Alteration of intracellular density is a pathological response in adrenaline-induced acute pulmonary edema in mice*
LIU Min, SHI Qin-qin, FU Han, WEI Jian-ge, LI Ju-xiang, ZHONG Ming, LIU Yu△
(1Department of Biochemistry, School of Medicine, Jinan University, Guangzhou 510632, China;2Vitalstrategic Research Institute, Shanghai 200041, China; 3Clinical Laboratory Center, TheFirst Affiliated Hospital of Jinan University, Guangzhou 510632, China; 4Dawnlight BiotechCompany, Guangzhou 510530, China. E-mail: xyuliu05@126.com)
Alteration of intracellular density is a pathological response in adrenaline-induced acute pulmonary edema in mice*
LIU Min1, SHI Qin-qin1, FU Han1, WEI Jian-ge2, LI Ju-xiang3, ZHONG Ming4, LIU Yu1△
(1DepartmentofBiochemistry,SchoolofMedicine,JinanUniversity,Guangzhou510632,China;2VitalstrategicResearchInstitute,Shanghai200041,China;3ClinicalLaboratoryCenter,TheFirstAffiliatedHospitalofJinanUniversity,Guangzhou510632,China;4DawnlightBiotechCompany,Guangzhou510530,China.E-mail:xyuliu05@126.com)
[ABSTRACT]AIM: To investigate the change of intracellular density as a pathological response in the lungs. METHODS: A mouse model of acute pulmonary edema (APE) was induced by overdose of adrenaline. The intracellular density alteration was determined by discontinuous sucrose gradient centrifugation of the lung homogenates. Lung wet/dry weight ratio was measured to evaluate the development of APE. Glycogen content, and the mRNA expression of IL-1β and IL-6 in the liver were quantitatively determined.RESULTS: After injection of adrenaline, change of intracellular density was observed in the lungs in a time- and dose-dependent manner, and this change was found to be significantly correlated with the lung wet/dry weight ratio. The mRNA levels of IL-1β and IL-6 in the liver significantly increased, with a peak at 1 h after injection of adrenaline.CONCLUSION: Alteration of intracellular density in the lungs is a pathological response, which can be induced by overdose of adrenaline. This pathological change could be used in the localization of tissues/cells targeted by drugs and in the study of pathogenesis in vivo.
[KEY WORDS]Acute pulmonary edema; Mice; Adrenaline
In previous studies, we employed discontinuous sucrose gradient centrifugation (DSGC) to separate cultured cells, in order to investigate the cells with diffe-rent sensitivities to drugs. Cultured human chronic myeloid leukemia cells K562 were separated by DSGC into 2 bands, one of which was located between the sucrose layers of 40% and 50% (W/V), and the other was between 50% and 60% (W/V). For convenience, a DSGC band of cells was named as “S” (for sucrose) with a number for the sucrose concentration of the layer on which the band stops. Therefore, the 2 bands of K562 cells by DSGC separation were named as “S50” and “S60”, respectively, and this nomination was also used for other bands of cells separated by DSGC. It was found that the number and position of the bands of K562 cells in DSGC changed when the culture conditions were changed or the cells were incubated with drugs or chemicals, resulting in disappearance of a normal band or appearance of a new band. This change of cells determined by DSGC, caused by drugs, pathological or environmental factors, was named as “density alteration in non-physiological cells” (DANCE)[1], since the change was in fact a result of the change of intracellular density of the cells in the band[2-3]. Later studies found that different DSGC bands of the cells from the same source were also different in expression of some genes[1, 4]. In addition, DANCE was not only found in cultured cells and microorganisms such as bacteria and fungi, but also found in tissue cells in the body. For example, transplantation of S180 tumor cells into mice caused significant DANCE response in the liver, suggesting that DANCE in the liver was a pathological event involved in tumor-body interaction[5]. However, whether DANCE can be caused in the body by drugs is not known. In the present study, we established a mouse model of acute pulmonary edema (APE) with overdose of adrenaline, in order to study DANCE in the lungs as a new pathological response to the drug in vivo.
APE is an acute disease resulted from fast and systemic pathological changes, especially in the lungs, mainly due to the accumulation of fluid in the air spaces and parenchyma of the lungs, which may cause severe symptoms such as acute hypoxia, respiratory distress, cardiac arrest and even death[6]. Clinically the most common cause of APE is heart failure or heart attack[7]; others may include hypertensive crisis, renal failure, infections, drugs, etc[8]. Drug-induced APE has been getting more and more attentions in clinics, since improper use of some drugs may cause severe APE[9], such as adrenaline, nifedipine, nicardipine, ethchlorvynol, etc[10-12]. Of the drugs that may cause APE, adrenaline is a well-known APE inducer, which is often used to establish animal model of APE[13]. Adrenaline acts on both alpha- and beta-adrenergic receptors of tissue cells, stimulates the heart to increase output, raises the blood pressure, and promotes glyco-genolysis in the liver[14]. The quick increase of blood pressure in the pulmonary circulation causes efflux of liquid from blood vessels into the air spaces and parenchyma of the lungs, and finally results in pathological changes of APE[15]. As a result of accumulation of fluid in the lungs, the wet/dry weight ratio of the lungs increases, and therefore, lung wet/dry weight ratio is often used to evaluate the development and severity of APE in animal studies.
MATERIALS AND METHODS
1Animals
The Kunming mice used in the current study were SPF grade, purchased from the Medical Laboratory Animal Center of South China Medical University [license number: SCXK(YUE)2011-0015], weighing 20.5~24.7 g. The mice were housed in the Laboratory Animal Center of Jinan University, with 3~5 mice per cage. The temperature of the animal rooms was controlled in the range of 24~26 °C. The mice were given free access to water and the standard mouse feeds provided by the Medical Laboratory Animal Center of South China Medical University, China.
