Recovery ofnickel,cobalt,copper and zinc in sulphate and chloride solutions using synergistic solvent extraction
Chu Yong Cheng*,Keith R.Barnard,Wensheng Zhang,Zhaowu Zhu,Yoko Pranolo
Minerals Resources Flagship,Karawara,WA 6152,Australia
1.Introduction
Currently,no commercial solvent extraction (SX) extractant is highly selective for metals such as nickel and cobalt.The development and commercialisation of new,highly selective extractants are very expensive.As a consequence,development of new commercial extractants to fulfil this specific role is unlikely.Therefore,the use of synergists,preferably commercially available reagents,to improve the selectivity of the existing extractants is an attractive alternative.Many synergistic SX(SSX)systems have been developed and tested in the past three decades[1].The SSX systems developed by the CSIRO SX team are described in detailbelow.
1.1.Separation ofnickel from calcium by SSX with Versatic 10 and CLX50(tested for Bulong Nickel,Australia)
Gypsum formation was a severe problemin Australia Bulong's nickel solvent extraction(SX)circuit(Fig.1).Versatic 10 was used to extract nickel,however,calcium was co-extracted due to their close pH isotherms[2–5].
The pH isotherm of nickel with Versatic 10 was significantly affected by the addition of Acorga 5443A or CLX50,a 3,5-pyridine ester(Fig.2).The pH50value of nickel was reduced by 0.60 and 0.93 pH units by adding 5%and 10%CLX50 to the organic solution,respectively(Table 1).The pH isotherm of calcium was not affected by the addition of CLX50.As a result,the ΔpH50(Ca–Ni)(the selectivity of Niover Ca)increased from 0.77 without CLX50 to 1.37 and 1.70 with the addition of 5%and 10%CLX50,respectively(Table 1)while the separation factors of Niover Ca increased from 47 to 258 and 1216,respectively,the extractions of Niat pH 6.4 increased from 63.3%to 89.5%and 97.4%,respectively(Table 2)while the extraction of Ca slightly decreased from 3.5%to 3.2%and 3.0%,respectively,indicating very strong synergistic effect.Due to the largely increased separation factor of Niover Ca,the formation ofgypsum should be eliminated if5%–10%CLX50 is added.
1.2.Separation of nickeland cobalt from manganese,magnesium and calcium by SSX with Versatic 10 and 4PC(tested for BHP Billiton,Australia)
High pressure acid leaching(HPAL)of nickellaterite ores results in solutions containing Ni,Co,Mn,Mg and Ca.The separation of Niand Co from Mn,Ca and Mg has been achieved using mixed sulphide precipitation(MSP)and mixed hydroxide precipitation(MHP)followed by releach and further separation using SX.Direct solvent extraction(DSX)processes have been developed to directly recover Niand Co from the neutralised leach solution without MSP and MHP and subsequent releach processes to save operating costs.The DSX processes are seen as having the greatest potentialin the future,provided a highly selective,stable and inexpensive extractantor extraction system is available.

Fig.1.A conceptual flow sheet of the Bulong process for the recovery ofnickeland cobalt from leach solution of nickellaterites.

Fig.2.The effect of synergist CLX50 on the pH isotherms of nickel and calcium with 0.5 mol·L-1 Versatic 10 acid at an aqueous to organic phase ratio(A/O)of1:1 and 23 °C.
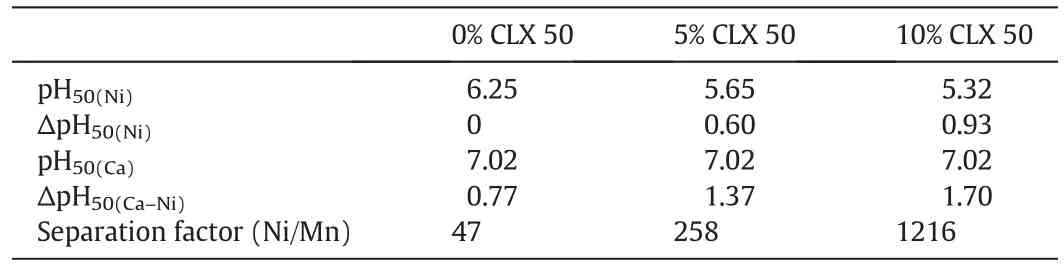
Table 1 pH50,ΔpH50 and separation factors with different CLX50 concentrations
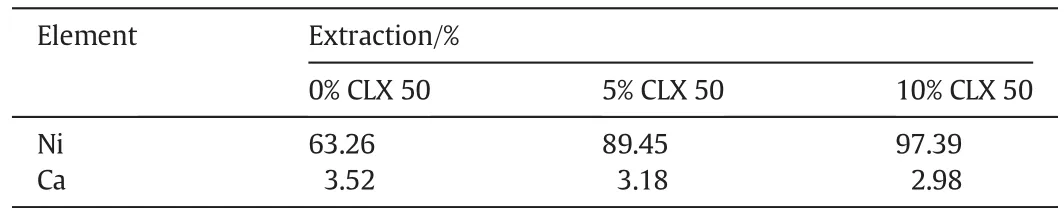
Table 2 Extraction of Niand Ca at pH 6.4 with different CLX50 concentrations
A synergistic system consisting of extractant Versatic 10 acid and a synergist decyl-4-pyridinecarboxylate(4PC)was developed to directly recover nickel and cobalt from sulphate leach solutions without intermediate precipitation and re-leach as for the MSP and MHP processes[4,6–10].It is impossible to separate Ni and Co from Mn,Mg and Ca(Fig.3)with the 8%Versatic 10 alone due to smallΔpH50(Mn–Ni)of0.3 pH units and ΔpH50(Mn–Co)of 0.2 pH units(Table 3).By adding 10%4PC to the Versatic 10 system,separation of nickel,cobalt,zinc and copper fromman ganese,magnesium and calcium could be achieved(Fig.4)with ΔpH50(Mn–Ni)of 1.9 pH units and ΔpH50(Mn–Co)of 1.1 pH units(Table 3).The separation factors of Niand Co over Mn increased from 3.9 and 1.7 to 1993 and 126,respectively.A pilot plant leach solution from BHP Billiton was tested using this system in semi continuous tests.Trials using this synergistic SX system resulted in almost>99.9%Co and Ni extracted,leaving Mn,Mg,Ca and Cl in the raf finate(Table 4).Some 96%of the co-extracted Mn,100%of the co-extracted Mg and 61%of the co-extracted Ca were scrubbed,leaving 2 mg·L-1manganese,no magnesium,and 4 mg·L-1calcium in the scrubbed organic solution(Table 5).
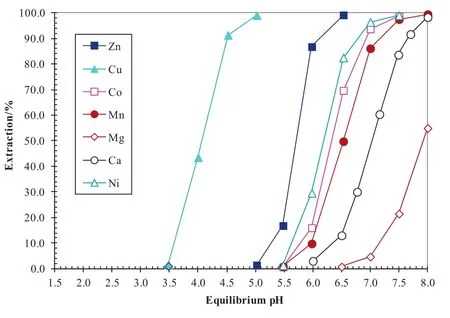
Fig.3.Extraction pH isotherms of metals with the synthetic laterite leach solution and 8%or 0.5 mol·L-1 Versatic 10 in Shellsol2046 at an A/O ratio of1:1 and 40 °C.

Table 3 pH50,ΔpH50 and separation factors with and without synergist 4PC
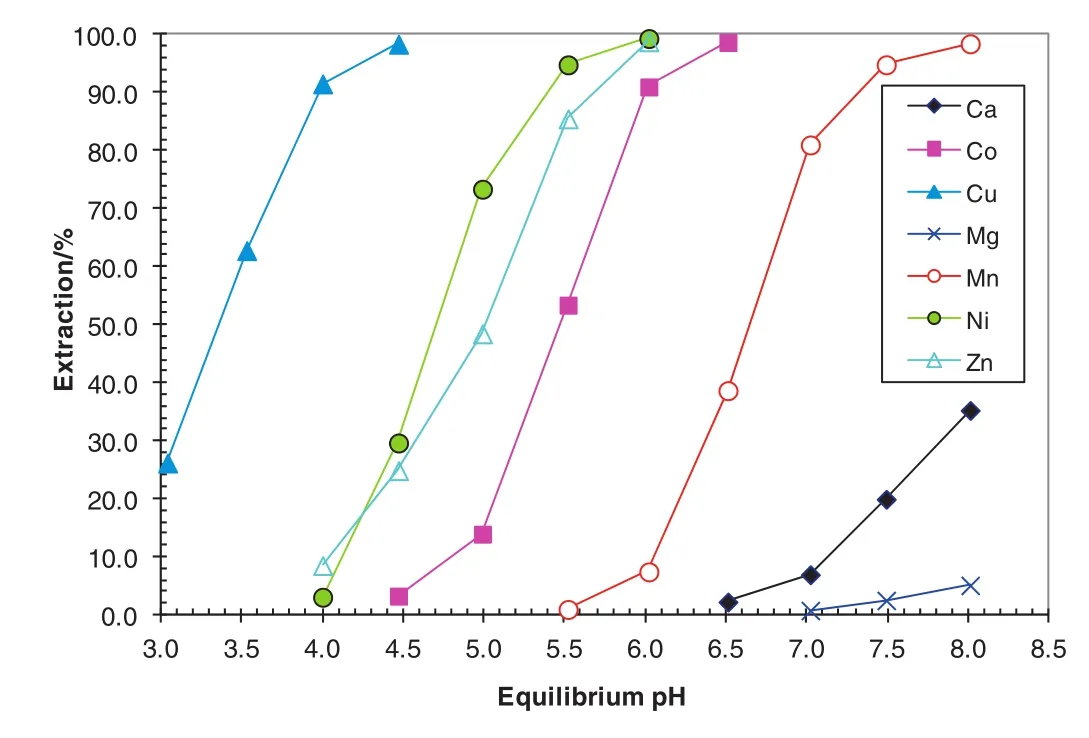
Fig.4.pHisotherms of metals with the 8%Versatic 10/10%4PC synergistic SXsystem for Ni and Co recovery using synthetic laterite leach solution.

Table 4 Semi-continuous extraction tests for the separation of Niand Co from Mn,Mg,Ca and Cl

Table 5 Semicontinuous scrubbing tests for the scrubbing of Mn,Mg and Ca from the loaded organic solution
The Versatic 10/4PC synergistic SX system also demonstrated very fast extraction and stripping kinetics,good phase separation characteristics and high chemicalstability[11].Using this synergistic SX system,a conceptual DSX flow sheet was developed for nickel laterites(Fig.5).
1.3.Separation of cobalt and zinc from manganese,magnesium and calcium by SSX with Versatic 10 and LIX63(piloted for the El Beleo project of Baja Mining,Canada)
The ElBoleo Cu–Co–Zn–Mn deposit is situated in the Baja Peninsula of Mexico[12].After copper recovery by SX and iron and aluminium removalby precipitation,the resultantsolution contains large amounts of manganese(up to 45 g·L-1).Due to the elevated manganese concentration,it is difficultto separate cobalt and zinc from manganese,magnesium and calcium using conventional SX processes.A novel DSX process has been developed to directly separate nickel,cobalt and zinc from manganese,magnesium and calcium using the SSXsystemsdeveloped by CSIRO with commercially available reagents[13–17].
