Catalytic kinetics of dimethyl ether one-step synthesis over CeO2–CaO–Pd/HZSM-5 catalyst in sulfur-containing syngas process☆
Ruizhi Chu ,Wenxin Hou ,Xianliang Meng ,*,Tingting Xu ,Zhenyong Miao ,Guoguang Wu ,Lei Bai
1 School of Chemical Engineering and Technology,China University of Mining&Technology,Xuzhou 221116,China
2 High-Tech Research Institute of China University of Mining and Technology,Lianyungang 222000,China
3 Department of Chemical and Biomedical Engineering,West Virginia University,Morgantown 26506,USA
1.Introduction
Interest in hydrogen production for fuel cell applications is steadily increasing due to environmental concerns[1,2].Among the various feed gases,DME proved to be a good alternative fuel to minimize the emissions of global warming gases and hazardous components such as SOx,NOxand particulate matter,because DME is similar in nature to liquefied petroleum gas(LPG)[3–5].
Until now,two synthesis methods of DME have been reported:approach one is the traditional methanol synthesis followed by a dehydration step[6];approach two is DME one-step synthesis fromsyngas under hybrid catalysts in the same reactor[7–10].DME one-step synthesis from sulfur-containing syngas attains more and more attention recently due to higher conversion and lower cost in comparison to methanol dehydration[11].The key issue of DME one-step synthesis from coal-derived syngas is catalytic stability of sulfur-tolerant[12].At present,the research work is focused on the sulfur-tolerant catalyst screening according to the DEM selection,activity or carrier effect[13,14].Although there are some researches on kinetics of DME one step synthesis from sulfur-containing syngas,most researchers tend to study on the kinetics of methanol synthesis or methanol dehydration individually.Such as,Popet al.[15]studied the intrinsic kinetics of methanol dehydration to DME in fixed bed reactor over H-SAPO-34 catalyst,and the experimental data were in good agreement with hyperbolic kinetic equation derived by Lu[16].Zhang[17]studied the intrinsic kinetics of methanol dehydration to DME and the effect of operation conditions on the conversion of methanol in fixed bed reactor over catalyst MD-2.The results indicate that the intrinsic kinetic equation based on the mechanism of Langmuir–Hinshelwood dissociation adsorption was reliable.However the pre-existing single reaction kinetic model could not be fully applicable in DME one-step synthesis from the reaction system,and different forms of kinetic equations or different parameter values could be obtained under different catalysts and different operating conditions.
Taking into account these results,the main objective of our study was to develop a sulfur-tolerant catalyst for DME one-step synthesis.After years of efforts,we have gained notable achievement in the preparation methods,modification technologies and sulfur-tolerant mechanism involving sulfur-tolerant Pd-based catalysts[14,18–20].The comparison of the stability of the synthetic catalyst and other common catalysts also has been presented in the previous literature[21].And the catalyst has good sulfur tolerance and catalytic activity.In this paper,we focused on the catalytic kinetics of DME one-step synthesis over the hybrid catalyst of CeO2–CaO–Pd/HZSM-5,and its macrokinetics model has been founded.Kinetic model for the methanol synthesis reaction and the dehydration of methanol were obtained separately according to reaction mechanism and Langmuir–Hinshelwood mechanism.Regression parameters were investigated by the method combining the simplex method and Runge–Kutta method.
2.Experimental
2.1.Catalyst preparation
5 g of HZSM-5(particle size 0.6–0.9 mm)was firstly impregnated into nitrate aqueous mixture solution of Ce3+and Ca2+in a 150 ml of flask at 50 °C for 2 h,the suspension was heated to 80 °C and vigorously stirred at the temperature until most of water was removed by evaporation followed by calcination in microwave oven with an indicated power 420 W for 1 h,then a series of CeO2–CaO/HZSM-5 carrier materials were obtained.Following the same process to supporting metal Pd on CeO2–CaO/HZSM-5 and then,nano-sized CeO2–CaO–Pd/HZSM-5 catalyst was obtained.
2.2.Catalytic test
Catalytic activity and sulfur tolerance of CeO2–CaO–Pd/HZSM-5 and kinetic experiments were carried out in gas phase in a fixed-bed microreactor,using a reaction tube with 5 mm of inner diameter.CeO2–CaO–Pd/HZSM-5 catalyst particles were loaded into the tube and the ratio of the total height of the catalyst bed with the upper and lower silica cotton and the catalyst particle size was greater than 50.The gas in the catalyst bed layer could be regarded as a flat pushing flow.The blank experiment results show that the reaction material and the silica filler have no effect on the catalytic reaction.The catalyst was heated to 220 °C under hydrogen flow at 1 °C·min-1and maintained the temperature for 2 h.Then H2+CO,N2,and H2S(volume ratio(H2+CO):N2:H2S=75:24.5:0.5)were introduced through a mass flow controller,slow step-up to desired pressure and temperature,to initiate the reaction.Reaction products exhaust through the valve after decompression,which leads to chromatographic analysis,or shorting.CO conversion(XCO,%)and product DME selectivity(SDME,%)were calculated by normalization.Specific calculation formula is as follows:

The content of each component was expressed in mole percent.
3.Results and Discussion
3.1.Catalytic activity and sulfur tolerance of CeO2-CaO-Pd/HZSM-5
As shown in Fig.1,an induction period exists at the initial stage of reaction over CeO2–CaO–Pd/HZSM-5 catalyst from sulfur-containing syngas.DME selectivity increases in the induction period and then reached to stable(after 10 h),which is consistent with the results of Ma et al.[22].Ma et al.explain that it is because of the elimination of the thin layers of reduced ceria covered on the Pd(1 1 1)active sites.It is well accepted that Pd(1 1 1)plane adjacent to ceria is the efficient active sites for methanol formation.Xue et al.[23]found the addition of Ca reduced the number of strong acid sites and enhanced the metal support interaction and increased the electronic surroundings of Pd sites,which maintained Pd in a partly oxidized(Pdδ+)state and consequently increased the activity for methanol.Naitoet al.[24]found that thin layers of additive component ceria covered on(1 1 1)plane of Pd particles could be removed by formed H2O during reaction,which was responsible for the increment of DME selectivity in the induction period.