2Reagents
Sucrose (C12H22O11, with a molecular weight of 342.3) was purchased from Sigma Chemicals Incorporation. HE staining kit was purchased from Beijing Labest Biotech Company. Sterile saline was purchased from the drug store of Oversea-Chinese Hospital, Guangzhou, China. Adrenaline hydrochloride (1 g/L) was purchased from Guangzhou Baiyun Shan Ming Xing Pharmaceutical Company. TRIzol and FastQuant cDNA kit were purchased from Invitrogen Biotech Company. SuperReal PreMix Plus (SYBR Green) was purchased from Tiangen Biotech Company. RPMI-1640 and fetal bovine serum were purchased from Gibco-BRL (Gaithersburg).
3Experimental methods
3.1Time-effect studyThe mice (n=119) were randomly divided into 9 experimental groups, including 1 control group (n=7) and 8 test groups (n=14, 2 of which were used for control, 6 for DANCE, biochemical and histological determinations, and other 6 for lung wet/dry weight ratio determination). The 8 test groups were labeled according to different time points, i.e. 0.5, 1, 2, 4, 6, 8, 12 and 16 h, respectively. The 7 mice in control group were not given any treatment. The 2 mice in each test group used for control were given saline at a single dose of 0.1 mL each mouse, and the other 12 mice in each test group were given adrenaline at a single dose of 5.0 μg/g body weight, via intrape-ritoneal injection. The 7 mice in control group were euthanized at the time point of 0 h, while those in the test groups (including 2 mice per group for control) were euthanized at different time points accordingly. The li-ver and the lungs of each mouse were harvested and washed in saline. The 7 mice in control group were used only for DANCE determination. One of the 2 mice for control in each test group was combined together as a control group for the lung wet/dry weight ratio mea-surement; another one was used as a control for DANCE determination of each test group, and for other determinations including real-time PCR, histological and biochemical determinations at each time point.
3.2Dose-effect studyA total of 49 mice were randomly divided into 4 experimental groups, including 1 control group (n=13, 7 of which for DANCE, biochemical and histological determinations and other 6 for lung wet/dry weight ratio determination), and 3 test groups (n=12, 6 of which for DANCE, biochemical and histological determinations and other 6 for lung wet/dry weight ratio determination). The 3 test groups were labeled according to the adrenaline doses, i.e. 2.5, 5.0 and 10.0 μg/g body weight, respectively. The mice in control group were given 0.1 mL saline each mouse, and those in each test group were given a single dose of adrenaline at different concentrations to reach the final doses as described above, respectively, via intraperitoneal injection. All mice were euthanized exactly 2 h after the drug injection. The liver and lung tissues were collected and analyzed as described previously.
Because both time-effect and dose-effect studies were performed in the same batch of mice and in the same experimental period, the test group at 2 h with adrenaline at dose of 5.0 μg/g body weight was used for both studies.
3.3Determination of DANCEAfter completion of the animal experiment, the mice were euthanized and the lung and liver tissues were collected. The right lung and the upper robe of the left lung of each mouse were cut into pieces and homogenized in 400 μL of saline with 25 vertical movements of the glass pestle (the lower robe of the left lung was used for histological examination). The lung homogenates were used for DANCE determination immediately after they were prepared.
Discontinuous sucrose gradient was prepared in a glass tube with 1.0 cm in diameter and 10 cm in length. Briefly, sucrose was dissolved in distilled water to make a series of concentrations: 30, 40, 50, 60 and 70% (W/V). 560 μL of each sucrose solution was gently added into the glass tube, in a sequence from high to low concentrations, to form 5 discontinuous layers with the layer of 70% at the bottom and that of 30% at the top. 200 μL of the lung tissue homogenate was carefully loaded on the top of the sucrose gradient, and the tubes were centrifuged at 1 600×gfor 5 min. The bands of the test samples separated by DSGC were respectively compared to those of the control. When the amount of a band of the test sample was 75% less than that of the control or even disappeared, it was identified as “DANCE”.
When the bands were used for microscopic examination, each band was carefully collected with a suction pipe, washed once in saline and centrifuged to remove the sucrose. The cell pellet was re-suspended in 100 μL saline, and the cell mixture was used to prepare smear glass slides for HE staining according to the instructions provided by the manufacturer.
3.4Histological examination of lung tissuesAfter completion of the animal experiment, the lower robe of the left lung was cut immediately, examined for visible lesions, and then inflated with 10% formaldehyde. The lung tissue was embedded in paraffin and then cut into 5-μm-thick sections. The sections were stained with hematoxylin and eosin, and the images of the slides were taken with a computer-equipped inverted microscope (Mshot MF 52) at a magnification of ×200.
3.5Determination of lung wet/dry weight ratioAPE in mice induced by overdose of adrenaline was assessed by the measurement of lung wet/dry weight ra-tio[15-16]. The lungs of each mouse were harvested, immediately blotted with filter paper, weighed for the wet weight of the lungs, dried in a 65 ℃ stove for 7 d, and then weighed again for the dry weight of the lungs. The lung wet weight to dry weight ratio was calculated for evaluation of the drug-induced APE.
3.6Glycogen determination in the mouse liverGlycogen content of the liver was measured by the anthrone method of van der Vies[17]. Briefly, 50 mg of hepatic tissue from the right robe of the liver was homogenized in 5 mL of 5% trichloracetic acid (TCA) and centrifuged at 6 000×gfor 10 min. The supernatant was collected and 1.0 mL of 10 mol/L KOH was added. The mixture was boiled for 60 min and neutralized with 0.5 mL glacial acetic acid. The mixture was then diluted with distilled water to a final volume of 10 mL, and 1.0 mL of the mixture was added to 2.0 mL of freshly prepared anthrone reagent (2 g/L anthrone in 18 mol/L H2SO4). Mix and heat the samples in a boi-ling-water bath for 10 min. The samples with green-brown color were measured colorimetrically at 650 nm against the blank (prepared simultaneously using 1.0 mL of 5% TCA instead of tissue sample). The absorbance was compared with the glucose standard of diffe-rent concentrations and the liver glycogen contents were calculated as mg/g liver tissue.