The metalpH isotherms with the SX system containing Versatic 10 acid alone,LIX63 alone and the novel SSX system consisting of LIX63 and Versatic 10 acid are shown in Figs.3,6 and 7,respectively.The ΔpH50of Co and Zn over Mn reached 2.5 and 1.6 pH units with the LIX63/Versatic 10 SSX system,respectively,indicating a very good separation of Co and Zn from Mn,Mg and Ca(Table 6).At pH 4.6,the separation factors ofCo and Zn over Mn reached 3067 and 291,respectively again,indicating a good separation of Co and Zn from Mn,Mg and Ca(Table 7).At pH 4.6,the Co and Zn extraction increased from zero with the Versatic 10 alone system and 4%and 1%with the LIX63 alone SX system increased to 99%and 98%with the LIX63/Versatic 10 SSXsystem,respectively,indicating very significant synergistic effect on their extraction(Table 7).
The DSX circuit performed very well in two demonstration pilot plantoperations[18,19].The overallrecovery of cobalt and zinc reached over 99%with a raf finate contained only 4 mg·L-1Zn and 1 mg·L-1Co.
The stability of the Versatic 10/LIX63 SSX system under conditions relevant to the El Boleo project has been investigated[20–22].While Versatic 10 is chemically stable,the hydroxyoxime extractant in LIX 63 issusceptible to degradation.Different degradationmechanisms predominate under extract and strip conditions in the synergistic system,with hydroxyoxime degradation occurring more readily under extract conditions at higher pH,an outcome attributable to metal(particularly manganese)complexation.Degradation under strip conditions is primarily associated with the presence of the organic acid,and to a lesser extent increasing aqueous acidity.A variety ofhydroxyoxime degradation products have been observed to form under different(extract,strip)operating conditions[1],although a detailed discussion of these lies beyond the scope of this paper.Importantly,none of these have been found to adversely affect metalselectivity or extraction kinetics[23,24].Degradation of hydroxyoxime was found to be minimised by operation at lower temperatures and judicious selection of operating pH to minimise manganese loading which was found to have a detrimentaleffect on hydroxyoxime stability.Operation at pH 4.5 resulted in low manganese loading(about 0.2 g·L-1)and a calculated oxime half life of 67 weeks[22].Zinc loading,cobalt loading and changes in pHbetween 4.5 and 6.0(excluding the resulting detrimental impact associated with increased with increased manganese loading)did notappear to have any adverse effect on oxime stability.Aconce ptual path way for the regeneration of active hydroxyoxime from the three major degradation products has been outlined elsewhere[25].Importantly,pilot plant studies of the DSX circuit[18,19]showed no sign of reagent degradation after the pilot plant operation,indicating good chemical stability under the pilot operating conditions.The Boleo plant is being built and the DSX process is expected to be put into production soon.
1.4.Separation of nickel cobalt from manganese,magnesium and calcium by SSX with Versatic 10,LIX63 and TBP(piloted for Rio Tinto,UK)
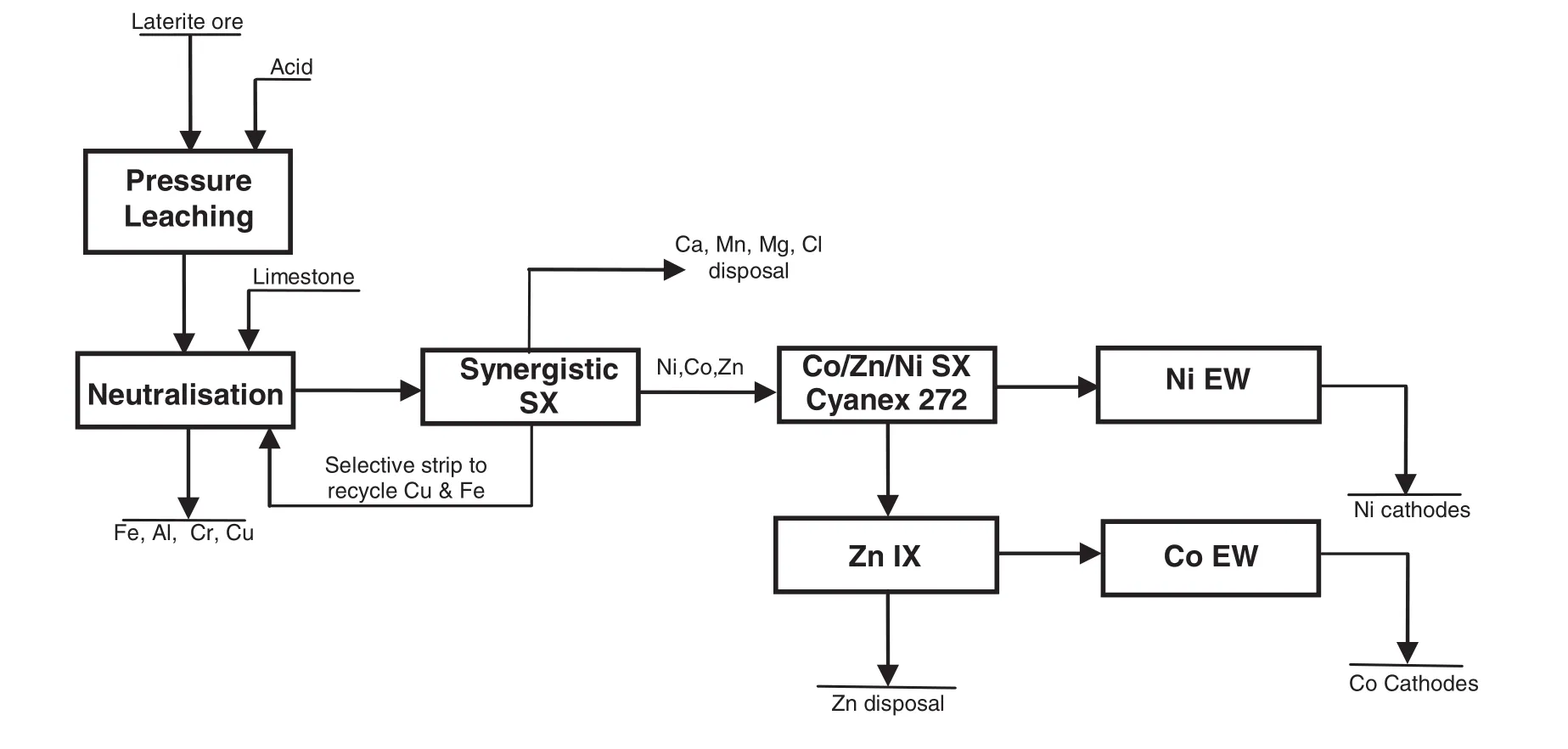
Fig.5.A conceptual flow sheet of the CSIRO DSX process for nickelsul phides.
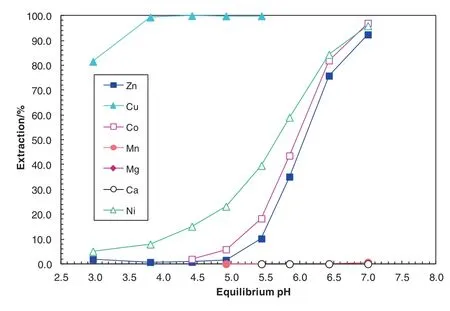
Fig.6.Extraction pH isotherms of metals with 0.5 mol·L-1 LIX 63 in Shellsol2046 at an A/O ratio of 1:1 and 40°C.
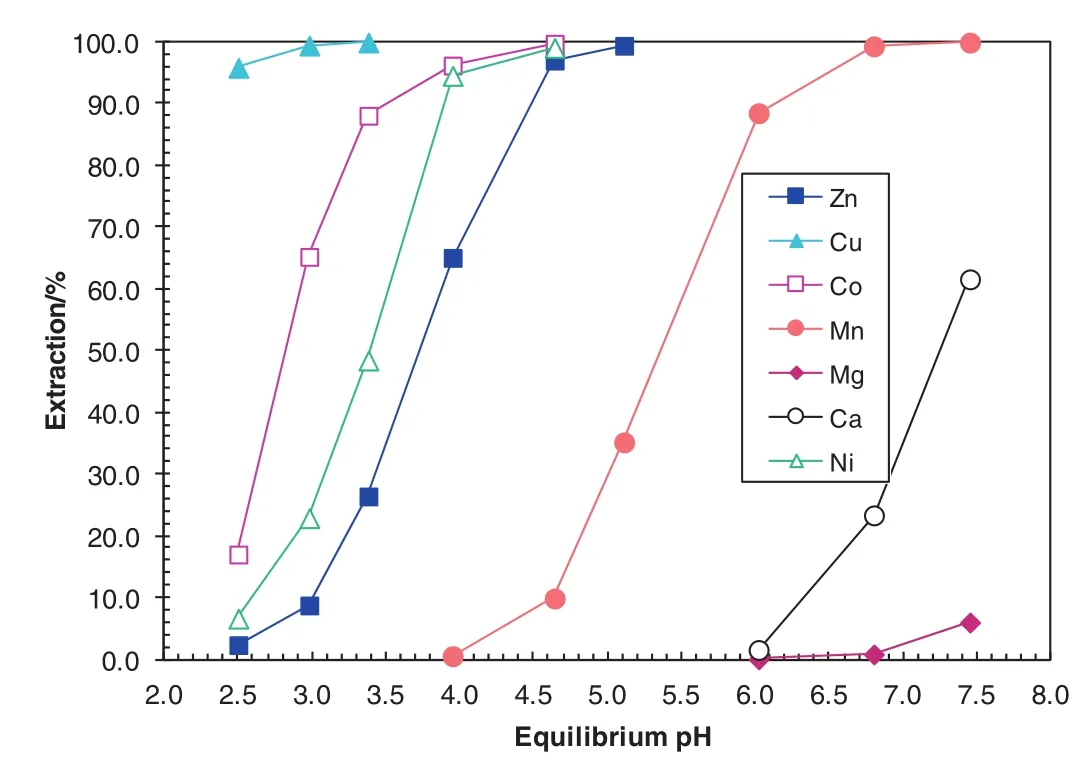
Fig.7.Extraction pH isotherms of metals in the synthetic laterite leach solution using the 0.5 mol·L-1 Versatic 10/0.35 mol·L-1 LIX 63 at an A/O ratio of1:1 and 40 °C.