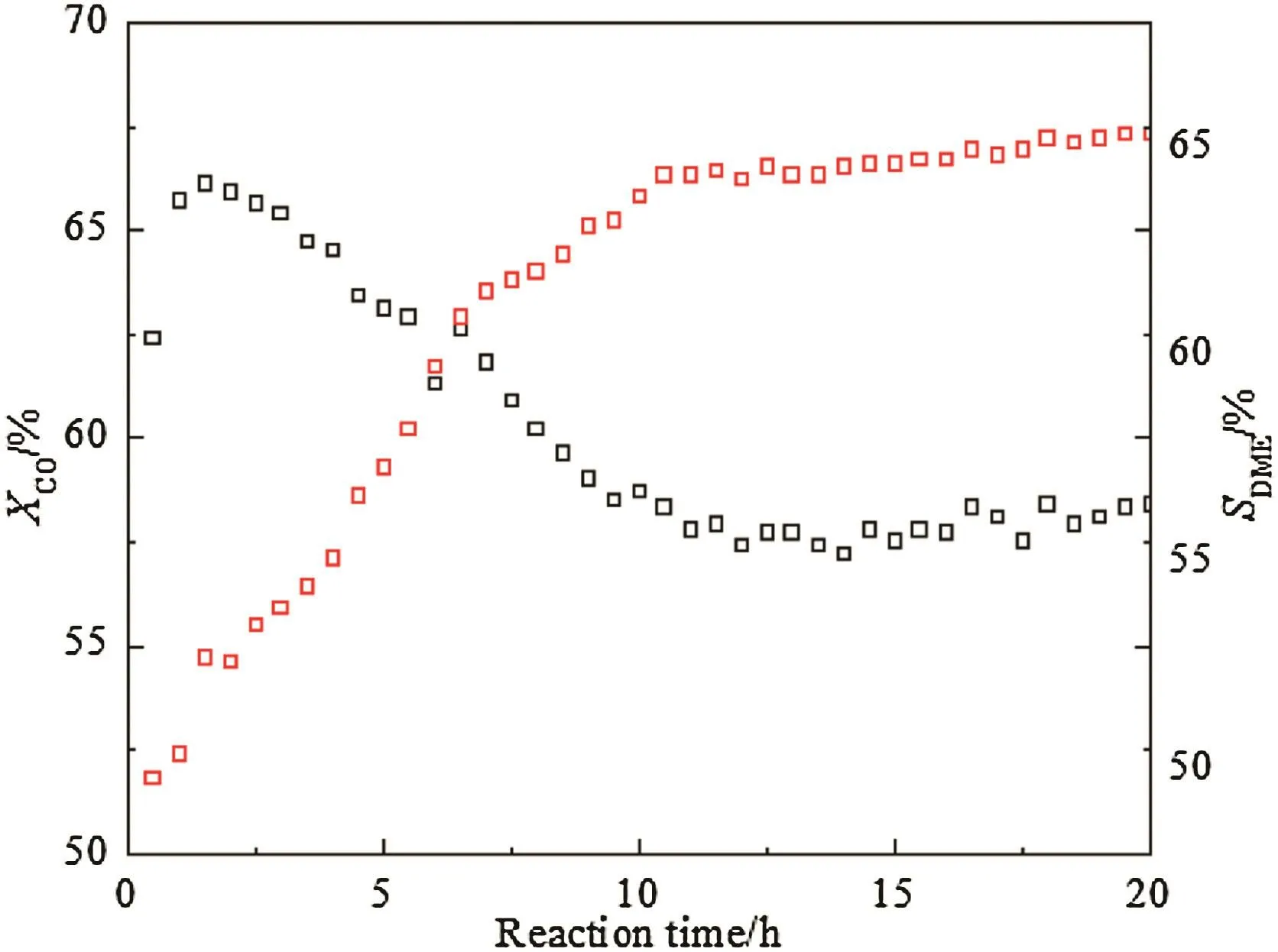
Fig.1.CO conversion(black)and DME selectivity(red)over CeO2–CaO–Pd/HZSM-5 for DME one-step synthesis from sulfur-containing syngas.
It could be concluded that the CeO2–CaO–Pd/HZSM-5 catalyst is very active and stable in sulfur-containing syngas and exhibited excellent catalytic performance for DME synthesis.In order to ensure that the kinetics experimental results are not significantly influenced by induction period and catalyst deactivation,the experimental data should be read after 10 h in the catalytic reaction.
3.2.Synthesis conditions of DME over CeO2-CaO-Pd/HZSM-5
3.2.1.Temperature
Fig.2 shows that CO conversion rate and DME selectivity increase with the rise of temperature,while DME selectivity decreases when the temperature is over 300°C.Conversely,methanol selectivity decreases significantly when the temperature is lower than 280°C and then stabilizes at 3%,and slight increases could be seen over 340°C.The selectivity of CO2presents no regular change under 300 °C,while it rises regularly above 300 °C.The data lead us to the conclusion that the optimal reaction temperature is 300°C.
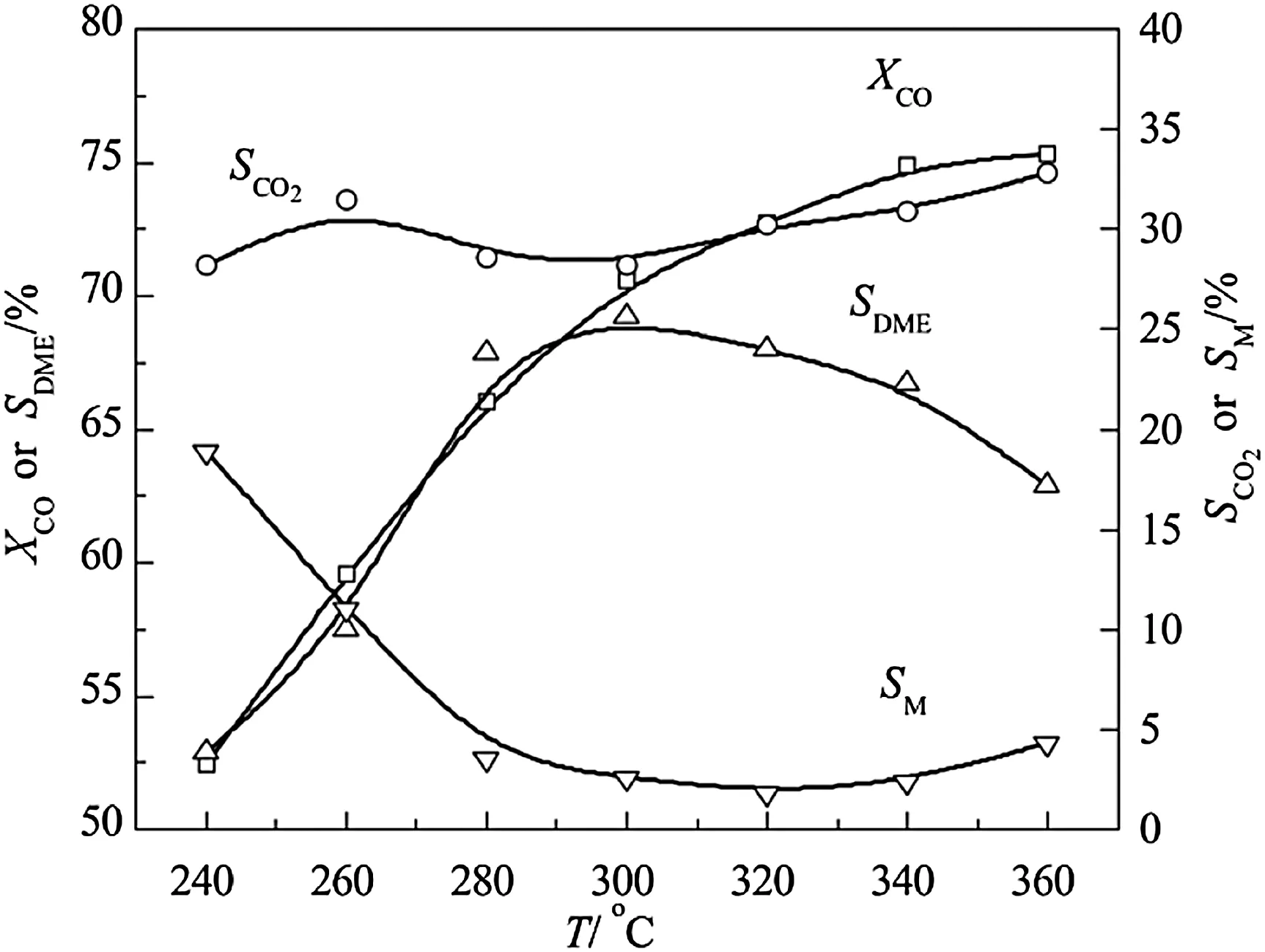
Fig.2.Effect of temperature on CeO2–CaO–Pd/HZSM-5 catalytic properties.Reaction condition:p=3.0 MPa,GHSV=1600 L·kg-1·h-1,n H2/n CO=2.0.
