Review on current advances,future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents☆
Zhiwu Liang*,Kaiyun Fu,RaphaelIdem,Paitoon Tontiwachwuthikul
Joint International Center for CO2 Capture and Storage(iCCS),ProvincialKey Laboratory for Cost-effective Utilization of Fossil Fuel Aimed at Reducing Carbon-dioxide Emissions,College of Chemistry and Chemical Engineering,Hunan University,Changsha 410082,China
1.Introduction
The rapid development of modern industrialization relies heavily on the combustions off ossilfuels(i.e.,coal,petroleum,and naturalgas)for energy supply,which is the contributor of83%of anthropogenic greenhouse gas emissions.Among them,carbon dioxide(CO2),methane(CH4)and nitrous oxide(N2O)account for 93%,6%and 1%,respectively[1].From the World Energy Outlook(WEO2013)projects,global CO2emissions from combustion of fuels will continue to grow from 31.3 Gt in 2011 to 37.2 Gtin 2035 unless tremendous efforts are made to control CO2emissions[1].To solve this serious environ mentalissue,governments and researchers around the world have been making greatefforts to reduce the globalat mospheric concentration of CO2.
Carbon capture and storage(CCS)[2],an attractive approach to reducing CO2emissions,has come into being and is expected to play a vitalrole in CO2emission mitigation in the foreseeable future untilthe development and application of low-carbon and even zero-carbon energy technologies can truly be achieved[3].The CCS process is an integration of various difficult technologies referring to CO2capture at the point of emission from various large CO2emitters,followed by the compression of gaseous CO2into liquid form,then followed by the transportofliquefied CO2by means of pipeline or ship,and eventually the storage of CO2in exploited coal and oil fields or the ocean floor.CCS is expected to reduce 20%of future world CO2emissions[4].So far,dozens of CCS projects have been proposed,with the intention of carrying out large demonstrations worldwide[2].However,the high cost of CO2capture,which primarily comes from the large energy requirement,greatly hampers the development of CO2capture technologies and the effective deployment of CCS projects[5].
The combustion off ossilfuels(mainly coal)for electricity generation and heating accounts for a large part(about 42%in 2011)of the total energy-related CO2emissions and is the main single large-CO2emitter,as shown in Fig.1[1].As a consequence,CO2capture from fossil fuelfired power plants is a critical factor to consider as the primary approach towards global CO2emission mitigation.
In a typicalcoal-fired power plant combustion flue gas,N2has the largest fraction ofcomponents,followed by CO2,H2O,O2and then several trace components of NOx,SOxand other compounds,as shown in Table 1[6].
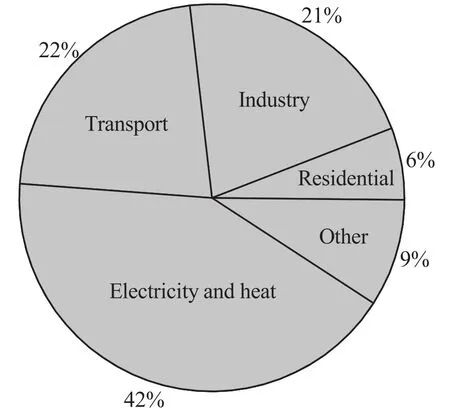
Fig.1.Emission of CO2 by activity sector.Data from[1].

Table 1 Typical flue gas composition in the coal-fired power plant[6]
So far,three strategies have been proposed to capture CO2,i.e.,postcombustion,pre-combustion and oxyfuelCO2capture.They have different working concepts and objectives,leading to different requirements of separation facilities and capture materials.Post-combustion CO2capture,which is at the end of the wet exhaust flue gas of power plants with fossil fuels burned completely with air,involves mainly the separation of CO2from a CO2/N2mixture.Pre-combustion strategy,which is a process with fossil fuels decarbonized prior to combustion,primarily captures CO2from a CO2/H2mixture.It is usually operated ata high pressure and a slightly elevated temperature.Oxyfuelcom bustion CO2capture refers to a combustion process offossilfuelwithout N2(i.e.using O2separated from air),with the combustion of fossilfuels in a nearly pure O2environment.As a consequence,the productofco mbustion is almost entirely CO2.The selection of a strategy depends on economic and reliability considerations.According to many assessments of the advantages and disadvantages of these strategies on the aspects of technology,economy,and environment,post-combustion CO2capture has been considered to be the most technologically mature and commercially viable option to capture CO2from fossil fuel-fired power plants,while the other strategies are either still in pilot testing or even at laboratory scale study[7–9].
It is to be noted that low CO2concentration in flue gas requires CO2capture materials with strong interaction between materials and CO2.Currently,a variety of materials have been considered for CO2capture[10],including amines[8],ionic liquids[11],metal–organic frameworks[12],solid porous adsorbents[13],membranes[14],etc.Since reactive solvents can combine CO2quickly by means of a chemical bond,the chemicalabsor ption method enables a high capture efficiency of CO2from flue gas and a nearly pure CO2product.According to the investigations by the Electric Power Research Institute,absorption-based processes account for the majority(60%)of post-combustion CO2capture technologies,followed by membrane(14%),mineralization(14%)and adsorption(12%)[15].It indicates that absorption-based technology has a high operation con fidence and is considered to be the mostattractive option to capture CO2from coal-fired power plants in the short-tomedium terms[7,8,16].