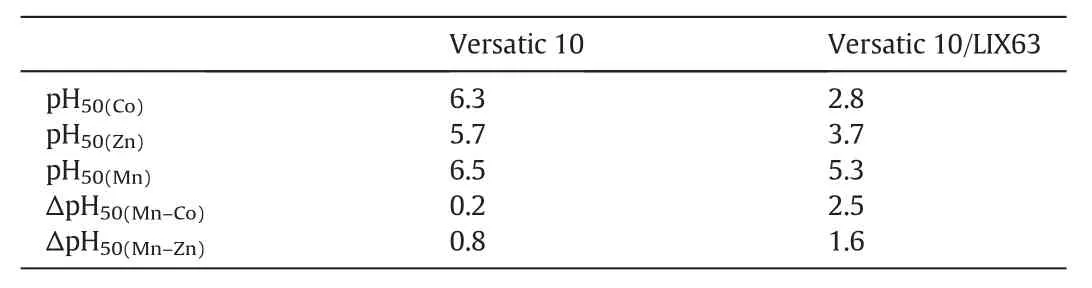
Table 6 Metal pH50 andΔpH50 values with the Versatic alone SX and the Versatic/LIX63 SSX systems

Table 7 Metalex tractions and separation factors with the SX and SSX systems at pH 4.6
The SSX system consisting of Versatic 10 and LIX63 was tried for the separation of Niand Co from Mn,Mg and Ca in laterite leach solutions,but suffered from slow stripping Nikinetics.It was found that the Ni stripping kinetics was largely increased by the addition of TBP in the LIX63/Versatic 10 SSX system as shown in Fig.8[14,26].Within 2 min,the stripping efficiency of nickel increased from 17.7%in the absence of TBP to 91%with 0.5 mol·L-1TBP addition.As a result,the stripping kinetics of nickel was fast enough for industrialoperations and another DSX process for the separation of Niand Co from Mn,Mg and Ca has been developed using the LIX63/Versatic 10/TBP SSX system.
The metal extraction pH isotherms were affected by the addition of TBP(compare Figs.7 and 9).The ΔpH50(Mn–Ni)values increased from 1.96 with no TBP to 2.62 with the addition of 0.5 mol·L-1TBP while the ΔpH50(Mn–Co)values decreased from 2.53 with no TBP to 2.11 with the addition of0.5 mol·L-1TBP(Table 8).The ΔpH50(Mn–Co)value is stillvery large as far as the separation of Co from Mn,Mg and Ca is concerned.As a result,the separation factor ofNiand Co over Mn was 4850 and 470,respectively,indicating a very good separation of both Niand Co from Mn,Mg and Ca.
Severalstability studies have also been undertaken on the Versatic 10/LIX63/TBP system[27–31].The addition of TBP to the Versatic 10/LIX63 system has been found to have no noticeable effect on hydroxyoxime stability or degradation behaviour(Fig.8).
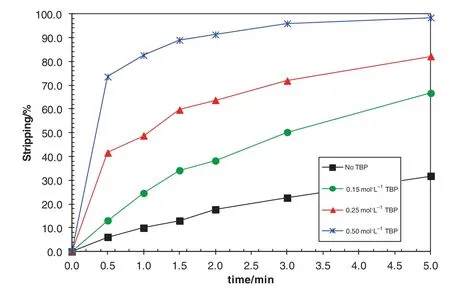
Fig.8.Effect of TBP concentration on the stripping kinetics ofnickel from loaded organic solution consisting of 0.5 mol·L-1 Versatic 10/0.35 mol·L-1 LIX 63/TBP at an A/O ratio of1:1 and 40°C.
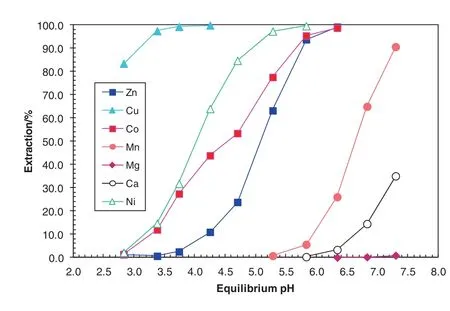
Fig.9.Extraction pH isotherms of metals in synthetic laterite leach solution using the 0.5 mol·L-1 Versatic 10/0.35 mol·L-1 LIX63/0.5 mol·L-1 TBP SSX system at A/O ratio of 1:1 and 40°C.
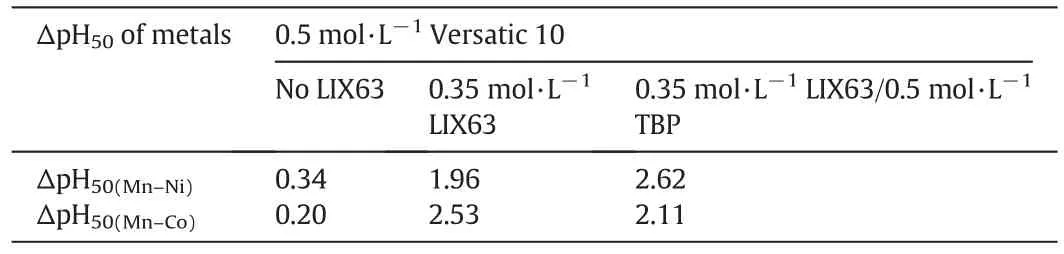
Table 8 ΔpH50(Mn–Ni)and ΔpH50(Mn–Co)values with the Versatic 10 alone,Versatic 10/LIX63 and Versatic 10/LIX63/TBP SSX systems
The addition of TBP to the Versatic 10/LIX63 system notonly greatly improved the nickelstripping kinetics,butalso improved its extraction kinetics(Table 9).The extraction of Niincreased from 72%with no TBP to 96%with 0.5 mol·L-1TBP addition within 2 min while the Co extraction was slightly reduced from 99%to 97%.
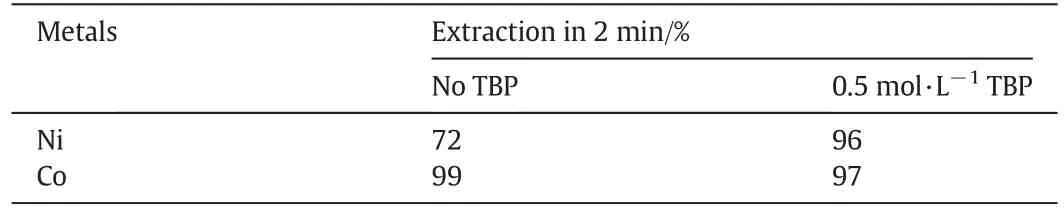
Table 9 Ni and Co extraction within 2 min with Versatic 10/LIX63 and Versatic 10/LIX63/TBP systems
The SSX system consisting of0.5 mol·L-1Versatic 10,0.45 MLIX63 and 1 mol·L-1TBP was used for the separation of Niand Co from Mn,Mg and Ca in a synthetic Rio Tinto leach solution containing(g·L-1):5.05 Ni,0.27 Co,1.57 Mn,30.4 Mg and 0.5 Ca in a piloting operation for 10 days[28,32–35].The pilot plant is shown in Fig.10.

Fig.10.Piloting plant using the Versatic 10/LIX63/TBP SSX system.
With four extraction stages at a pH pro file of 5.5/5.8/6.0/6.3,both nickeland cobalt were almost completely extracted(Table 10).The nickel and cobalt concentration in the raffinate was lower than the detection limit of 0.2 mg·L-1.The manganese,magnesium and calcium concentrations in the loaded organic solution were 34,8 and 1 mg·L-1,respectively.
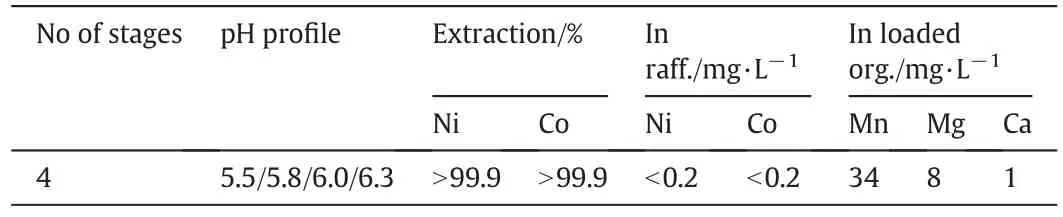
Table 10 Niand Co extraction and separation from Mn,Mg and Ca at an O/A ratio of2 and 40°C
With a pH pro file of5.4/5.0 and an organic to aqueous phase ratio(O/A)of 9.2,the manganese scrubbing efficiency was over 96%and the concentrations of manganese,magnesium and calcium were only 1 mg·L-1(Table 11).
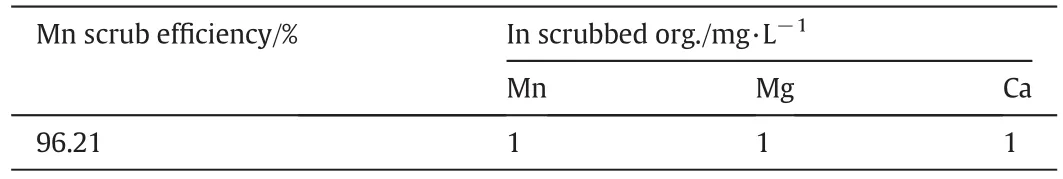
Table 11 Scrubbing manganese,magnesium and calcium
A strip solution containing 50 g·L-1sulphuric acid and 55 g·L-1nickelwas used for Niand Co stripping at an O/A ratio of10 and 40°C in three stages.It was found that the over 98.0%Niand 99.5%Co were stripped and the residual nickel concentration in the stripped organic solution was only 64 mg·L-1(Table 12).The nickel concentration in the loaded strip liquor(LSL)reached 83 g·L-1,resulting in a ΔNi of over 31 g·L-1.The pH of the loaded strip liquor was 3.1 and the loaded strip liquor contained <1 g·L-1acid.Therefore,the loaded strip liquor would be suitable as a feed to a Cyanex 272 SX circuit in which cobalt would be separated from nickel(Table 12).

Table 12 Nickel and cobalt stripping with three stripping stages
The fully continuous pilot plan to peration was conducted successfully with the synthetic Rio Tinto leach solution after iron removal.Using four extraction stages,two scrubbing stages and three stripping stages,almostall Niand Co were extracted.>96%Mn scrubbed and over 98%Ni and>99%Co stripped.The SSX system was suitable for the recovery of Ni and Co from laterite leach solutions after iron removal.
1.5.Separation of cobalt and nickelby the difference in extraction and stripping kinetics(piloted with CESL,Canada)
The difference in stripping kineticsofNiand Co also wasfound in extraction kinetics with the SSX system consisting of Versatic 10 and LIX63.This means that not only the stripping kinetics of Ni is slower than that of Co,but also the extraction kinetics of Ni is slower than that of Co.This property could be used for the separation of a small amount of Co from large amount of Niin sulphate solutions in attempt to replace the Cyanex 272 SX system due to its co-extraction of Mg,resulting in consumption of base and therefore high operating cost(Fig.11)[36].With the SSX system containing Versatic 10 and LIX63 and a synthetic solution containing 20 g·L-1Niand 1 g·L-1Co,the primary goals of the research were to achieve(1)a Ni/Co ratio of>700 in the raffinate,(2)Co recovery above 95%and(3)a Co/Niratio of>1 in the Co productsolution.In extraction,to overcome back mixing in ordinary mixers,pipe reactors were used for mixing due to its plug flow and minimum back mixing.In stripping,ordinary mixer-settlers were used due to very slow nickelstripping kinetics.