3.7Real-time PCR analysis of IL-1β and IL-6Total RNA was extracted from the hepatic tissue with TRIzol, and the first strand of cDNA was synthesized using FastQuant cDNA kit following the manufacturer’s instructions. 1.0 μL cDNA (80 ng) was used for quantitative real-time PCR analysis for the mRNA levels of IL-1β and IL-6 in the liver. The primers for IL-1β were 5’-GCC ACC TTT TGA CAG TGA TGA G-3’ (forward) and 5’-ATG TGC TGC TGC GAG ATT TG-3’ (reverse); the primers for IL-6 were 5’-AGT GGC TAA GGA CCA AGA CC-3’ (forward) and 5’-ATA ACG CAC TAG GTT TGC CG-3’ (reverse); and the primers for the internal control β-actin were 5’-TGT TAC CAA CTG GGA CGA CAT G-3’ (forward) and 5’-CTG GAT GGC TAC GTA CAT GGC T-3’ (reverse). Quantitative real-time PCR was performed under the following conditions: 1.0 μL of cDNA (80 ng) was mixed with 10 μL of 2×PCR SuperReal PreMix Plus, 0.5 μL of each primer (10 μmol/L) and 1.0 μL of 50×ROX reference dye, and RNase-free ddH2O was added to a final volume of 20 μL. Real-time PCR was run at 95 ℃ for 15 min, followed by 39 cycles as 95 ℃ for 10 s, 60 ℃ for 20 s, and 72 ℃ for 32 s. The relative mRNA levels of the 2 cytokines were calculated by the 2-ΔΔCtmethod, i.e. both mRNA levels of IL-1β and IL-6 were compared to the level of β-actin in each sample.
3.8Determination of DANCE in K562 cellsTo study DANCE in lung tissues directly caused by adrenaline, it may be better to use the lung-derived cell lines. However, most lung-derived cell lines (e.g. mouse lung fibroblasts, epithelial cell, and macrophages) are adherent cells and need trypsin digest or scratching in preparation, which is not suitable in this study. Here we selected a suspension cell line, human chronic myeloid leukemia cells (K562), to determine DANCE in cells directly caused by adrenaline. K562 cells were cultured in RPMI-1640 complemented with 10% fetal bovine serum, 5% CO2, 98% humidity at 37 ℃. The culture medium was changed every 2 d. For DANCE determination, K562 cells were normally cultured for 48 h and then harvested and washed twice in saline. The cells (1×109/L, 2 mL/well) were incubated in the culture medium with adrenaline at 0, 100, 200 or 400 mg/L. After 48 h of incubation, the cells were collected, and centrifuged at 500×gfor 5 min to remove the culture medium. The cells were re-suspended in 200 μL of PBS, and the cell mixtures were used for microscopic examination and DANCE determination as described above.
4Statistical analysis
Sigma Stat 8.0 software was used to calculate the means and standard deviations. One-way ANOVA was performed for the difference among the test groups and control group of different time points and different doses of adrenaline, and LSD-ttest was used for the comparison of the means between groups. Pearson correlation coefficients and P value were calculated for correlations between the parameters of different groups.
RESULTS
1DSGC separation of normal lung homogenates
Normal mouse lung homogenates were separated by DSGC into 4 bands, named S40 through S70 as shown in Figure 1 (S30 was the liquid phase of the sample). It was found that under microscope, the DSGC bands of the lung homogenates mainly consisted of tissue fragments and each of which consisted of a certain number of cells. Generally, no significant difference in cell morphology, cell number in the fragments, and tissue fragment size among the 4 DSGC bands was observed. It was found that among the 4 bands of the lung homogenates, S40 was the largest and band S70 was the smallest in amount of tissue fragments (Figure 1).
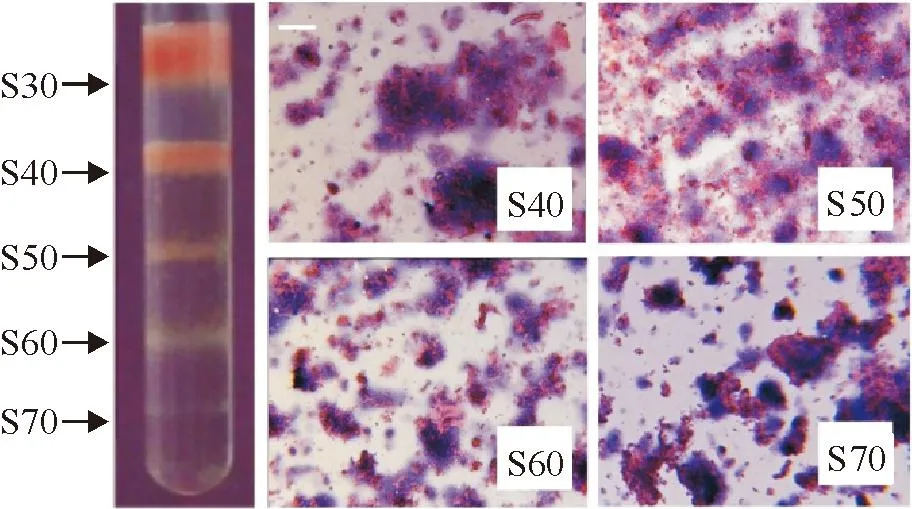
Figure 1.DSGC bands and the tissue fragments of the lung homogenates (HE staining, the scale bar=50 μm).