Methanol synthesis reaction,methanol dehydration reaction and water–gas shift reaction are carried out simultaneously on the catalyst during DME one-step synthesis from syngas.In terms of thermodynamics,STD total reaction is a reversible exothermic,the equilibrium conversion should be reduced when the reaction temperature rises.However,the experimental results show that the increase of temperature is favorable to the reaction conversion rate,which contradicts thermodynamics.Since the different characteristics of three reactions,we think bifunctional catalyst which has one active center for methanol synthesis and water–gas shift reaction and the other for methanol dehydration is required for STD reaction.The spatial arrangement of the two kinds of active centers on the catalyst surface and the porous structure of the catalyst support make the reaction always kinetically controlled.Elevating temperature accelerates the movement of molecules,increases the number of activated molecules,and accelerates the reaction to direction of the DME.Thus,higher reaction temperature would improve the yield of DME in suitable temperature range.Nevertheless,the total reaction is close to thermodynamic equilibrium values[25]and DME is easy to be decomposed at high temperature.Therefore,the reaction temperature should not be higher than 300°C.
3.2.2.Pressure
Fig.3 indicates that CO conversion rises rapidly and reaches a maximum at 4 MPa then subsequently declines sharply with increasing pressure.The stoichiometric coefficient of direct synthesis of DME from CO and H2is reduced,consequently,elevating pressure facilitates CO conversion.However,since the number of molecules for methanol synthesis reaction reduces,quantity of generated methanol increases and excess methanol could not promptly dehydrate with the increase of pressure,which leads to reverse inhibited methanol synthesis reaction,so CO conversion reduces at high pressure.In addition,the number of molecules remains the same pre/post methanol dehydration reaction so that pressure has little effect on the reaction.DME selectivity slightly changes and reaches a higher level at 3–4 MPa just because of the effect of synergy.
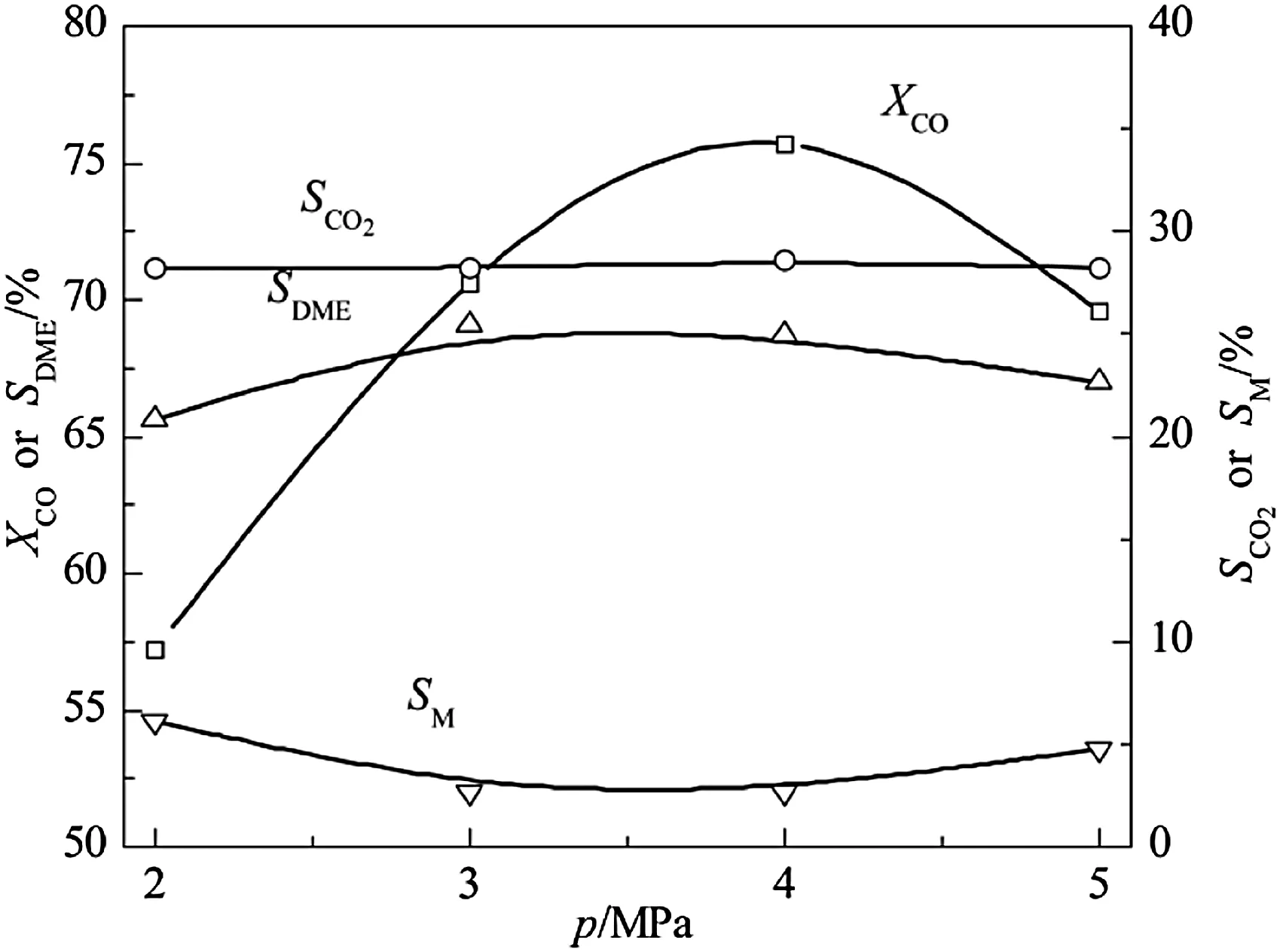
Fig.3.Effect of pressure on CeO2–CaO–Pd/HZSM-5 catalytic properties.Reaction condition:T=300 °C,GHSV=2000 L·kg-1·h-1,n H2/n CO=2.0.