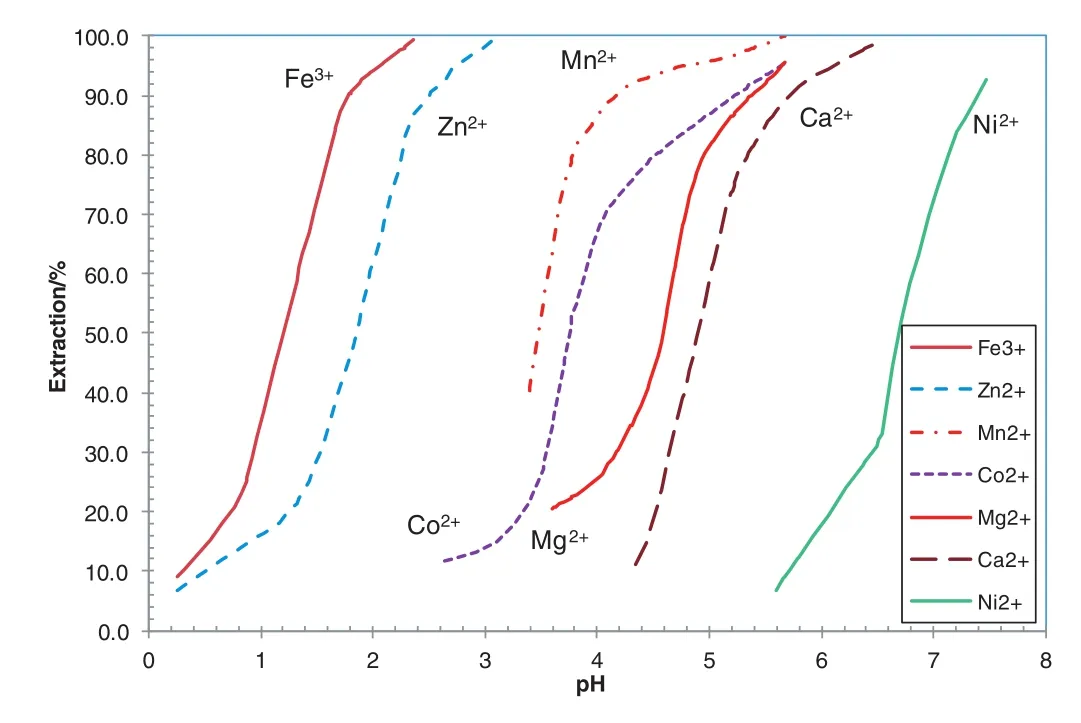
Fig.11.MetalpH isotherms with 0.6 mol·L-1 Cyanex 272 at A/O ratio 1 and 50 °C(based on[36]).
With the SSX system containing 0.25 mol·L-1Versatic 10 and 0.25 mol·L-1LIX63 in Shellsol D70,the Co and Niextraction kinetics under 30 °C at pH 4.5 at different times was shown in Fig.12[37–39].The Co extraction from the synthetic solution was 44%after 60 s,compared to 2.7%Niextracted.This was for a single stage extraction with an A/Oof1,demonstrating excellent selectivity under these conditions.The Co extraction peaked at60 s and then declined thereafter due to the crowding effect ofNi.The Niextraction continued to increase slowly.As increasing the operating temperature hastened metal extraction and thus the onset of cobalt crowding by nickel,the extraction should be operated at a low temperature such as 30°C.
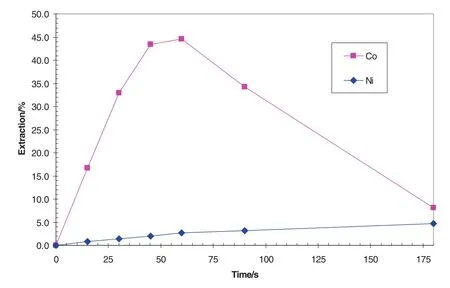
Fig.12.Co and Niextraction vs.residence time at A/O 2:1 and 30 °C with 0.25 mol·L-1 Versatic 10 and 0.25 mol·L-1 LIX 63 in ShellsolD70.
The stripping kinetics ofCo was much faster than thatof Ni(Fig.13).After 3 min,over 93%Co,but only 12%Niwas stripped,indicating with limited acid available,the stripping of Co was much faster than that of Ni.It was also found that the Nistripping in a settime increased with increasing temperature.Therefore,controlling temperature and acidity can be used to encourage selective stripping.

Fig.13.Stripping kinetics of Co and Niusing 3 g·L-1 sulphuric acid at 30 °C and an A/O ratio of1:1.
In semicontinuous tests,with a two-stage series(relative to aqueous solution)/parallel(relative to organic solution)extraction mode at pH 4.5,30°C and an A/O ratio of 1:1 in both stages,the Co extraction reached 97.8%,the Ni/Co ratio in the raf finate 1100 and the Co/Niconcentration ratio in the loaded organic solution 0.46 in average.With a selective strip step,the Co/Niconcentration ratio in the loaded strip liquorreached 4.9.These results exceeded the primary goals setat the beginning of the test work.
In fully continuous tests(Fig.14),with a two-stage series/parallel extraction mode,extraction stage 1 performed very welleven with organic solutions containing up to 0.5 g·L-1ofresidual Ni.After second stage of extraction,the Co/Niconcentration ratio in the finalraf finate reached 621,which is close to the target of 700.This target should be reached readily ifthe residualNiin the stripped organic was much lower.
The Co strip efficiency reached 85%with a strip A/O ratio of 1:20 using a strip solution containing 40 g·L-1sulphuric acid.The Co/Niconcentration ratio reached 2.0–2.9 in the loaded strip liquor(Co product solution)in most cases,indicating that after Co selective stripping the target of a Co/Niratio ofmore than 1 in the Co product solution was easily achieved.
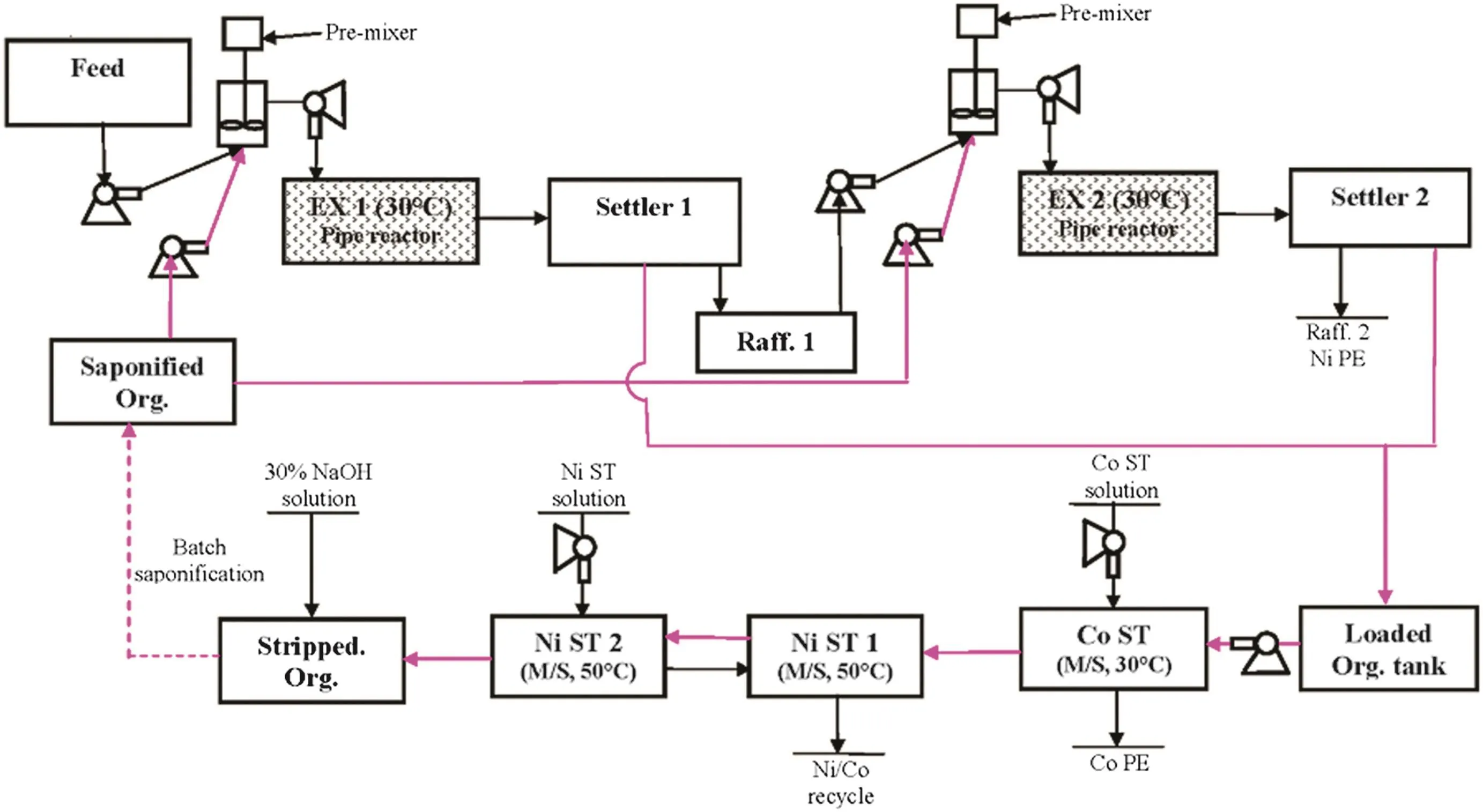
Fig.14.A schematic offully continuous operation flow sheet.
In most cases,the Nistripping efficiency was in the range of 70%–88%and that for Co over 99%with almost no Co left in the stripped organic solution.With the highest Ni stripping efficiency of 88%and only 0.256 g·L-1Nileft in the stripped organic solution,the Ni/Co concentration ratio in the finalraf finate could reach 700.
The Mg co-extraction was in the range of 0–0.02%,resulting in the Mg concentration of 0–22 mg·L-1based on the Mg concentration in the feed solution of11 g·L-1.The co-extraction of Mg was much higher with the Cyanex 272 system[36].AtpH 4.4,80%Co was extracted with co-extraction of40%Mg(Fig.11),indicating a large amountofbase will be saved with the SSX system based on extraction and stripping kinetics.
1.6.Synergistic SX for the separation ofiron,zinc and copper from nickel and cobalt(piloted for Minara Resources,Australia)
In the Murrin Murrin operation of Minara Resources,Australia,a HPAL-MSP-Re-leach-SX process is used to recover Niand Co from laterite ores.The precipitated crude mixed nickeland cobalt sulphides are re-leached by pressure oxidation.The leach solution contains high concentration of nickeland cobalt and smallamount of iron,copper and zinc.After iron removalby hydroxide precipitation,the copper is precipitated as copper sulphide with nickelsulphide.The zinc is then separated from cobalt and nickelin SX circuit 1 using Cyanex 272 and the cobalt is separated from nickel in SX circuit 2,again,using Cyanex 272.Due to the incomplete separation of copper,the cobalt product quality is affected.