2DANCE in the lungs and the lung wet/dry weight ratio
In the mice intraperitoneally injected with adrenaline, DANCE in the lungs was positively determined by DSGC, with the band S40 disappeared or mostly reduced as compared with that of the control (Figure 2). Because no new band was found at the position of S30 (compared to the control), the results suggested that the original band S40 might move down, so called “DANCE-down”, as a result of increased mass density in the tissue fragments due to the pathological changes in the lungs. It was found that the incidence of DANCE in the lungs in each test group varied with the time after injection of the drug, with a peak (83%) at the time-point of 2 h and a recovery (0%) after 16 h. The result showed a typical time-dependent fashion (Figure 3A).
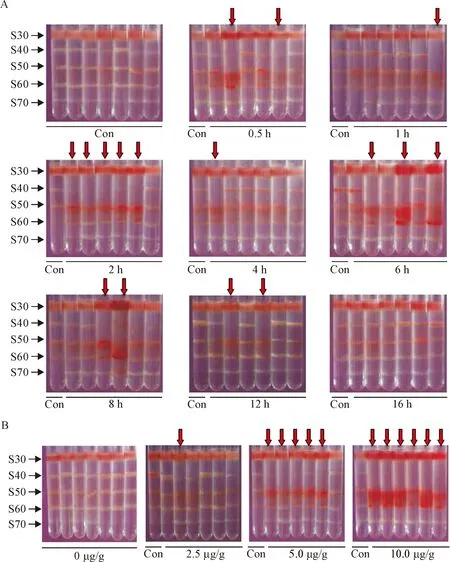
Figure 2.DANCE determined in the lungs after adrenaline injection. A: time-dependent change of DANCE in the lungs after a single dose of adrenaline at 5.0 μg/g body weight; B: dose-dependent change of DANCE in the lungs 2 h after injection of adrenaline. Con: control; arrows indicate DANCE in the lungs (band S40 decreased by 75% or more when compared to that of the control).
In the dose-effect experiments, we found that only 1 of the 6 drug-treated mice (17%) exhibited DANCE in the lungs when the dose of adrenaline was 2.5 μg/g body weight, but 5 mice (83%) were found for the dose of 5.0 μg/g body weight, and 6 mice (100%) for 10.0 μg/g body weight. Obviously, the incidence of DANCE in the lungs significantly increased with the dose of adrenaline, showing a good dose-dependent fashion (Figure 3B).
In the time-effect experiments, the lung wet/dry weight ratio was found significantly increased after injection of adrenaline, with a peak at the time point of 2 h and a recovery phase after 12 h (Figure 3D). Pearson correlation analysis showed that the DANCE incidence in the lungs was significantly correlated with the lung wet/dry weight ratio (P<0.05), with a correlation
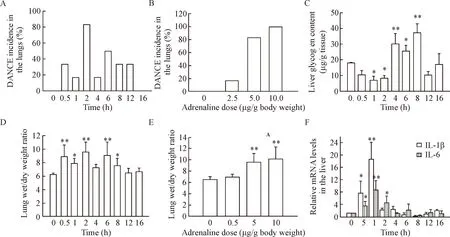
Figure 3.DANCE incidence in the lungs and pathological changes in the lungs and liver. A, C, D, F: time-dependent changes of DANCE incidence in the lungs, liver glycogen content, lung wet/dry weight ratio and cytokine mRNA levels in the liver, respectively, after adrenaline injection at a single dose of 5.0 μg/g body weight; B, E: DANCE incidence in the lungs and the lung wet/dry weight ratio, respectively, determined 2 h after adrenaline injection at different doses. Mean±SD. n=6.*P<0.05,**P<0.01 vs 0 h or 0 μg/g.
coefficient of 0.81 (Figure 4A). In addition, the dose-effect experiments showed that the lung wet/dry weight ratio significantly increased with the dose of adrenaline, exhibiting the highest level at the dose of 10.0 μg/g body weight (Figure 3E). Pearson correlation analysis showed that the DANCE incidence in the lungs was well correlated with the lung wet/dry weight ratio corresponding to the increase in adrenaline dose (P<0.01) (Figure 4B).
It is interesting to know that the red color was found in the bands S50 and/or S60 in almost all samples that exhibited DANCE (Figure 2). The red color was from red blood cells in the tissue mixtures of the bands, and the presence and density of the red color appeared to be proportional to the severity of the disease as evaluated by the lung wet/dry weight ratio measurement. Whether the blood cells in the tissues were rela-ted to DANCE in the lungs is not clear.
3Biochemical and molecular responses in the liver
Since liver is an important organ in response to adrenaline, and the metabolism changes of liver glycogen are indicative of physiopathology, we investigated the effects of overdose of adrenaline on hepatic glycogen content. The results showed that after injection of the drug at a single dose of 5 μg/g body weight, the liver glycogen content decreased first, significantly lower than the control group (P<0.05), to the lowest level of (7.05±0.60) mg/g wet tissue, forming a reverse peak at the time point of 1 h. Then the liver glycogen content increased, significantly higher than the control group (P<0.05), forming a normal peak of (37.19±5.78) mg/g wet tissue at the time point of 8 h (Figure 3C). These results suggested a time-dependent change in the liver glycogen content caused by the drug. When the DANCE incidence in the lungs was compared with the liver glycogen content, it was found that both had 2 peaks (the first peak of the liver glycogen content at 1 h was a reverse peak), but no significant correlation was found between the 2 pathological changes. It looks like that the change of the liver glycogen content was 1 h earlier than DANCE in the lungs (Figure 4C).