3.2.3.H2/CO mole ratio
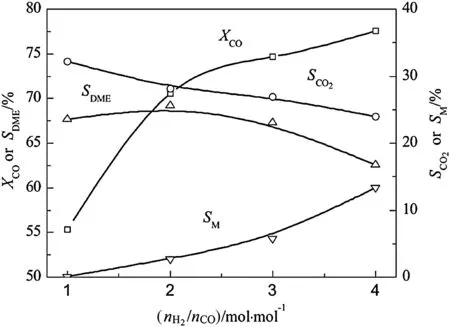
Fig.4.Effect of H2/CO on CeO2–CaO–Pd/HZSM-5 catalytic properties.Reaction condition:T=300 °C,p=3.0 MPa,GHSV=2000 L·kg-1·h-1.
As exhibited in Fig.4,it can be seen that CO conversion on the catalyst improves as the H2/CO mole ratio increases.Especially,CO conversion rises up fastest when the H2/CO in 1–2 and then tends to slow.Methanol selectivity increases with the increase of H2/CO,while CO2conversion decreases.DME selectivity reaches the maximum when the H2/COis 2 and then decreases obviously.Keeping the pressure constant and increasing H2/CO are detrimental to water gas shift reaction.When the accumulated water vapor too late to translate,it is inevitable to restrain methanol synthesis reaction and the growth of CO conversion slow down.Indeed,superfluous water vapor would also inhibit the methanol dehydration reaction,which leads to reduction of DME and CO2selectivity.3.2.4.GHSV
The reaction contact time of syngas and catalyst is shortened due to the increasing space velocity with the increase of space velocity,which reduces the selectivity of DME and CO2and the CO conversion,and increases the selectivity of methanol(Fig.5).The increasing selectivity of methanol owes to the untimely completion of methanol dehydration.From the thermodynamic point of view,increasing space velocity has little influence on water–gas shift reaction[26],and the methanol dehydration reaction and the water–gas shift reaction always maintain equilibrium.As a consequence,both the selectivity of DME and CO2fell off in the same range.
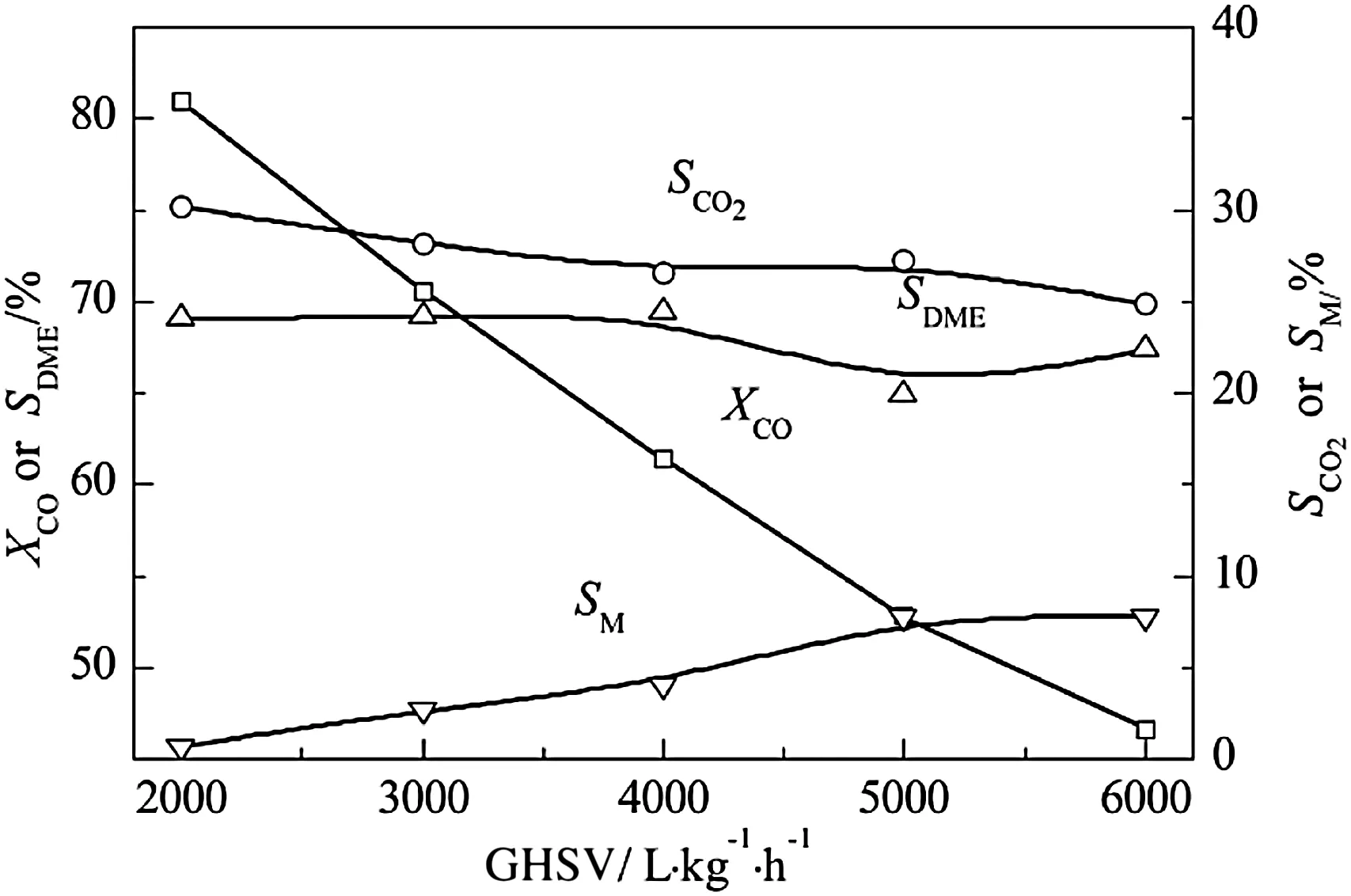
Fig.5.Effect of GHSV on CeO2–CaO–Pd/HZSM-5 catalytic properties.Reaction condition:T=300°C,p=3.0 MPa,n H2/n CO=2.0.