The Minara process liquor taken from its Murrin Murrin NickelOperation after iron precipitation before copper removal contained 126 g·L-1Ni,8.1 g·L-1Co,0.17 g·L-1Cu,1.83 g·L-1Zn and 0.002 g·L-1Fe.The primary and most important objective of the project was to obtain raffinate solutions containing <0.3 mg·L-1Cu and Fe from which a high quantity cobalt product containing <50 mg·L-1Cu and Fe could be achieved.As shown in Fig.15,atpH 3.5,the separation of Fe and Zn from Niand Co is complete,however,the separation of Cu from Niand Co is impossible.An SSX system was developed for Minara Resources to separate Fe,Cu and Zn from Niand Co in SX circuit1 by the addition of another extractant LIX84 to the existing Cyanex 272 organic solution(Fig.16).AtpH 3.5,allFe and almostallCu and Zn were extracted with very little Niand Co co-extracted[40–43].The Cu extraction increased significantly from 2.8%with no LIX84 to 97.9%with the addition of 15%LIX84(Table 13).The separation factor of Cu over Co increased from 8 with no LIX84 to 11,000 with the addition of15%LIX84.The separation factor of Zn over Co decreased from 9600 to 8800,but it is still very high.With this synergistic system,a very easy separation of Fe,Cu and Zn from Niand Co could be obtained.
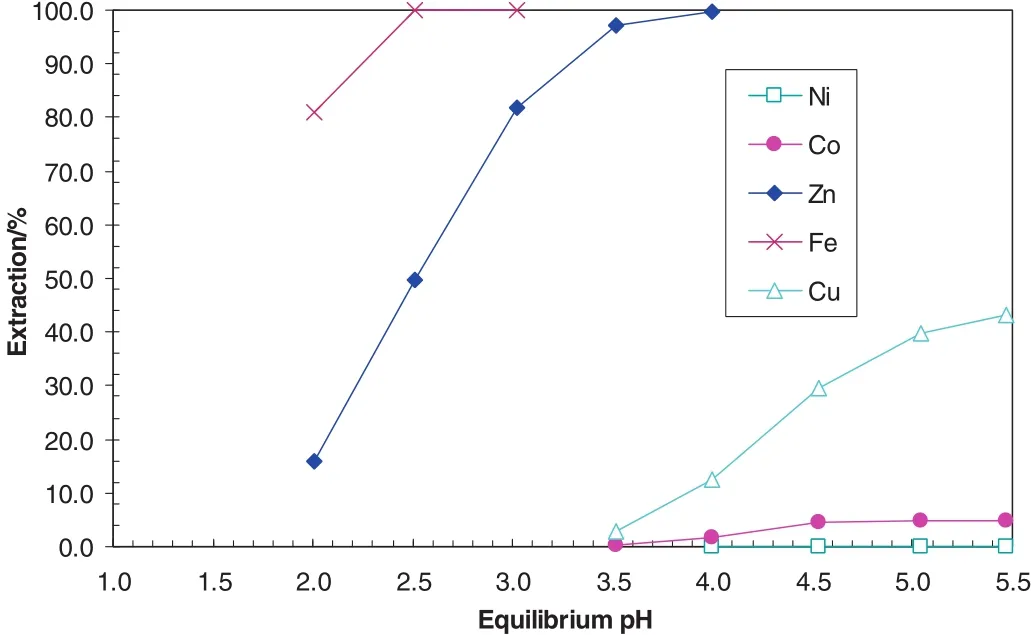
Fig.15.MetalpH isotherms with organic system consisting of17%Cyanex 272 in ShellSol 2046 at A/O ratio of6.5.
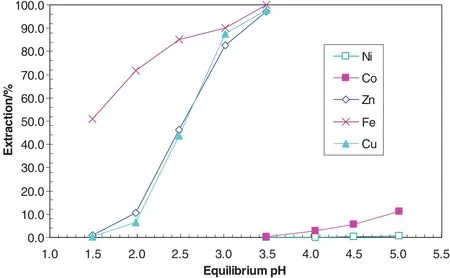
Fig.16.Metalp Hiso therms with the mixed system consisting of17%Cyanex 272 and 15%LIX84 in ShellSol2046 at A/O ratio of5.

Table 13 Zinc and copper extraction and separation factors over Co and Ni
Apiloting was carried out with two stages of extraction,one stage of scrubbing and two stages of stripping.After extraction in the pH range of3.5–4.0 and A/O ratio of 2–7,less than 0.3 mg·L-1of Cu and Fe and 0.2–0.5 mg·L-1Zn in the raf finate were achieved with >99%Fe,Cu and Zn removal(Table 14).The co-extraction of Niand Co was in the range of 0.04%–0.1%and 0.04%–1.0%,respectively.

Table 14 Effect ofpH and A/O flow ratio on extraction of metals in piloting
Using a diluted loaded strip liquor containing Fe,Cu and Zn,85%–90%Ni and >90%Co were scrubbed in the pH range of 2.8–3.3 at a wide range of O/A ratios of2–7.The strip solution contained 80 g·L-1H2SO4and the pH was controlled by dosing 100 g·L-1H2SO4.The stripping efficiency of Cu and Zn was in the range of 96%–99%,and for Fe,50%–90%at pH 1.2–2 in most of cases.
The batch,semi-continuous and fully continuous testresults demonstrated the new organic system with the mixed Cyanex 272 and LIX 84 extractants is suitable for the separation ofCu,Fe and Zn from Niand Co.The primary goal to obtain a cobalt product solution containing<0.3 mg·L-1Cu and Fe has been achieved.The system offers a wide range of operating windows for pH controland A/O ratios with very smallloss of Co and Niin the overallcircuit.
1.7.Separation of cadmium and zinc with a novelSSX system consisting of HRJ-4277 and Cyanex 471X
The removalof cadmium by solvent extraction has severala dvantages over zinc powder cementation,which include potential lower capitaland operating costs and less environmentalpollution.However,to this point,no commercialrea gents can be used to selectively remove cadmium from zinc in industrialscale.
The metalpH50andΔpH50values were determined and compared for a number oforganic systems to selectan SSX system for the separation of Cd from Zn in a synthetic solution containing 2.5 g·L-1ofeach metal[44–46].The largestΔpH50(Zn–Cd)value of1.53 pH units was obtained with HRJ-4277(nonylsalicylic acid)/Cyanex 471X(tri-isobutyl phosphine sulphide or TIBPS)system,indicating good separation of Cd from Zn with this system(Fig.17 and Table 15).At pH,the extraction of Cd increased from 3.9%to 94.7%,but that of Zn,only from 3.7%to 7.2%,resulting in a separation factor ofCd over Zn of612,again,indicating a good separation(Table 16).
In a three-stage batch continuous extraction test,>99%Cd and only 0.4%Zn were extracted.The loaded organic solution contained 3.87 g·L-1Cd and only 12 mg·L-1Zn.In a two-stage batch continuous scrubbing test,all the co-extracted Zn was removed with the scrubbed organic containing<1 mg·L-1Zn.Nearly 99%ofcadmium was stripped in two batch contacts with 20 g·L-1sulphuric acid.Excellentseparation of Cd and Zn was achieved with this SSX system.
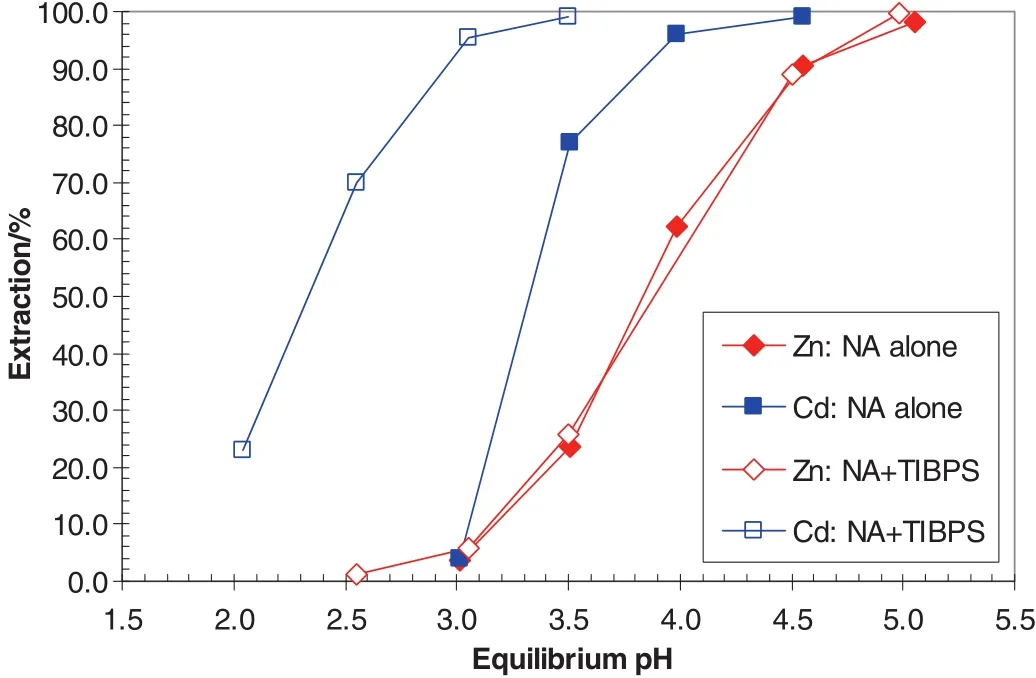
Fig.17.Extraction pH isotherms of cadmium and zinc with 0.5 mol·L-1 HRJ-4277(NA)alone and 0.5 mol·L-1 HRJ-4277(NA)and 0.5 MCyanex 471X(TIBPS)in Shellsol2046.
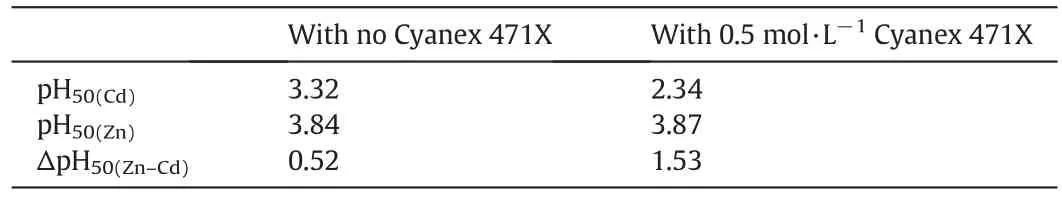
Table 15 pH50 andΔpH50 of metals with HRJ-4277 and Cyanex 471X in Shellsol D70

Table 16 Metal extraction at pH 3 and separation factor(SP)of Cd over Zn with HRJ-4277 and Cyanex 471X in ShellsolD70
1.8.Separation of copperfrom iron in chloride solution using an SSX system and transfer of copper to sulphate solution for electrowinning
Copper chloride hydrometallurgy has advantages such as higher copper leaching kinetics and recovery and less environmental concern compared to smelting and leaching with other media such as sulphuric acid.However,the electro winning of copper from chloride solutions was very problematic due to the difficulties in cell design and the handling of a copper powder product.If the copper can be transferred from chloride to sulphate solutions for conventional electro winning,the new process will have the advantages of copper leaching in chloride solutions and copper electro winning in sulphate solutions[47,48].
With the SSX system consisting of 0.3 mol·L-1LIX 63 and 0.33 mol·L-1Versatic 10 in Shellsol D70,the extraction of copper was independent of the solution acidity between 0.1 and 0.5 mol·L-1HCl with a constant value(Fig.18).However,the extraction of iron was slightly acidity dependent,showing an increasing trend when the solution acidity increased.Aftera single contact,over95%Cu butonly 14%Fe were extracted.The extraction of aluminium and zinc was negligible.This clearly shows that the SSX system can be used to separate copper from other metals,including Fe(III),in chloride solutions with a few extraction stages.