For the study of molecular response in the liver to overdose of adrenaline, real-time PCR was carried out to measure the mRNA levels of cytokines IL-1β and IL-6 in the mouse liver, using β-actin as an internal control. The melting peaks of β-actin, IL-1β and IL-6 were determined and satisfactory specificities were obtained (Figure 4E). The mRNA expression of both
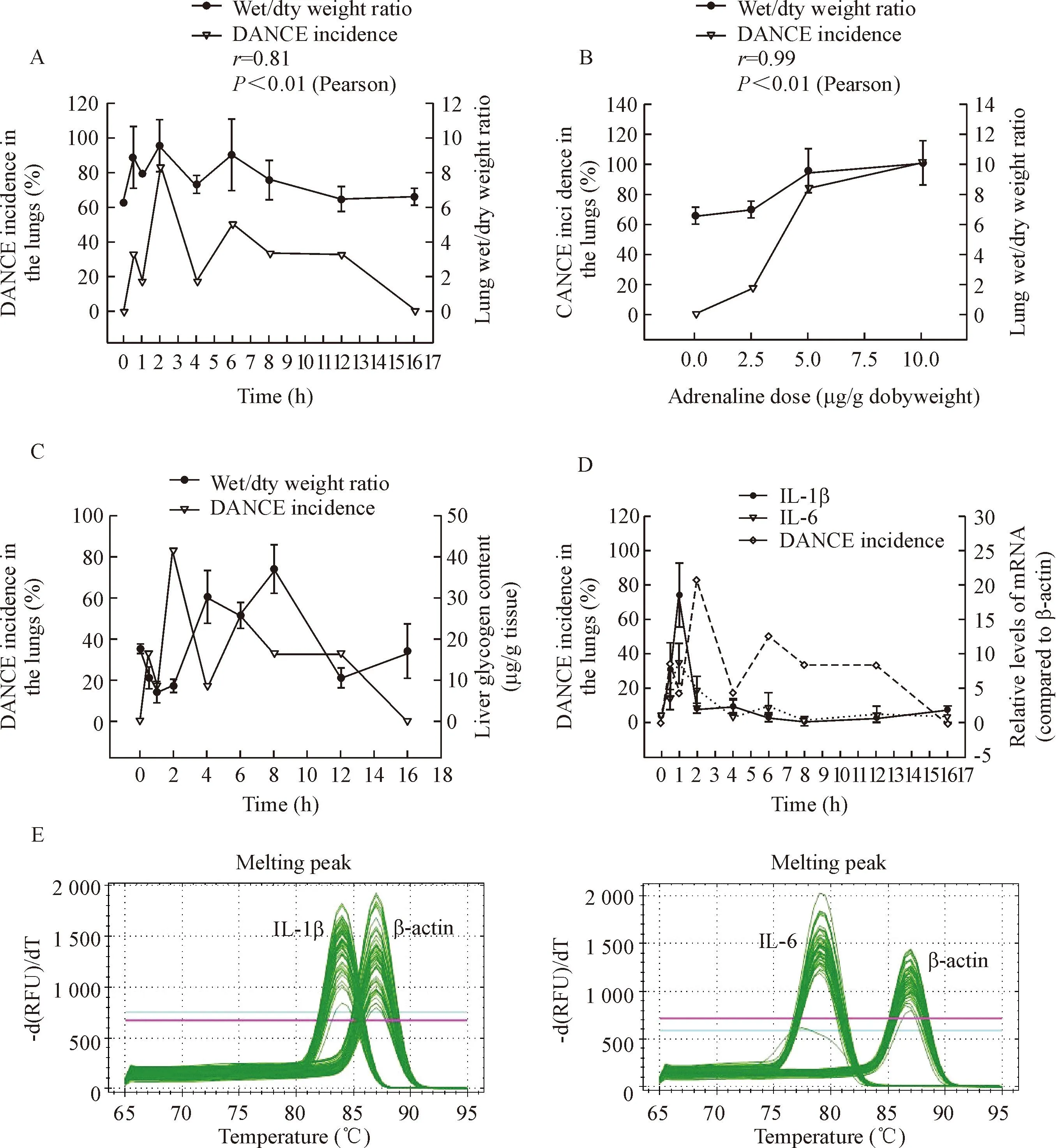
Figure 4.Correlation between DANCE incidence in the lungs and the pathological changes in the lungs and liver. A: DANCE incidence in the lungs and lung wet/dry weight ratio after injection of adrenaline at a single dose of 5.0 μg/g body weight; B: DANCE incidence in the lungs and lung wet/dry weight ratio 2 h after adrenaline injection at different doses; C: DANCE incidence in the lungs and the liver glycogen content; D: DANCE incidence in the lungs and mRNA expression of IL-1β and IL-6 in the liver after adrenaline injection at a single dose of 5.0 μg/g body weight; E: melting peaks of real-time PCR analysis for determination of IL-1β and IL-6 mRNA levels in the mouse liver after injection of adrenaline at a single dose of 5.0 μg/g body weight.
cytokines increased sharply soon after injection of adrenaline at a single dose of 5 μg/g body weight, reaching a peak at the time point of 1 h (Figure 3E). The peak level of IL-1β mRNA was found to be (18.60±5.73) folds, while that of IL-6 mRNA was (8.74±3.02) folds of that of β-actin, while both cytokines were not detectable in normal control group (P<0.05). The time-dependent changes of the mRNA expression of both IL-1β and IL-6 showed a short time of increase at 1 h, followed by a fast recovery to the baseline 4 h after the drug injection. Pearson correlation analysis showed that the changes of both cytokine expression in the liver were significantly correlated to each other, with a correlation coefficient of 0.895 (P<0.01) (Figure 4D).
DANCE incidence in the lungs was found to be not significantly correlated to the 2 proinflammatory cytokine levels in the liver. However, both cytokines exhibited their peaks at the time point of 1 h, the same as the first peak change of the liver glycogen content, indicating that the general biochemical and molecular responses to adrenaline in the liver were earlier than DANCE in the lungs, and both cytokines shared the same mode of mRNA expression in the liver (Figure 4D).