3.3.Kinetic mechanism and model
Kinetic model of bifunctional catalyst particles CeO2–CaO–Pd/HZSM-5 is established under the conditon(240–300 °C,3–4 MPa,nH2/nCO=2:1 and space velocity 2000–6000 L·kg-1·h-1)based on the above experimental results of technological conditions.
3.3.1.Kinetics experimental conditions
As exhibited in Fig.6(a),CO conversion remains stable when the particle size of the catalyst is less than 0.6 mm,which implies that the effect of internal diffusion has been excluded.Fig.6(b)demonstrates the effect of external diffusion on CO conversion by changing space velocity under the loading of 0.5 g and 0.7 g,respectively.When the space velocity is less than 6 × 10-4kg·h·L-1,namely more than 1600 L·kg-1·h-1,CO conversion of the two kinds of catalysts loading are basically the same,indicating the influence of external diffusion has been eliminated.
3.3.2.Kinetics reaction model building
According to the actual situation,some assumptions made as follows in order to simplify the reaction kinetic equation:
1.CO is the main raw material for the production of methanol,methanol synthesis from CO2and other side effects are ignored.Thus,the reaction system contains three reactions[27]:
Methanol synthesis from syngas:
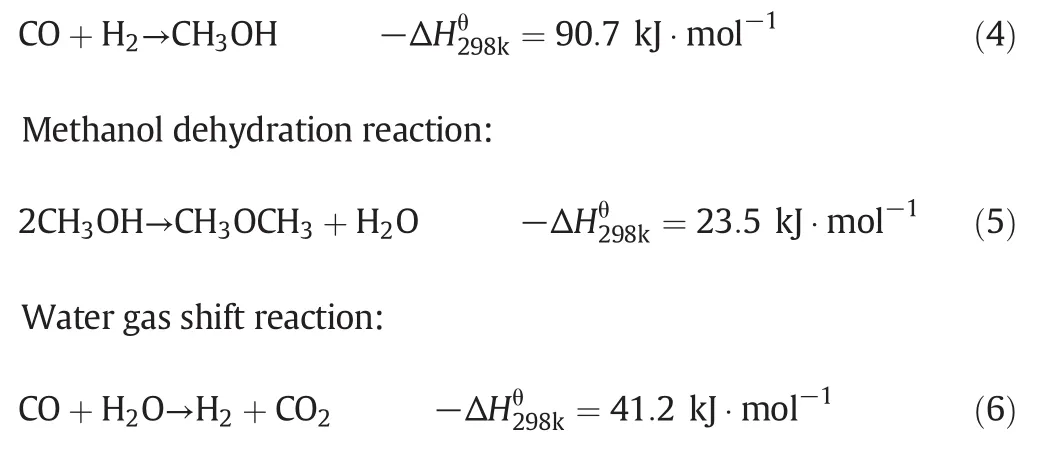
2.Metal Pd is the active center of methanol synthesis and water gas shift reaction.By contrast,HZSM-5 surface is the active center of the methanol dehydration reaction.
3.The water gas shift reaction is rapid equilibriumprocess,the reaction is always in an equilibrium state.
4.The surface reaction of the adsorbed species is identified as the controlling step of the reaction,and the adsorption and desorption are in an equilibrium state.
3.3.2.1.Surface reaction mechanism and rate equation of methanol synthesis.Methanol synthesis and water gas shift reaction occur mainly on Pd surface.There are chiefly two active centers on the catalyst,C1is adsorption center of CO,CO2,CHO,CH2O,CH3O and CH4O,C2is the adsorption center of H2,H and H2O.The possible mechanisms of the reaction are as follows:

In the above process,the adsorptions are all weak adsorption except for CO,CO2,H2and H2O.Taking Formula(e)as rate controlling step for the synthesis of methanol and water gas shift reaction,the other steps are considered as equilibrium.The rate equation for the methanol synthesis reaction can be deduced.

3.3.2.2.Surface reaction mechanism and rate equation of methanol dehydration.Methanol dehydration reaction occurs mainly on HZSM-5 surface,and primary adsorption species on the adsorption center(HX)are CH3OH,CH3+and CH3OHCH3+.The reaction mechanism proceeds according to the Langmuir–Hinshelwood type of molecules adsorption[28].
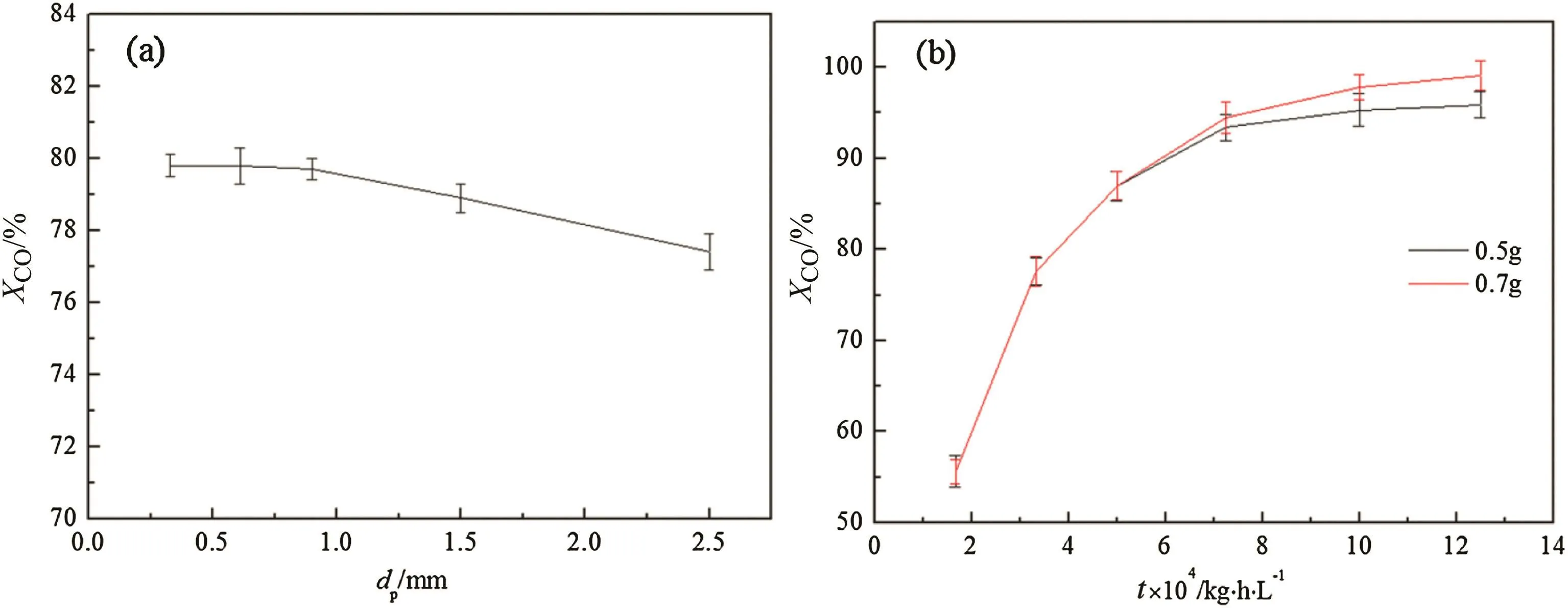
Fig.6.Effect of(a)catalyst particle size and(b)space–time on CO transforming-rate.Reaction condition:T=300 °C,p=3.0 MPa,n H2/n CO=2:1.