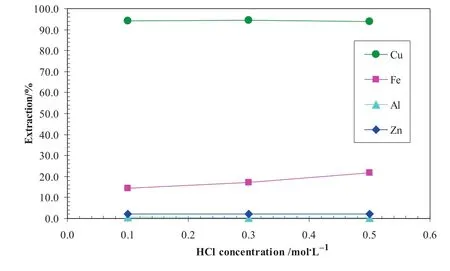
Fig.18.Effect of HClconcentration on metal extraction with the SSX system consisting of 0.3 mol·L-1 LIX63 and 0.33 Versatic 10 at A/Oratio of1:2 and 40 °C.Aqueous solution:Cu 15 g·L-1,Fe(III)10 g·L-1,Zn 3 g·L-1,Al(III)2 g·L-1 and CaCl2 350 g·L-1.
The co-extracted iron was scrubbed with a scrub solution containing 350 g·L-1CaCl2and 0.2 mol·L-1HCl.A totalof4 batch scrubbing tests were successively conducted at an A/Oratio of1:4 and 40°C.As shown in Fig.19,after the first two scrubbing stages,over 99.8%Fe(III)was scrubbed with <1 mg·L-1Fe(III)left in the organic solution.About 11%Cu was also scrubbed in each of the first two scrubbing stages.In a counter-currentoperation,all the scrubbed copper would be extracted again by the organic solution in the subsequent extraction stages.
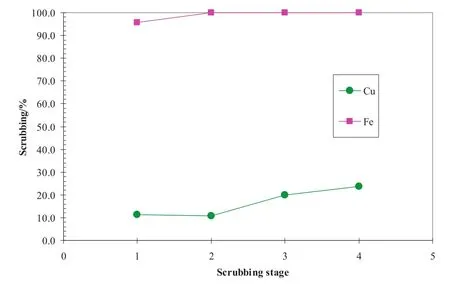
Fig.19.Scrubbing iron from the loaded organic solution with chloride solution in 4 batch scrubbing stages.Loaded organic solution:6.8 g·L-1 Cu,0.32 g·L-1 Fe(III).Scrub solution contained 350 g·L-1 CaCl2 and 0.2 mol·L-1 HClat A/O ratio 1:4 and 40 °C.
The chloride scrubbing pHisotherm is shown in Fig.20.AtpH 4,over 99.5%chloride was scrubbed with only 39 mg·L-1Clleftin the scrubbed organic solution,while the scrub efficiency of copper was only 0.75%.In a counter-current operation,the scrubbed copper would be extracted again by the organic solution in the subsequent scrub stages.
With the above solvent extraction circuit,copper can be separated from iron in chloride solutions,transferred to sulphate solution and recovered as pure copper cathodes by conventional electro winning.A proposed flow sheet is shown in Fig.21.
This process,consisting of chloride leaching,copper solvent extraction and electro winning,and iron hydrolysis would have the following main features:
·Hydrochloric acid leaching provides faster leaching kinetics and higher copper recovery compared with sulphuric acid leaching;
·Copper is readily separated from iron in the high chloride concentration solution by the novel SSX system;
·Iron is readily scrubbed from the copper-loaded SSX system with a weak acid(0.2 mol·L-1HCl)solution;
Fig.20.Scrubbing chloride from the loaded organic solution with water under different scrubbing pH values at an A/O ratio of1:2 and 40 °C.Loaded organic solution:6.55 g·L-1 Cu,8.82 g·L-1 Cl.Scrub solution:water.The solution pH adjusted with 30%NaOH solution.
·Chloride is scrubbed with water in a neutralpH range,
·Copper is readily stripped from the loaded SSX system to obtain loaded strip liquor suitable for conventional electro winning;
·Iron hydrolysis is used to regenerate HCl and chloride salts for leaching and to obtain an environmentally friendly product of iron oxide;
·The raf finate is recycled to the leach process to close the circuit.
1.9.Separation and recovery of copper,nickel,cobalt and zinc in chloride solutions by SSX
To recover Cu,Ni,Co and Zn from chloride leach solutions,three solventextraction circuits are required[49–51].In the copper circuit,a SSX system consisting of 0.3 mol·L-1LIX 63,0.3 mol·L-1Versatic 10 and 0.7 mol·L-1TBP in Shellsol D70 was used.After a single contact with the synthetic solution containing(g·L-1)Cu 12,Ni8,Co 0.7,Zn 0.07,Mn 1.6 and CaCl2300 in the pH range of 0.14–0.8 at an A/O ratio of 1:2 and 40°C,over 95%Cu was extracted by the SSX system(Fig.22).Some 12%Niand negligible Co,Mn and Ca were co-extracted.
To scrub the impurities,metaland chloride scrubbing pH isotherms were obtained(Fig.23).The loaded organic solution contained 5.57 g·L-1Cu,0.52 g/L Ni,0.032 g·L-1Zn and 3 mg·L-1Co and 0.4 mg·L-1Mn and Ca.Water was used as scrub solution.It was found that in the pH range of 3.0–3.5,90%–92%Zn,80%–90%Cl,88%Co and 70%–86%Niwere scrubbed while only 0–11%Cu was scrubbed in a single contact.In a counter current operation,the co-extracted zinc,cobalt,nickeland chloride should be readily scrubbed in this pH range studied.
The key to the success of the SSX system is the scrubbing of chloride so that the copper can be transferred from a chloride solution to a sulphate solution for electro winning.The require ment for chloride concentration in the copper electrolyte normally is below 30 mg·L-1.Although 92%Clwas scrubbed atpH 4 in Fig.23,to make sure that the chloride can be removed to meet the requirement of conventionalcopper electrow inning,a two-stage successive chloride scrubbing test was conducted.The loaded organic solution containing 7.53 g·L-1Cu and 8.08 g·L-1Clwas scrubbed twice successively using water with pH adjusted to 4.0 at an A/O ratio of1:4 and 40°C(Table 17).After the first scrubbing,97%Clwas scrubbed with about 0.25 g·L-1Clremaining in the organic solution.After the second scrubbing,99.6%Clwas scrubbed with only 29 mg·L-1Clremaining in the organic solution.In a counter current scrubbing operation,it is estimated that 3 scrub stages would reduce the chloride concentration to less than 10 mg·L-1.After scrubbing and stripping with copper spentelectrolyte,the loaded strip liquor would be suitable for copper conventionalelectrowinning in terms of chloride concentration in the advanced copper electrolyte.
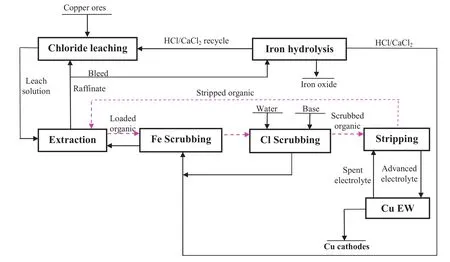
Fig.21.A schematic of a proposed process flow sheet for the separation of copper from iron and the recovery of copper from chloride solutions by a synergistic solvent extraction system and conventionalelectro winning.
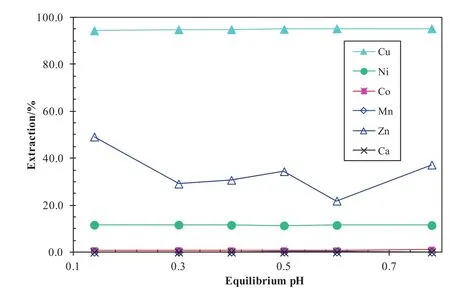
Fig.22.Metalextraction pHisotherms with the SSXsystem and the feed solution containing 300 g·L-1 CaCl2 using an A/O ratio of 1:2 at 40 °C.

Fig.23.Scrubbing pHisotherms ofelements with the SSXsystematan A/Oratio of1:2 and 40°C.

Table 17 Chloride scrubbing at pH 4.0,an A/O ratio of1:4 and 40°C
After copper was extracted by the SSX system,nickel,cobalt,manganese and zinc remained in the raf finate.In the cobalt-zinc SX circuit,an organic solution consisting of20%Alamine 336(tri-octyl/decylamine)and 20%TBP in Shellsol D70 was mixed with a synthetic solution simulating the composition of the copper-depleted raf finate containing(g/L)Ni9.0,Co,0.76,Zn 0.10,Mn 1.1,Ca 108 and Cl200 at an A/O ratio of1:1 and 40°C to determine metal extraction pHisotherms(Fig.24).It can be seen that the metal extraction order was Zn>Co>Mn≫Ni/Ca.In the pH range of 0.5–3.5,87%–90%Co,89%–99%Zn and 37%–43%Mn were extracted and almost no nickeland calcium extracted.The separation factors of zinc and cobalt over nickel were in the range of 1.8 × 104–3.7×105,indicating that cobalt and zinc can be separated from nickel and calcium very well.
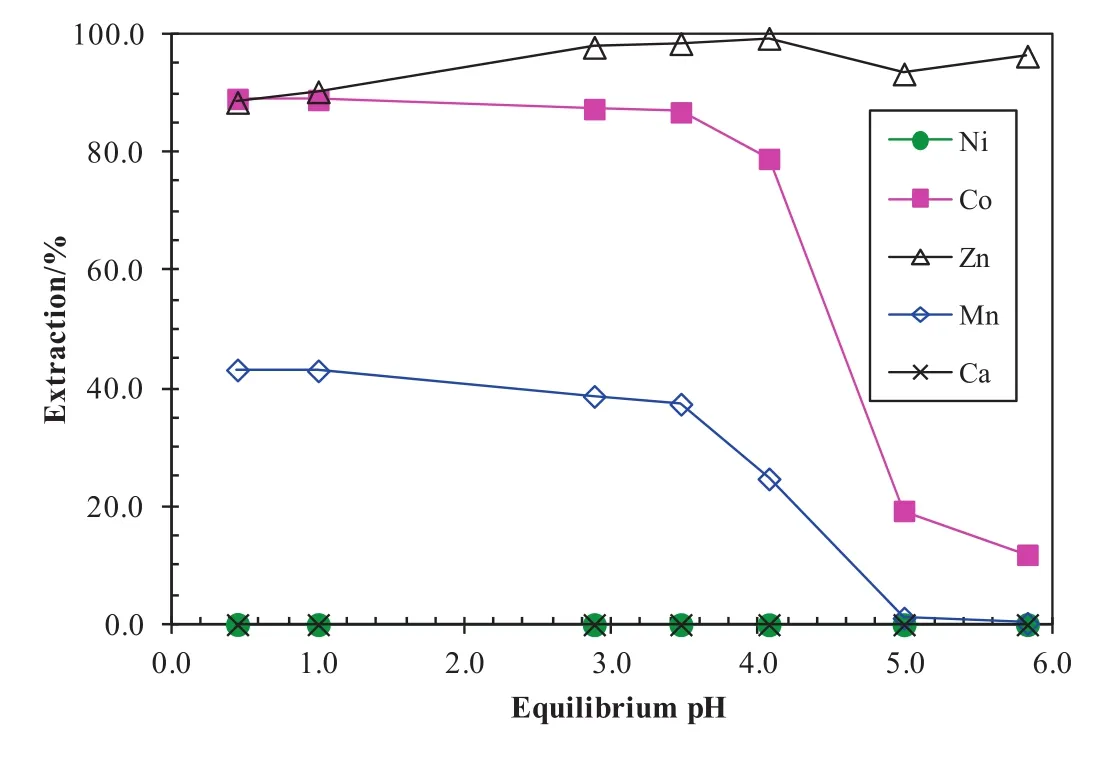
Fig.24.Metalextraction pH isotherms with the Alamine 336 and TBP system at an A/O ratio of1:1 and 40°C.