4Histological changes of the lungs
HE staining of the lung tissues showed that after injection of adrenaline at a single dose of 5 μg/g body weight, and at the time points of 0.5, 1 and 2 h, the mice had significant retention of blood cells in the lung tissues (Figure 5). This was even more severe in those lungs with DANCE detected. The severe retention of blood cells in the lung tissues was in good agreement with the presence of red color in the bands S50 and S60 in DANCE determination (Figure 2). However, whe-ther the severe retention of blood cells in the lung tissues promoted DANCE in the lungs is not known. Compared to the control, the air spaces (labeled as “A”) of the lungs in the mice injected with adrenaline were significantly reduced, while the parenchyma area (labeled “C”) were evidently enlarged, indicating a severe collapse of the lungs. However, these changes were not observed in the lungs at the time point of 16 h, suggesting a histological recovery in the lungs 16 h after the drug injection.

Figure 5.Microscopic graphs of the lung tissues stained with HE. A: air space (parts); B: arrows indicate accumulation of blood cells (parts); C: parenchymal tissues (parts). The scale bar=100 μm.
When the lungs with DANCE were compared with the lungs without DANCE in the same group given the drug at the same dose and at the same time point, the microscopic images of the lungs with DANCE appeared to be higher in the density of tissue structures, characterized by smaller air spaces, thicker parenchyma area, and higher retention of blood cells. This highly increased density of pulmonary tissue structures could be one of the mechanisms for DANCE in the lungs.
5Prediction of DANCE in the lungs for all time points
In the present experiments only a few of the time points were determined for DANCE in the lungs. We predicted the incidence of DANCE in the lungs for all time points based on the data obtained. From the first peak of DANCE incidence in the time course, which showed a duration of about 4 h (from 0.5~4.5), we hypothesize that the testable duration time of each DANCE in the lungs should be about 2 h (4/2). Putting the detected DANCE incidences and the hypothesized duration time of DANCE in the lungs together, we might be able to predict DANCE incidence for each time point as shown in Figure 5. The predicted curve for DANCE incidence in the lungs exhibited 3 peaks at 2, 6.5 and 12 h, respectively. However, each mouse might have two occurrences of DANCE in the lungs through the experiment (Figure 6).

Figure 6.Prediction of the number per group of mice with DANCE in the lungs at different time points. A: predicted and detected curves for the number per group of mice with DANCE in the lungs based on C; B: based on the detected numbers, DANCE (bars in red) in the lungs was predicted to occur and continue for 2 h (hypothesized) during the experiments; C: predicted number per group of mice with DANCE in the lungs at different time points based on B.
6DANCE in cultured cells induced by adrenaline
To avoid irritations such as trypsin digest or scratching before DSGC, we selected a suspension cell line K562 to study DANCE in the cells directly induced by adrenaline. K562 cells were incubated with adrenaline at the concentrations of 0, 100, 200 and 400 mg/L. When the drug reached 400 mg/L, the cells exhibited “DANCE-up” response, with band S60 disappeared instead of the band S40 as seen in the lungs. Microscopic examination showed that when the drug reached 200 mg/L, a significant number of K562 cells died, leaving visible cell debris, which were found even more at 400 mg/L (Figure 7), suggesting that DANCE was directly caused by adrenaline in cultured cells. However, as a response to adrenaline, DANCE in the cultured cells was different from that in the lungs, and the latter might be not from the direct interaction between the lung tissues and the drug.
DISCUSSION
In this study, we found that DANCE was induced by drug in vivo. Our experiments suggested that DANCE in the mouse lungs induced by overdose of adrenaline was a pathological response. However, the molecular mechanisms for DANCE in the lungs are not clear. Based on the present study, we would like to raise the following points for discussion:

Figure 7.DANCE in the K562 cells (A) and the microscopic changes (B) caused by adrenaline (×400). The hollow arrow indicates DANCE in the cells (band S60 decreased by 75% or more when compared to that of the control).
DANCE was a pathological response in the lungs. From the present study, we found that DANCE in the lungs was well consistent with the change of lung wet/dry weight ratio, which reflected edema changes in the lungs. Importantly, DANCE in the lungs exhibited a good dose-dependence and a typical time-course on adrenaline. These results demonstrated that DANCE in the lungs occurred with the change of lung wet/dry weight ratio, suggesting that DANCE in the lungs was a result of the pathological changes of APE in the lungs.
Adrenaline is a sympathomimetic hormone. It decreases vasodilatation and increases vascular permeability, leading to efflux of intravascular fluid. In addition, adrenaline increases heart rate, blood pressure, and hepatic glycogenolysis, leading to hyperglycaemia. Therefore, administration of exogenous adrenaline in amounts greatly exceeding physiological concentrations may produce APE[18]. In case of APE, the blood is not adequately removed from the pulmonary circulation. High blood pressure and increased vascular permeability cause injuries to the lung vasculature and parenchyma, resulting in efflux of fluid from the blood vessels to the air spaces and parenchyma of the lungs, and accumulation of a great quantity of liquid in the lungs, which can be determined as an increase in the lung wet/dry weight ratio. Therefore, increase in the lung wet/dry weight ratio indicates the development and severity of APE[19].
The cause for increase in lung wet/dry weight ratio could also be, at least a part, for DANCE in the lungs. Accumulation of liquid in the lungs is a result of increased pulmonary vascular permeability and outflow of water from the blood, which cause high pressure in the pulmonary tissues. We hypothesize that efflux of fluid from cells might also occur due to the high pressure in the lungs and increased permeability of pulmonary cell membrane. The latter might be caused by local hypoxia as a result of decreased oxygen exchange, such as reported by Bailey et al[20].Therefore, efflux of water from cells might be a cause for increase of mass density in the cells, which should contribute to DANCE response in the lungs.
It is interesting to know that lots of blood cells (red color) were observed in DSGC bands S50 and S60 of the lungs with DANCE, and the more blood cells in the bands were the higher dose of adrenaline. The amount of blood cells in the bands seemed to be an index of severity of APE. In a same dose of adrenaline, the lungs with DANCE showed more blood cells, smaller space area and thicker parenchyma than those without DANCE, suggesting that the mice with DANCE in the lungs had severer pathological changes in the lungs. However, whether accumulation of blood cells in the lungs or efflux of water from the pulmonary cells or both caused DANCE need further studies.