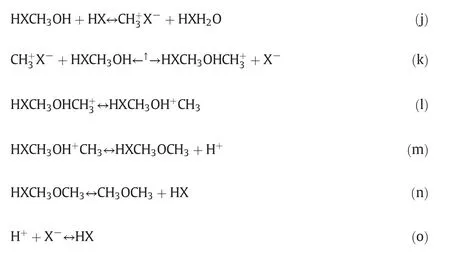
The rate controlling step for the dehydration reaction of methanol is described by Formula(k),the other steps are considered as equilibrium.The following expression is used to derive the rate equation for the dehydration of methanol based on Langmuir–Hinshelwood mechanism[28,29]:.

Herek11andk21in Formulas(4)and(5)are reaction rate constant to be solved.Equilibrium constantsKeqm1andKeqm2are the function of temperature as follows:
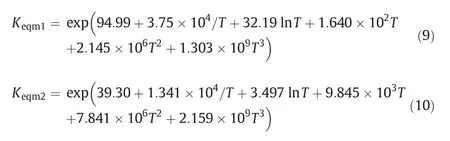
The model contains two rate constants and eight adsorption constants,and each constant could be expressed by the Arrhenius equation and the Van't Hoff equation.Twenty parameters contained in the model are to be confirmed.

3.3.3.Kinetics experiment data
The data in Table 1 are used to calculate partial pressure.

Table 1Results of kinetics experiment
3.3.4.Parameter estimation
According to the assumption,the two independent reactions of Formulas(4)and(5)exist in the system.Taking M and DME as the key components,CO conversion is labeled asXCO.The mole fractions of Mand DME are expressed in terms ofYMandYDME,respectively.Material balance calculation of reaction layer volume element is conducted.

Plug Eqs.(22)and(23)into Eq.(20)and Eq.(21)respectively:


Numerical integration of the Formulas(24)and(25)are calculated by using Runge–Kutta method[30,31],which obtains the reaction rate of outlet key component.And then the parameter estimation of the model is performed with the simplex method[32].The model parameters obtained are shown in Table 2.
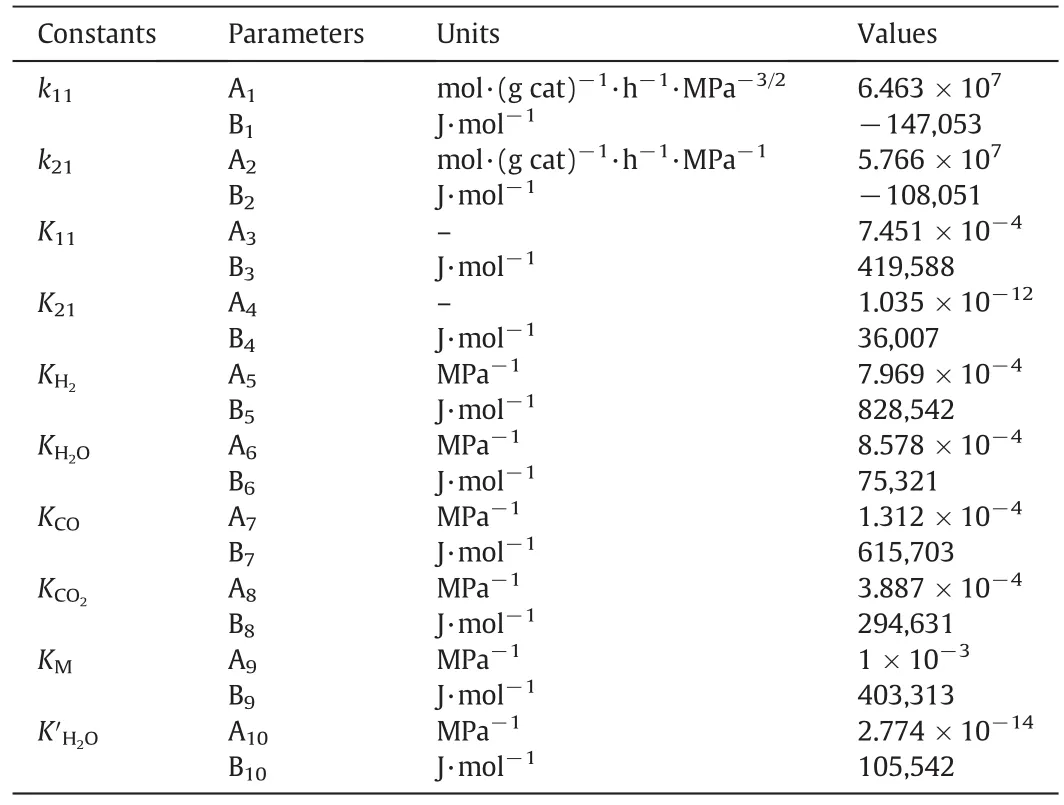
Table 2Kinetic parameters for dimethyl ether synthesis
3.3.5.Model validation
In order to investigate the fitting ability of the model,the relative error of outlet key component concentration CO,CH3OH and DME between the experimental and calculated date is plotted in Fig.7.It could be clearly seen that the relative errors of CO and DME are all less than 5%while the relative error of methanol is less than 11%except for a few points.According to the information gathered above,we may reach the conclusion that the kinetic model could be well correlated with experimental data for DME one-step synthesis catalyzed by CeO2–CaO–Pd/HZSM-5.
