesis of sodium 5-nitroguaiacolate,Hebei Chem.Eng.Ind.30(2007)6–8.
[5]H.R.Gao,X.J.Ren,S.X.Jing,L.Z.Xu,Synthesis of 5-nitroguaiacol sodium salt,J.Qingdao Univ.Sci.Technol.24(2003)330–332.
[6]M.J.Cheng,Study on the synthesis of sodium 5-nitroguaiacol Ma.Eng(Thesis)Zhengzhou Uni.,China,2008.
[7]C.R.Zhou,H.F.Wang,X.H.Shi,W.Feng,Determination of specific heat capacity and combustion enthalpy of naproxen,Chem.Eng.38(2010)93–96.
[8]C.R.Zhou,G.P.Li,X.W.Han,D.G.Jiang,Determination for enthalpies of combustion of high-carbon fatty alcohols by static bomb calorimetry,Adv.Mater.Res.396–398(2011)1023–1028.
[9]X.W.Han,C.R.Zhou,X.H.Shi,Determination of specific heat capacity and standard molar combustion enthalpy of taurine by DSC,J.Therm.Anal.Calorim.109(2012)441–446.
[10]L.Atanasova,G.Baikusheva-Dimitrova,G.Gospodinov,Specific heat capacity and thermodynamic properties of CuTeO3and HgTeO3,J.Therm.Anal.Calorim.118(2014)493–497.
[11]S.Q.Miao,H.P.Li,G.Chen,Temperature dependence of thermal diffusivity,specific heat capacity,and thermal conductivity for several types of rocks,J.Therm.Anal.Calorim.115(2014)1057–1063.
[12]P.Andreu-Cabedo,R.Mondragon,L.Hernandez,R.Martinez-Cuenca,L.Cabedo,J.E.Julia,Increment of specific heat capacity of solar salt with SiO2nanoparticles,Nanoscale Res.Lett.9(2014)582–593.
[13]D.G.Jiang,C.R.Zhou,F.Wang,H.P.Li,D.Y.Wei,Study on standard combustion enthalpy and heat capacity for cis-and trans-1,2-cyclohexanediol,Chem.Eng.33(2005)63–68.
[14]G.W.H.Höhne,W.F.Hemminger,H.J.Flammersheim,Differential scanning calorimetry,Encycl.Nanotechnol.40(2005)402–407.
[15]ISO 11357–4,Plastics-differential scanning calorimetry(DSC)—Part 4:Determination of specific heat capacity,ISO,2005.
[16]C.S.Yang,P.S.Ma,S.Q.Xia,The specific heats of aqueous mixtures of acetic acid with water measured with DSC,J.Chem.Eng.Chin.Univ.16(2002)479–483.
[17]R.Pilař,P.Honcová,P.Koštál,G.Sádovská,L.Svoboda,Modified stepwise method for determining heat capacity by DSC,J.Therm.Anal.Calorim.118(2014)485–491.
[18]ASTM norm E1269–11,Standard test method for determining specific heat capacity by differential scanning calorimetry,ASTM Int.,Pennsylvania,2011.
[19]P.Nancarrow,M.Lewis,L.Abouchacra,Group contribution methods for estimation of ionic liquid heat capacities:Critical evaluation and extension,Chem.Eng.Technol.4(2015)632–644.
[20]X.F.Dong,L.G.Fang,L.Chen,Physical property estimation principle and computer calculation,Chemical Industry Press,Beijing,2006.
[21]P.S.Ma,Chemical engineering data,China Petrochemical Press,Beijing,2003.
[22]M.A.V.R.D.Silva,J.I.T.A.Cabral,Standard molar enthalpies of formation of 5-and 6-nitroindazole,J.Therm.Anal.Calorim.100(2010)457–464.
[23]M.A.V.R.D.Silva Silva,L.M.P.F.Amaral,Standard molar enthalpies of formation of some methylfuran derivatives,J.Therm.Anal.Calorim.100(2010)375–380.
[24]D.J.Zhu,Y.H.Xie,F.M.Mei,H.W.Wang,G.X.Li,Measurement of standard molar combustion enthalpy of methyl phenyl carbonate,Chem.Eng.35(2007)38–40.
[25]L.Yang,S.Zhuang,D.F.Dai,S.Wang,Z.W.Feng,X.Z.Wang,The heat release calculation methods of polymer and experiments,Fire Sci.Technol.30(2011)181–182.
[26]R.Pilar,J.Pachman,R.Matyáš,P.Honcová,D.Honc,Comparison of heat capacity of solid explosives by DSC and group contribution methods,J.Therm.Anal.Calorim.121(2015)683–689.
[27]L.L.Chen,C.R.Zhou,X.H.Shi,Standard combustion enthalpy and thermal capacity of some nitrogenous organisms,J.Chem.Eng.Chin.Univ.23(2009)729–734.
[28]N.An,X.H.Shi,C.R.Zhou,Determination of the standard combustion enthalpy of trans-ferulic acid,Chem.Eng.35(2007)43–45.
[29]P.S.Ma,Y.H.Li,Chemical engineering thermodynamics,Chemical Industry Press,Beijing,2005.
[30]R.Holze,T.Jacob,S.Schunke,P.Neudecker,D.Willbold,Physical chemistry,nachrichten aus der chemie,2012.
[31]Physical chemistry teaching and research section of Tianjin University,physical chemistry,Higher Education Press,Beijing,2009.
 Chinese Journal of Chemical Engineering2016年12期
Chinese Journal of Chemical Engineering2016年12期
- Chinese Journal of Chemical Engineering的其它文章
- Hemicellulose in corn straw:Extracted fromalkali solution and produced 5-hydroxymethyl furfural in HCOOH/HCOONa buffer solution☆
- Molar volume of eutectic solvents as a function of molar composition and temperature☆
- Statistical mechanics and artificial intelligence to model the thermodynamic properties of pure and mixture of ionic liquids☆
- A comprehensive fractal char combustion model☆
- Development of a bifurcation analysis approach based on gPROMS platform☆
- Catalytic kinetics of dimethyl ether one-step synthesis over CeO2–CaO–Pd/HZSM-5 catalyst in sulfur-containing syngas process☆