DANCE in the lungs was different from the responses in the liver. The liver is an important organ of the body in response to adrenaline, because the hormone is involved in regulation of metabolisms in the liver especially the metabolism of carbohydrates. Our present study showed that overdosed adrenaline caused fast responses in the liver, including decrease in glycogen content and increase in pro-inflammatory cytokine expression (IL-1β and IL-6). All these responses in the liver reached their peaks at 1 h after the drug injection, suggesting that the responses in the liver to adrenaline were 1 h faster than DANCE and wet/dry weight ratio change in the lungs, which reached their first peaks at the time-point of 2 h.
Obviously, the liver and the lungs showed different modes of response to the drug, or different mechanisms for their responses. DANCE in the lungs might be caused mainly by the pathological changes of the lung edema as described previously, such as possible water efflux from the cells, extravasation of fluid, protein, red blood cells into the interstitial and alveolar space[21], and condensed tissue structures in the lungs (Figure 5). In other words, DANCE in the lungs was indirectly caused by adrenaline, because direct interaction between cells and the drug might result in DANCE-up change such as that seen in the K562 cells. How-ever, adrenaline may directly act on the liver cells through its receptor and thereby cause pathophysiological changes of metabolism in hepatic cells, including glyco-genolysis and pro-inflammatory cytokine expression. Theoretically, these biochemical and molecular responses in the liver were induced by adrenaline via the PKA signal transduction pathway, and therefore faster than those in the lungs.
There might be 2 phases in response to adrenaline. From the time-course study, we found that most of the mice (5/6) exhibited DANCE in the lungs in 2 h after injection of adrenaline, and subsequently DANCE was detected in the lungs between the time points of 6 h and 12 h. Based on DANCE in the lungs and biological responses in the liver, we suggest 2 reaction phases in the body in response to adrenaline-induced APE. The first reaction phase could be from time 0 to 5 h, in which APE was quickly established, the first DANCE occurred in the lungs, and glycogenolysis sharply increased with cytokines (IL-1β and IL-6) highly expressed in the liver. These early reactions in the lungs and in the liver formed an acute phase response in the body. In this phase the mice risked the highest death rate during the experiment. The second reaction phase could be from time 5 to 14 h, in which mRNA levels of IL-1β and IL-6 in the liver recovered to their base lines, the liver glycogen content increased due to hyperglycemia[22], and DANCE in the lungs occurred again for the second time. The second reaction phase could be mainly the combination of pulmonary and hepatic pathological changes. As reported by Kolnes et al[23], after degradation of the liver glycogen and increase of blood glucose in the first reaction phase, glycogenesis in the liver was strongly stimulated by hyperglycemia, resulting in increase of the liver glycogen content[24]. These results were in good agreement with the report by Jensen et al[25].
Compared to the reactions of the first phase, the second phase did not involve the two cytokines, and therefore, the first phase could be an inflammation-like[16], acute response, and the second phase could be a non-inflammation-like, sub-acute response. Patholo-gically, the first phase reactions might be destruction-prone and the second phase could be a recovery-prone response. For DANCE in the lungs, each mouse exhibited a DANCE response in each of the 2 phases, in other words, each mouse might have 2 DANCE responses during the experiment as hypothesized. Unlike the first DANCE forming a sharp peak at the time of 2 h, the second DANCE in the lungs distributed in a longer range of time (about 14 h). Therefore, the second DANCE in the lungs in the second phase might reflect the individual differences in the mice in response to overdose of adrenaline.
The present study demonstrated that DANCE in the lungs (the band S40 disappeared or mostly reduced in DSGC) was well correlated with the increase in lung wet/dry weight ratio, which reflected the development and severity of APE. We hypothesize that in the lungs the band S40 is the sensitive and responding tissue/cells to overdosed adrenaline, and further study with band S40 might reveal the pathological mechanisms of APE. Based on the present and other studies of DANCE[3-5], it might be reasonable to suggest that those organs/tissues/cells with DANCE caused by drugs, toxins, or pathogens could serve as targets for further study of pathological mechanisms, and determination of DANCE in the body might be useful in localization of those organs/tissues/cells that are sensitive and responding to drugs.
ACKNOWLEDGEMENTS
We thank Professor JIANG Guang-yu for her assistance with the histological analysis.
[REFERENCES]
[1]Liu Y, Wei JG, Wu BB, et al. Density alteration in non-physiological cells[J/OL]. Nature Precedings(2011-10-17)[2016-04-21]. http://precedings.nature.com/documents/6541/version/1.
[2]Guo T, Liu F, Liu Y, et al.In-situdetection of density alteration in non-physiological cells with polarimetric tilted fiber grating sensors[J]. Biosens Bioelectron, 2014, 55:452-458.
[3]Wu B, Liu Y. Study of DANCE in K562 cells and its biochemical mechanism[EB/OL]. (2013-05-15)[2016-04-21]. http://www.paper.edu.cn/html/releasepaper/2013/05/152/.
[4]Wu XH, Liu Y. The relationship between DANCE and the change of cell metabolism mode[EB/OL]. (2013-05-09)[2016-04-21]. http://www.paper.edu.cn/html/releasepaper/2013/05/91/.
[5]Zhu XY, Liu M, Li JX, et al. DANCE in tumor-bearing mouse liver cells and the mechanism[J]. Highlights of Sciencepaper Online, 2015, 8(7):699-706.
[6]Lane SM, Maender KC, Awender NE, et al. Adrenal epinephrine increases alveolar liquid clearance in a canine model of neurogenic pulmonary edema[J]. Am J Respir Crit Care Med, 1998, 158(3):760-768.
[7]Alwi I. Diagnosis and management of cardiogenic pulmonary edema[J]. Acta Med Indones, 2010, 42(3):176-184.