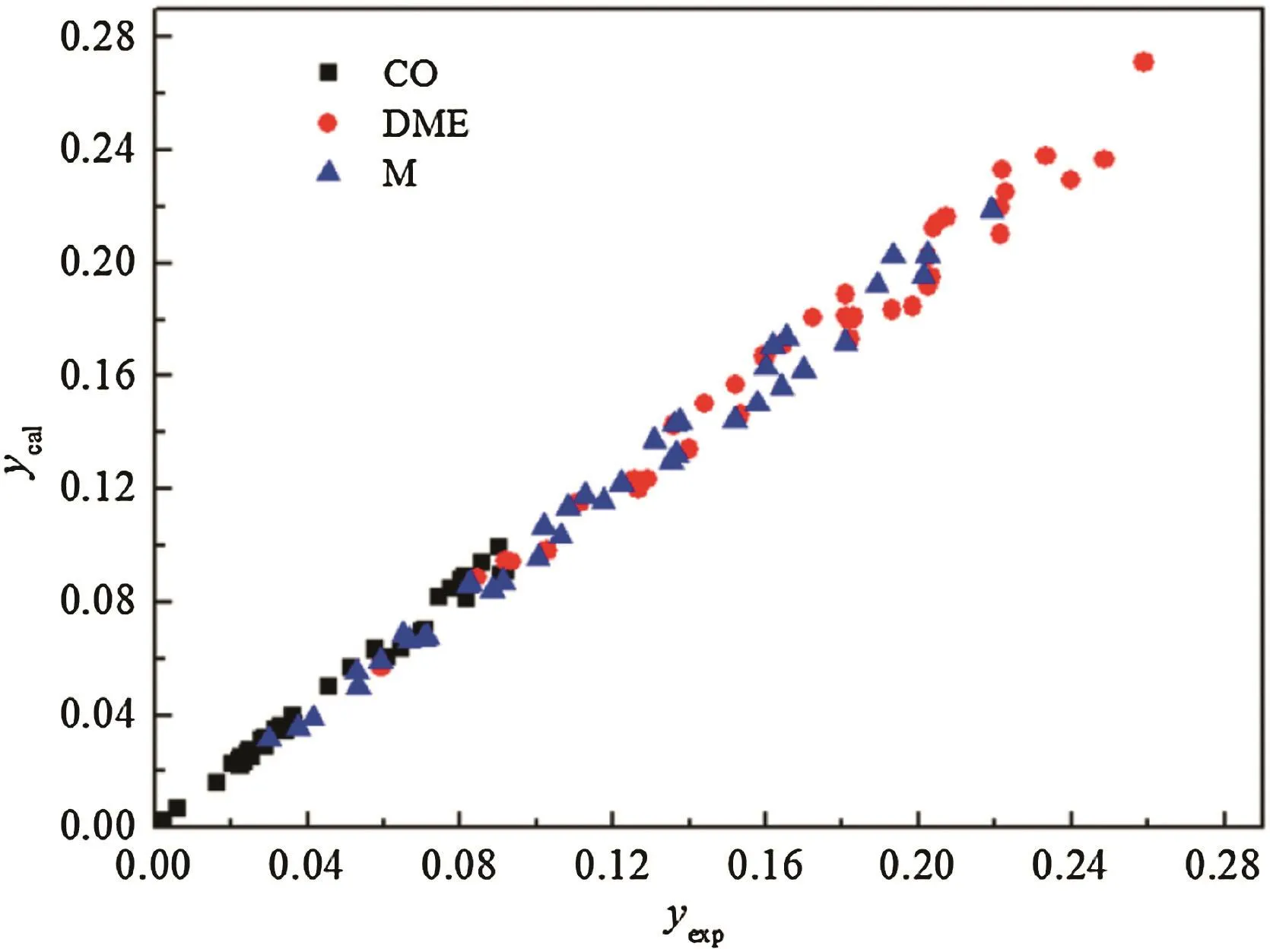
Fig.7.Mole fraction ofCO,CH3OH(M)and DME between the experimental and calculated date.
4.Conclusions
In the present work,the nano-sized CeO2–CaO–Pd/HZSM-5 catalyst exhibits excellent catalytic stability for the reaction of sulfur-containing syngas to dimethyl ether.The optimal technological conditions for DME one-step synthesis reaction are 240–300 °C for temperature,3–4 MPa for pressure,2000–3000 L·kg-1·h-1for space velocity and 2–3 for H2/CO mole ratio.Noble metal Pd is the active center of methanol synthesis and water gas shift reaction,while the HZSM-5 surface is the active center of the methanol dehydration reaction that has been assumed.Based on the assumptions,the optimal technological conditions were used to establish kinetic equation of hybrid catalyst of CeO2–CaO–Pd/HZSM-5.By combining the simplex method and Runge–Kutta method to investigate regression parameters,statistical tests show that the model is reliable,and the calculated values are in good agreement with the experimental data.
Nomenclature
A pre-exponential factor,mol·(g cat)-1·h-1·MPa-3/2,mol·(g cat)-1·h-1·MPa-1,MPa-1
B negative value of activation energy,J·mol-1
Ciadsorption center
dpcatalyst particle size,mm
Fimolar flow,mol·h-1
K adsorption constant
Keqmequilibrium constants
kreaction rate constant
ppressure,MPa
Rmolar gas constant,J·mol-1·K-1
Siselectivity of species i,%
STYDMEDME space–time yield,mmol·g-1·h-1
Ttemperature,°C
tspace–time,kg·h·L-1
wmass of catalyst,g
XCOCO conversion,%
Yimole fractions of speciesi yiconstitution of tail gas
γ reaction rate,mol·g-1·h-1
standard molar reaction enthalpy,kJ·mol-1
τ reaction time,h
Superscripts
Mmethanol
[1]A.V.Pattekar,M.V.Kothare,A microreactor for hydrogen production in micro fuel cell applications,J.Microelectromech.Syst.13(2005)7–18.
[2]R.Retnamma,A.Q.Novais,C.M.Rangel,Kinetics of hydrolysis of sodium borohydride for hydrogen production in fuel cell applications:A review,Int.J.Hydrog.Energy36(2011)9772–9790.
[3]N.Jamsran,O.Lim,A study on the autoignition characteristics of DME-LPG dual fuel in HCCI engine,Heat Transf.Eng.1–38(2016).
[4]C.Arcoumanis,C.Bae,R.Crookes,E.Kinoshita,The potential of di-methyl ether(DME)as an alternative fuel for compression–ignition engines:A review,Fuel87(2008)1014–1030.
[5]K.Sato,Y.Tanaka,A.Negishi,T.Kato,Dual fuel type solid oxide fuel cell using dimethyl ether and liquefied petroleum gas as fuels,J.Power Sources217(2012)37–42.
[6]Y.Y.Zhu,S.R.Wang,X.L.Ge,Q.Liu,Z.Y.Luo,K.F.Cen,Experimental study of improved two step synthesis for DME production,Fuel Process.Technol.91(2010)424–429.
[7]Y.Han,H.Zhang,Modeling and simulation of production process on dimethyl ether synthesized from coal-based syngas by one-step method,Chin.J.Chem.Eng.17(2009)108–112.