The raf finate of the cobalt–zinc SXcircuitcontained(g·L-1)Ni10,Mn 1.1,Ca 124 and Cl240.Since only a small part of nickel forms weak neutral complex with chloride ions,the SSX system consisting of LIX63,Versatic 10 and TBP in ShellsolD70 could be,again,used in the nickelcircuitto extract the nickelin the chloride solution as ifin sulphate solutions[14,17].With the SSX system consisting of 0.3 mol·L-1LIX63,0.3 mol·L-1Versatic 10 and 0.7 mol·L-1TBP in ShellsolD70,over 98%Niwas extracted with only negligible manganese and calcium extracted in a single contact at pH 4.5,an A/O ratio of1:4 and 40°C(Fig.25).The co-extracted chloride should be negligible.After stripping the nickelusing spent sulphate electrolyte,the loaded nickelstrip liquorshould be suitable for conventional electro winning.
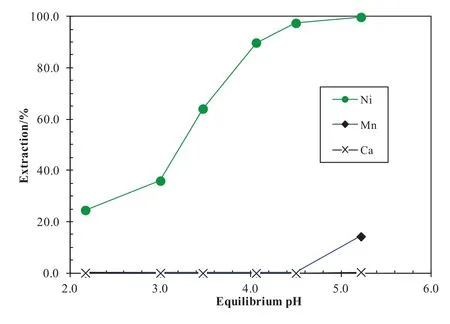
Fig.25.Metalextraction pH-isotherms with the SSXsystematan A/Oratio of1:4 and 40°C.
With the three circuits,copper,cobalt,nickeland zinc can be separated and directly recovered from chloride solutions in the proposed flow sheet(Fig.26).This process has the following main features and advantages:
(1)A process flow sheetthateffectively and efficiently separates and recovers copper,cobalt,nickeland zinc from chloride solutions.
(2)The product solutions bearing copper and nickel from their respective SSX circuits are suitable for conventionalelectrowinning.
(3)Capitaland operating costs are saved due to the use of SSX systems and conventionalelectrowinning processes.
(4)The finalraffinate is suitable for recycling to the leach process without treatment.
(5)Environment pollution is expected to be considerably reduced compared with other processes due to the closed cycles.
2.Conclusions
Highly selective SX extractants for metals are rare and the development and commercialisation of new,highly selective extractants is prohibitively expensive.Therefore,the use of synergists,preferably commercially available reagents,to improve the selectivity of the existing extractants is an attractive alternative.A number of SSX systems including(1)Versatic 10/CLX50,(2)Versatic 10/4PC,(3)Versatic 10/LIX63,(4)Versatic 10/LIX63/TBP,(5)Cyanex 272/LIX84 and(6)HRJ-4277/Cyanex 471X,have been developed by the SX technology team of CSIRO to recover nickel,cobalt,zinc and copper from sulphuric and chloride leach solutions.Signi ficant synergistic effect has been determined and possible applications demonstrated.
Acknowledgements
The authors would like to thank CSIROresearchers forrevie wing this paper and providing valuable comments.The past contributions of CSIRO staff members including Mark Urbani,Davood Moradkhani,Michael Davies,Nicki Turner,Nick Kelly,Denies Shiers,Dave Robinson,Peter Miovski,Russell Pennifold as well as others including David Jones(CESL),Keith Mayhew(CESL),Tannice McCoy(CESL),David Dreisinger(UBC),GeoffBoddy(Rio Tinto)and Mark Godfrey(Rio Tinto)to the systems outlined in this review are gratefully acknowledged.The support of the CSIRO Mineral Resources Flagship and various industrial companies is acknowledged.
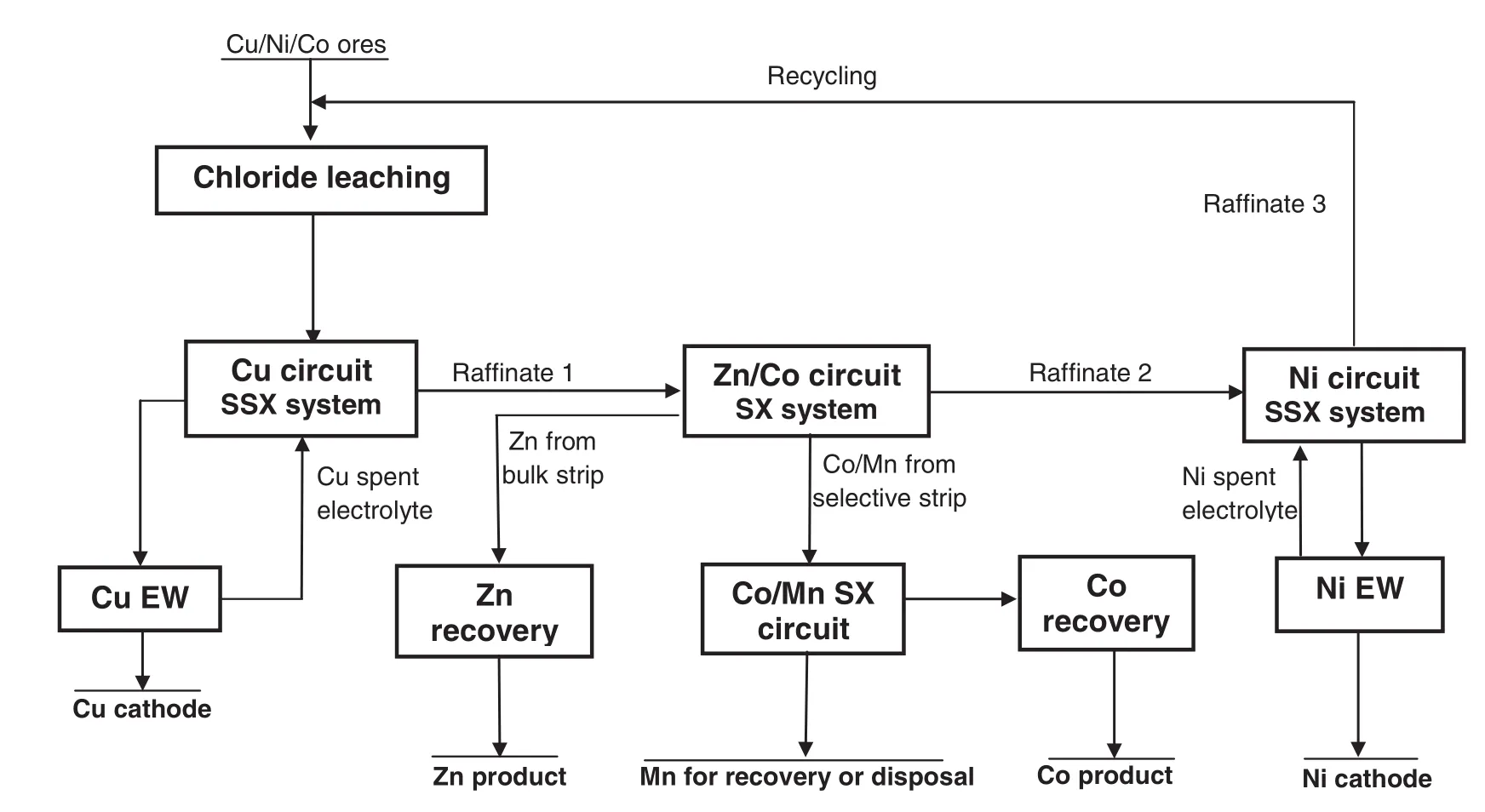
Fig.26.A proposed process flow sheet for the recovery of copper,cobalt,zinc and nickel from chloride solutions.
[1]C.Y.Cheng,K.R.Barnard,W.Zhang,D.J.Robinson,Synergistic solvent extraction of nickel and cobalt:a literature review of recent developments,Solvent Extr.Ion Exch.29(2011)719–754.
[2]C.Y.Cheng,M.G.Davies,Separation ofnickel from calcium using synergistic solvent extraction,Proceedings of International Symposium of Solvent Extraction,Bhubaneswar,India,September,2002 2002,pp.71–88.
[3]C.Y.Cheng,M.D.Urbani,Recovery of nickel and cobalt from leach solutions by direct solvent extraction,ALTA Nickel/Cobalt-8,Perth,May,20022002.
[4]C.Y.Cheng,M.D.Urbani,Puri fication of laterite leach solutions by direct solvent extraction,Yazawa International Symposium on Metallurgical and Materials Processing:Principals and Technologies,TMS Annual Meeting,San Diego,USA,vol.3 March 2003,pp.251–265.
[5]C.Y.Cheng,SX application for nickel and cobalt:pros and cons of existing processes and possible future development,Proceedings of ALTA SX/IX World Summit,ALTA Metallurgical Services,Perth,Australia,May,2003.
[6]C.Y.Cheng,M.D.Urbani,M.Houchin,Synergistic solvent extraction and its potential application to nickeland cobalt recovery,Hydrometallurgy 2003—Fifth International Conference in Honour of Professor Ian Ritchie,Leaching and Solution Puri fication,Vancouver,Canada,vol.1 August 2003,pp.787–798.
[7]C.Y.Cheng,M.D.Urbani,The recovery of nickeland cobalt from leach solutions by solvent extraction:practice,research and development,International Conference on Hydrometallurgy(ICHM)October 16–19,2004,Xian,China,Abstracts 2004,p.20.
[8]C.Y.Cheng,M.D.Urbani,The recovery of nickeland cobalt from leach solutions by solvent extraction:process overview,recentresearch and development,Proceeding of ISEC 2005,Beijing,China September 2005,pp.503–526.
[9]C.Y.Cheng,K.R.Barnard,M.D.Urbani,Application of synergistic solvent extraction for metal recovery,ALTA Ni/Co 2005,ALTA Metallurgical Services,Perth,Australia,May 2005.
[10]C.Y.Cheng,K.R.Barnard,M.D.Urbani,Improving plantoperation using synergistic SX technology and organic monitoring,The First Extractive Metallurgy Operators'Conference,Brisbane,Australia November 2005,pp.59–66(accepted for publication).
[11]K.R.Barnard,M.G.Davies,C.Y.Cheng,Stability of the Versatic 10-decyl-4-pyridinecarboxylate system under continuous SX conditions:chemical analysis and phase disengagement studies,Hydrometallurgy 74(2004)47–56.
[12]D.Dreisinger,C.Y.Cheng,W.Zhang,Y.Pranolo,Development of Boleo process flow sheet and its direct solvent extraction(DSX)circuit,Proceedings of the International MineralProcessing Conference,Brisbane Australia September 2010,pp.309–317.
[13]C.Y.Cheng,M.D.Urbani,Solvent extraction process for separation cobalt and/or manganese from impurities in leach solutions.Patent Publication No.WO 2005/073415 A1(2005).