[8]Koma T, Yoshimatsu K, Nagata N, et al. Neutrophil depletion suppresses pulmonary vascular hyperpermeability and occurrence of pulmonary edema caused by hantavirus infection in CB-17 SCID mice[J]. J Virol, 2014, 88(13):7178-7188.
[9]Lee-Chiong T Jr, Matthay RA. Drug-induced pulmonary edema and acute respiratory distress syndrome[J]. Clin Chest Med, 2004, 25(1):95-104.
[10]Abbas OM, Nassar AH, Kanj NA, et al. Acute pulmonary edema during tocolytic therapy with nifedipine[J]. Am J Obstet Gynecol, 2006, 195(4):e3-e4.
[11]Glauser FL, Smith WR, Caldwell A, et al. Ethchlorvynol (Placidyl)-induced pulmonary edema[J]. Ann Intern Med, 1976, 84(1):46-48.
[12]Campbell RL, Bellolio MF, Knutson BD, et al. Epinephrine in anaphylaxis: higher risk of cardiovascular complications and overdose after administration of intravenous bolus epinephrine compared with intramuscular epinephrine[J]. J Allergy Clin Immunol Pract, 2015, 3(1):76-80.
[13]Uraoka M, Nakajima Y, Kurita T, et al. Landiolo, an ultra short acting beta1-blocker, improves pulmonary edema after cardiopulmonary resuscitation with epinephrine in rats[J]. J Anesth, 2010, 24(1):67-72.
[14]Dunser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress[J]. J Intensive Care Med, 2009, 24(5):293-316.
[15]Parker JC, Townsley MI. Evaluation of lung injury in rats and mice[J]. Am J Physiol Lung Cell Mol Physiol, 2004, 286(2):L231-L246.
[16]Aninat C, Seguin P, Descheemaeker PN, et al. Catecholamines induce an inflammatory response in human hepatocytes[J]. Crit Care Med, 2008, 36(3):848-854.
[17]Van der Vies J. Two methods for the determination of glycogen in liver[J]. Biochem J, 1954, 57(3):410-416.
[18]Behonick GS, Novak MJ, Nealley EW, et al. Toxicology update: the cardiotoxicity of the oxidative stress metabolites of catecholamines (aminochromes)[J]. J Appl Toxicol, 2001, 21(Suppl 1):S15-S22.
[19]Van den BE, Bem RA, Bos AP, et al. The effect of TIP on pneumovirus-induced pulmonary edema in mice[J]. PLoS One, 2014, 9(7):e102749.
[20]Bailey DM, Kleger GR, Holzgraefe M, et al. Pathophysiological significance of peroxidative stress, neuronal damage, and membrane permeability in acute mountain sickness[J]. J Appl Physiol, 2004, 96(4):1459-1463.
[21]Minnear FL, Kite C, Hill LA, et al. Endothelial injury and pulmonary congestion characterize neurogenic pulmonary edema in rabbits[J]. J Appl Physiol, 1987, 63(1):335-341.
[22]Kurose T, Seino Y, Nishi S, et al. Mechanism of sympathetic neural regulation of insulin, somatostatin, and glucagons secretion[J]. Am J Physiol, 1990, 258(1 Pt 1):E220-E227.
[23]Kolnes AJ, Birk JB, Eilertsen E, et al. Epinephrine-stimulated glycogen breakdown activates glycogen synthase and increases insulin-stimulated glucose uptake in epitrochlearis muscles[J]. Am J Physiol Endocrinol Metab, 2015, 308(3): E231-E240.
[24]Asfar P, Hauser B, Radermacher P, et al. Catecholamines and vasopressin during critical illness[J]. Crit Care Clin, 2005, 22(1):131-149.
[25]Jensen J, Ruge T, Lai YC, et al. Effects of adrenaline on whole-body glucose metabolism and insulin-mediated regulation of glycogen synthase and PKB phosphorylation in human skeletal muscle[J]. Metabolism, 2011, 60(2):215-226.
细胞内密度改变是肾上腺素诱导小鼠急性肺水肿的一种病理反应
刘敏1,石琴琴1,傅涵1,韦建鸽2,李菊香3,钟鸣4,刘誉1△
(1暨南大学医学院生化教研室, 广东 广州 510632;2上海华斯泰医学咨询有限公司, 上海 200041;3暨南大学第一附属医院检验科, 广东 广州 510632;4广州市东来生物科技有限公司, 广东 广州 510530)
[摘要]目的:研究病理条件下肺的细胞内密度改变反应。方法: 以高剂量肾上腺素腹腔注射法制备小鼠急性肺水肿模型,经不连续蔗糖密度离心法测定肺组织匀浆的细胞内密度改变。定量测定肺的湿/干重比值、肝糖原含量、肝脏炎症因子IL-1β和IL-6 的mRNA相对表达水平,分析急性肺水肿的发展及其病变程度。结果: 经腹腔注射肾上腺素后,小鼠肺组织细胞内密度发生显著改变,其发生几率呈时间和剂量依赖性,且与肺的湿/干重比值呈正相关关系。肝脏IL-1β和IL-6的mRNA表达水平显著升高,在注射药物1 h达到高峰。结论: 在肾上腺素诱导的小鼠急性肺水肿中,肺的细胞内密度改变是体内的一种病理反应。这种病理改变可能应用于药物对体内组织细胞作用的定位及其病理学研究。
[关键词]急性肺水肿; 小鼠; 肾上腺素
[Received date]2016- 03- 02[Accepted] 2016- 04- 18
*[Foundation item]Supported by the Science Foundation of the Ministry of Science and Technology of China (No.12C26214405312).
Corresponding author△Tel: 020-85220256; E-mail: xyuliu05@126.com
[CLC number]R285.5; R363
[Document code]A
doi:10.3969/j.issn.1000- 4718.2016.05.001
[中图分类号]R285.5; R363[文献标志码]A
杂志网址: http://www.cjpp.net
[Article ID]1000- 4718(2016)05- 0769- 12
·论著·