[8]F.S.Ramos,A.M.D.D.Farias,L.E.P.Borges,J.L.Monteiro,M.A.Fraga,E.F.Sousa-Aguiar,L.G.Appel,Role of dehydration catalyst acid properties on one-step DME synthesis over physical mixtures,Catal.Today101(2005)39–44.
[9]A.García-Trenco,A.Martínez,The influence of zeolite surface-aluminum species on the deactivation of CuZnAl/zeolite hybrid catalysts for the direct DME synthesis,Catal.Today227(2014)144–153.
[10]S.Papari,M.Kazemeini,M.Fattahi,Modelling-based optimisation of the direct synthesis of dimethyl ether from syngas in a commercial slurry reactor,Chin.J.Chem.Eng.21(2013)611–621.
[11]G.R.Moradi,S.Nosrati,F.Yaripor,Effect of the hybrid catalysts preparation method upon directsynthesis of dimethyl ether from synthesis gas,Catal.Commun.8(2007)598–606.
[12]J.H.Flores,D.P.B.Peixoto,L.G.Appel,R.R.D.Avillez,M.I.P.D.Silva,The influence of different methanol synthesis catalysts on direct synthesis of DME from syngas,Catal.Today172(2011)218–225.
[13]D.Feng,Y.Zuo,Steam reforming of dimethyl ether over coupled catalysts of CuO–ZnO–Al2O3–ZrO2and solid-acid catalyst,Chin.J.Chem.Eng.17(2009)64–71.
[14]R.Z.Chu,Controllable preparation of sulfur-tolerant Pd catalysts under microwave irradiation and application of these catalysts in one-step synthesis of dimethyl ether,J.China Coal Soc.37(2012)711–712(in Chinese).
[15]G.Pop,G.Bozga,R.Ganea,N.Natu,Methanol conversion to dimethyl ether over H-SAPO-34 catalyst,Ind.Eng.Chem.Res.48(2009)7065–7071.
[16]W.Z.Lu,L.H.Teng,W.D.Xiao,Simulation and experiment study of dimethylether synthesis from syngas in a fluidized-bed reactor,Chem.Eng.Sci.59(2004)5455–5464.
[17]L.Zhang,H.Zhang,W.Ying,D.Fang,Dehydration of methanol to dimethyl ether over γ-Al2O3,catalyst:Intrinsic kinetics and effectiveness factor,Can.J.Chem.Eng.91(2013)1538–1546.
[18]R.Z.Chu,Z.C.Zhang,Y.F.Liu,X.L.Meng,Z.M.Zong,X.Y.Wei,Study on preparation and catalytic properties of Pd/γ-Al2O3catalysts in one-step synthesis of dimethyl ether,Appl.Mech.Mater.66–68(2011)1404–1409.
[19]X.L.Meng,R.Z.Chu,B.Qin,E.W.Yue,T.T.Chen,X.Y.Wei,Influence of compound additive CeO2–MxOyon catalytic performance of Pd/γ-Al2O3catalyst for one-step synthesis of dimethyl ether,Energy Sources Part A37(2015)870–877.
[20]R.Z.Chu,X.L.Meng,X.Y.Wei,Z.M.Zong,Z.C.Zhang,Y.F.Liu,Preparation Method of Dual Function Type Palladium Based Catalyst Under Microwave Condition,CN102389793A,2012.
[21]R.Z.Chu,T.T.Xu,X.L.Meng,G.G.Wu,Mechanism of reaction of CeO2–CaO–Pd/HZSM5 catalyst in the syngas process in the presence of sulfur containing impurities,Prog.React.Kinet.Mech.41(2016)235–244.
[22]Y.Ma,Q.Ge,W.Li,H.Xu,Methanol synthesis from sulfur-containing syngas over Pd/CeO2catalyst,Appl.Catal.B Environ.90(2009)99–104.
[23]X.Li,X.W.Wang,M.Zhao,J.Y.Liu,M.C.Gong,Y.Q.Chen,Chin.J.Catal.32(2014)1739–1746.
[24]S.Naito,T.Kasahara,T.Miyao,Transformation of methane formation sites into methanol formation ones during CORH2,reaction over Pd/CeO2in its SMSI state,Catal.Today74(2002)201–206.
[25]H.J.Chen,C.W.Fan,C.S.Yu,Analysis,synthesis,and design of a one-step dimethyl ether production via a thermodynamic approach,Appl.Energy101(2013)449–456.
[26]A.Basile,S.Curcio,G.Bagnato,S.Liguori,S.M.Jokar,A.Lulianelli,Water gas shift reaction in membrane reactors:Theoretical investigation by artificial neural networks model and experimental validation,Int.J.Hydrog.Energy40(2015)5897–5906.
[27]G.R.Moradi,J.Ahmadpour,F.Yaripour,J.Wang,Equilibrium calculations for direct synthesis of dimethyl ether from syngas,Can.J.Chem.Eng.9999(2011)1–8.
[28]S.J.Royaee,C.Falamaki,M.Sohrabi,S.S.A.Talesh,A new Langmuir–Hinshelwood mechanism for the methanol to dimethyl ether dehydration reaction over clinoptilolite–zeolite catalyst,Appl.Catal.A Gen.338(2008)114–120.
[29]L.Tong,L.Chen,Y.Ye,Z.Qi,Kinetic studies on the dimerization of isobutene with Ni/Al2O3as a catalyst for reactive distillation process,Chin.J.Chem.Eng.23(2015)520–527.
[30]G.Tasi,D.Barna,Analytical and numerical computation of error propagation of model parameters,J.Math.Chem.49(2011)1322–1329.
[31]M.Poorabdollah,M.H.Beheshty,M.Vafayan,Kinetic modeling of nanoclay reinforced unsaturated polyester resin,Polym.Compos.32(2011)1265–1273.
[32]G.Dimarco,L.Pareschi,Exponential Runge–Kutta methods for stiff kinetic equations,SIAM J.Numer.Anal.49(2010)2057–2077.
 Chinese Journal of Chemical Engineering2016年12期
Chinese Journal of Chemical Engineering2016年12期
- Chinese Journal of Chemical Engineering的其它文章
- Hemicellulose in corn straw:Extracted fromalkali solution and produced 5-hydroxymethyl furfural in HCOOH/HCOONa buffer solution☆
- Molar volume of eutectic solvents as a function of molar composition and temperature☆
- Investigation on molar heat capacity,standard molar enthalpy of combustion for guaiacol and acetyl guaiacol ester
- Statistical mechanics and artificial intelligence to model the thermodynamic properties of pure and mixture of ionic liquids☆
- A comprehensive fractal char combustion model☆
- Development of a bifurcation analysis approach based on gPROMS platform☆