[14]C.Y.Cheng,Solvent extraction of nickel and cobalt with synergistic systems consisting of carboxylic acid and aliphatic hydroxyoxime,Hydrometallurgy 84(2006)109-107.
[15]C.Y.Cheng,W.Zhang,Y.Pranolo,Recovery of cobalt and zinc from Boleo leach solutions using the CSIRO DSX process,ALTA Ni/Co,PerthMay 2007.
[16]C.Y.Cheng,W.Zhang,Y.Pranolo,Recovery of cobalt and zinc from Boleo leach solutions using the CSIRO DSX process,Proceedings of ISEC 2008,Solvent Extraction:Fundamentals to IndustrialApplications,Tucson,USA September,2008,pp.163–168.
[17]C.Y.Cheng,W.Zhang,Y.Pranolo,Separation of cobalt and zinc from manganese,magnesium and calcium using synergistic solvent extraction system consisting of Versatic 10 and LIX63,Solvent Extr.Ion Exch.28(2010)604–624.
[18]Anon,Boleo Pilot Plant Program at SGS Lake field Research Successfully Completed,News release of Baja Mining Corp.,Toronto,December 2004.
[19]Anon,Baja Mining Phase 2 Pilot Plant Program Successfully Completed,News release of Baja Mining Corp.,Toronto,July 2006.
[20]K.R.Barnard,LIX®63 stability in the presence of Versatic 10 under proposed commercialextract and strip conditions,part I:operation at high temperature,Hydrometallurgy 91(2008)1–10.
[21]K.R.Barnard,N.L.Turner,LIX®63 stability in the presence ofVersatic 10 underproposed commercialextractand strip conditions,partII:oxime isomerinter-conversion and the effect ofoxime degradation products on selected physicalproperties,Hydrometallurgy 91(2008)11–19.
[22]K.R.Barnard,N.L.Turner,D.W.Shiers,LIX®63 stability in the presence of Versatic 10 under proposed commercialextract and strip conditions,part III:effect ofmanganese and cobalt loading on oxime stability at 30 °C,Hydrometallurgy 104(2010)268–277.
[23]K.R.Barnard,M.N.Tsuntsaeva,Identi fication of four additionalhydroxyoxime degradation products formed in the LIX 63/Versatic 10 synergistic system and their effect on metalselectivity,Solvent Extr.Ion Exch.31(1)(2013)79–94.
[24]K.R.Barnard,N.J.Kelly,D.W.Shiers,T.M.McCoy,D.L.Jones,K.E.Mayhew,The effect of LIX 63 hydroxyoxime degradation products on kinetics-based metal extraction,Solvent Extr.Ion Exch.32(1)(2013)95–110.
[25]K.R.Barnard,Current understanding of LIX®63/Versatic 10 synergistic solvent extraction system chemistry,in:F.Valenzuela L.,B.A.Moyer(Eds.),Gecamin Conference for Mining,Santiago,Chile,October 2011 Chapter 7,Paper 162.
[26]C.Y.Cheng,M.D.Urbani,Solvent extraction process for separation cobalt and/or nickel from impurities in leach solutions.Patent Publication No.WO 2005/073416 A1(2005).
[27]K.R.Barnard,M.D.Urbani,The effect of organic acids on LIX®63 stability under harsh strip conditions and isolation of a diketone degradation product,Hydro metallurgy 89(2007)40–51.
[28]C.Y.Cheng,G.Boddy,W.Zhang,G.Godfrey,K.R.Barnard,D.J.Robinson,Y.Pranolo,Z.Zhu,W.Zhang,L.Zeng,N.L.Turner,T.N.Hill,Separation of nickel and cobalt from manganese,magnesium and calcium by synergistic solvent extraction—from batch tests to pilot plant operation,Proceedings of the International Mineral Processing Conference,Brisbane Australia September 2010,pp.285–297.
[29]K.R.Barnard,N.L.Turner,Hydroxyoxime stability and unusual cobaltl oading behaviour in the LIX 63-Versatic 10-tributylphosphate synergistic system under synthetic laterite conditions,Hydrometallurgy 109(2011)29–36.
[30]K.R.Barnard,N.L.Turner,The effect of temperature on hydroxyoxime stability in the LIX 63-Versatic 10-tributylphosphate synergistic solvent extraction system under synthetic nickellaterite conditions,Hydrometallurgy 109(2011)245–251.
[31]N.L.Turner,K.R.Barnard,The effect of individual complexed metals on hydroxyoxime stability in the LIX 63-Versatic 10-tributylphosphate synergistic solvent extraction system under synthetic nickel laterite extract conditions,Hydrometallurgy 117–118(2012)79–85.
[32]C.Y.Cheng,G.Boddy,W.Zhang,M.Godfrey,D.J.Robinson,Y.Pranolo,Z.Zhu,L.Zeng,W.Wang,Direct solvent extraction for recovery ofnickeland cobalt from Rio Tinto laterite leach solutions,Proceedings of International Symposium,Hydrometallurgy of Nickel and Cobalt 2009,Canada 2009,pp.243–254.
[33]C.Y.Cheng,G.Boddy,W.Zhang,M.Godfrey,D.J.Robinson,Y.Pranolo,Z.Zhu,W.Wang,L.Zeng,A DSX process for Niand Co recovery from Rio Tinto laterite leach solutions using SSX technology—from batch tests to pilot plant operation,ALTA Nickel/Cobalt,Perth AustraliaMay 2009.
[34]C.Y.Cheng,G.Boddy,W.Zhang,M.Godfrey,D.J.Robinson,Y.Pranolo,Z.Zhu,W.Wang,Recovery of nickeland cobalt from laterite leach solutions using direct solvent extraction:part 1—selection of a synergistic SX system,Hydrometallurgy 104(2010)45–52.
[35]C.Y.Cheng,G.Boddy,W.Zhang,M.Godfrey,D.J.Robinson,Y.Pranolo,Z.Zhu,L.Zeng,W.Wang,Recovery of nickel and cobalt from laterite leach solutions using direct solvent extraction:part 2—semi-and fully-continuous tests,Hydrometallurgy 104(2010)53–60.
[36]Anon,Cyanex 272 Extractant,Cytec Industries Inc.,2007
[37]K.E.Mayhew,T.M.McCoy,D.L.Jones,K.R.Barnard,C.Y.Cheng,W.Zhang,D.J.Robinson,Kinetic separation of Co from Ni,Mg,Mn and Ca via synergistic solvent extraction,Solvent Extr.Ion Exch.29(2010)755–781.
[38]T.M.McCoy,K.E.Mayhew,D.L.Jones,K.R.Barnard,C.Y.Cheng,D.J.Robinson,W.Zhang,Separation ofCo from Niin an impure sulphate solution,part1—extraction,in:F.Valenzuela L.,B.A.Moyer(Eds.),Gecamin Conference for Mining,Santiago,Chile,October 2011 Chapter 2,Paper 56.
[39]T.M.McCoy,K.E.Mayhew,D.L.Jones,K.R.Barnard,C.Y.Cheng,D.J.Robinson,W.Zhang,Separation of Co from Niin an impure sulphate solution,part 2—stripping,in:F.Valenzuela L.,B.A.Moyer(Eds.),Gecamin Conference for Mining,Santiago,Chile,October 2011 Chapter 2,Paper 57.
[40]C.Y.Cheng,M.Rodriguez,Upgrading cobaltproduct using synergistic solventextraction technology at Murrin Murrin Operation ofMinara Resources,ALTANi/Co,Perth,AustraliaMay 2006.
[41]C.Y.Cheng,W.Zhang,M.Rodriguez,Separation ofCopper,Iron and Zinc from Cobalt and Nickelin Minara Process Liquor by Synergistic Solvent Extraction:Pilot Plant Operation,ALTA Ni/Co,Perth,May,2007.
[42]C.Y.Cheng,W.Zhang,Puri fication,separation and recovery of metals by synergistic solvent extraction,Proceedings of EMC,Dusseldorf,Germany,June 11–14 2007.
[43]W.Zhang,C.Y.Cheng,M.Rodriguez,Y.Pranolo,Separation of copper,iron and zinc from cobalt and nickelwith mixed Cyanex 272 and LIX84 extractants,Proceedings of ISEC 2008,Solvent Extraction:Fundamentals to Industrial Applications,Tucson,USA September 2008,pp.177–182.
[44]D.Moradkhani,M.D.Urbani,C.Y.Cheng,The separation ofcadmium from zinc with a novel synergistic mixture of nonylsalicylic acid and tri-isobutylphosphine sulphide,Hydrometallurgy 78(2005)129–136.
[45]C.Y.Cheng,D.Moradkhani,M.D.Urbani,M.Askari,D.Bastani,The separation of cadmium from zinc with a novel synergistic system and the synergistic mechanism interpreted by a new slope analysis method,Proceeding of ISEC 2005,Beijing,China September 2005,pp.527–539.
[46]C.Y.Cheng,W.Zhang,D.Moradkhani,The recovery of metals from leach solutions by synergistic solvent extraction,HydroProcess Conference,Iquique,Chile October 2006,pp.3–16.
[47]C.Y.Cheng,Z.Zhu,W.Zhang,A novel synergistic solvent extraction system for separating copper from iron in strong chloride solutions and transferring it into sulphate solutions for recovery,ALTA Ni/Co/Cu Conference,Perth,Australia,ALTA Metallurgical Service,May 28–30 2010.
[48]Z.Zhu,W.Zhang,C.Y.Cheng,A novel synergistic solvent extraction system for separating copper from iron in strong chloride solutions and transferring it into sulphate solutions for recovery,Hydrometallurgy 113–134(2011)155–159.
[49]C.Y.Cheng,Z.Zhu,W.Zhang,A DSX Process to Recover Copper,Nickel,Cobalt and Zinc from Chloride Solutions by Synergistic Solvent Extraction,ALTA Copper,Perth Australia,ALTA Metallurgical Service,May 2009.
[50]Z.Zhu,C.Y.Cheng,W.Zhang,A novel synergistic solvent extraction process for the recovery of copper,nickel,cobalt and zinc from chloride solutions,in:F.Valenzuela L.,B.A.Moyer(Eds.),Gecamin Conference for Mining,Santiago,Chile,October 2011,Chapter 7,Paper 169,October 2011.
[51]Z.Zhu,W.Zhang,Y.Pranolo,C.Y.Cheng,Separation and recovery of copper,nickel,cobalt and zinc in chloride solutions by synergistic solvent extraction,Hydrometallurgy 127–128(2012)1–7.
 Chinese Journal of Chemical Engineering2016年2期
Chinese Journal of Chemical Engineering2016年2期
- Chinese Journal of Chemical Engineering的其它文章
- Relationship between breakthrough curve and adsorption isotherm of Ca(II)imprinted chitosan microspheres for metaladsorption☆
- Experimental study on the effects of big particles physical characteristics on the hydraulic transport inside a horizontal pipe
- Investigation of extraction fraction in con fined impinging jet reactors for tri-butyl-phosphate extracting butyric acid process☆
- Experimental evaluation and modeling of liquid jet penetration to estimate droplet size in a three-phase riser reactor
- Photorheologically reversible micelle composed ofpolymerizable cationic surfactant and 4-phenylazo benzoic acid☆
- Review on current advances,future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents☆