This review first seeks to provide technical and economical assessments of chemical absorption technologies for the post-combustion CO2capture.It also looks atpresentprocesses,challenges and improved strategies.In addition,particular attention is directed to wards the issues of fundamental research and recent advancements associated with chemical absorption technology,so as to illustrate the behavior of post-combustion CO2capture using chemical absorption methods.Furthermore,this review will also look at emerging trends in postcombustion CO2capture using chemical solvents in the near to mid-term.
2.Current and Emerging Chemical Solvents for CO2 Capture
2.1.Conventional chemical absorption technologies and their challenges
The absorption of CO2into aqueous amine solutions is among the most effective technologies for CO2capture from coal-or natural gas fired power plants[17].Monoethanol amine(MEA),a primary amine,has been industrially employed for over 50 years.It is regarded as a state-of-the-art process,with the operating conditions of 20%–30%(by mass)aqueous MEA,0.2–0.35 mol·mol-1lean loading,and inlettem perature of approximately 40°C[8].These operation values are based on a compromise among different parameters of MEA,including solubility,kinetics,regeneration,degradation and corrosion.MEA is very reactive with CO2,cheap and easy to produce.However,it still faces numerous challenges,such as high energy consumption for solvent regeneration,low capacity to absorb CO2,poor thermal stability and high corrosivity.
In the pastdecades,several principalamines have been commercially employed to overcome the limitations of MEA,including diethanolamine(DEA),N-methyldiethanolamine(MDEA),2-amino-2-methyl-1-propanol(AMP)[17]and ammonia(NH3)[18,19].For MEA,DEA and MDEA,their performance in terms of CO2absorption capacity,absorption rate and heat of absorption can be ranked as:MEA~DEA<MDEA,MEA>DEA>MDEA and MEA<DEA<MDEA,respectively[17].Since these amines have their own drawbacks,specialty amines named hindered amines have emerged.AMP,a primary sterically hindered amine,reacts with CO2directly to form a carbamate in a way similar to MEA and DEA,with a considerable reactivity.Meanwhile,AMP contains larger substituents,which make its carbamate unstable and easy to form a bicarbonate(which can free AMP to react with CO2again)[10,20].NH3has a higher CO2absorption capacity and a lower heat of reaction compared to MEA.In addition,NH3can also be used to remove NOx,SOxand mercury from flue gas in a coal-fired power plant.However,its low absorption rate and high volatility present a significant obstacle to achieve a low-cost CO2capture.Therefore,the absorption of CO2into NH3should be carried out at a low temperature to reduce solvent loss.
Clearly,no single solvent exists that possesses allexcellent features for CO2capture.Therefore,research attentions should turn towards the develo pment of promising solvents with both high CO2capture performance and low operation cost.
2.2.Emerging chemicalsol vents for CO2 capture
In the recent years,blending of commercialsol vents has been considered as potentialappl ications in CO2absorption.The system combines the advantages of primary/secondary amines(fast kinetics)and tertiary/sterically hindered amines(high absorption capacity and low energy consumption to strip CO2).Studies on blended solvents,such as MEA–MDEA[21],DEA–MDEA[22]and AMP–piperazine(PZ)[23],have demonstrated a great improvement on the performance in terms of kinetics,solubility,mass transfer,as well as regeneration energy.A pilotplantteston a mixture of4 mol·L-1MEA/1 mol·L-1MDEA,carried outat the InternationalTest Centre for CO2Capture,University ofRegina,Canada,has shown that the MEA–MDEA solution consumes a much less heat-duty than single MEA solution and maintains the chemical stability of the solvent.Besides,the use of PZ–potassium carbonate(K2CO3)is also near the commercial stage.Experimental results from pilot plant have shown that the CO2absorption rate of5 mol·L-1K+/2.5 mol·L-1PZ is 1–1.5 times faster than thatof7 mol·L-1MEA,while the heatduty requirement for stripping CO2may be slightly higher than that with MEA[24].
More recently,attention has turned to new promising solvents,examples are some novelsterically hindered amines such as 2-((2-aminoethyl)amino)ethanol(AEEA)[25],2-(diethylamino)ethanol(DEEA)[26],4-diethylamino-2-butanol(DEAB)[27–29],diethylenetriamine(DETA)[30–33],and piperidine(PIP)[34].It is found that structural modi fication of an absorbent has greateffect on improving the amine performance to capture CO2.The introduction ofsubstituents atα-carbon,making MEA become AMP for example,has been employed as a strategy to promote the hydrolysis of carbamate to form bicarbonate,enabling higher CO2loadings[35].In addition,Singh et al.[36]have found that for most absorbents,an increase in chain length of substituent on the nitrogen atom is in favor of absorption capacity but adverse to the initial absorption rate(possibly because it would slow down the formation of carbamate)[36].They have also pointed out that(1)alkyland amine groups are the most noticeable substituted functional groups(atα-carbon)to enhance the absorption rate and capacity of amine absorbents;(2)hydroxyl groups increase absorption capacity but decrease absorption rate;and(3)aromatic amines substituted with alkyl groups at the 2nd and 5th positions present higher absorption capacity and rate[37].Their efforts towards revealing the effects of structurally variant amines on the initial CO2absorption rate and capacities provide valuable guidance to make a promising absorbent for CO2capture.Therefore,by means of screening and designing potentialamine solvents and modifying their chemical structures,it is possible to obtain a more desirable absorbent[34].
Biphasic solvent constitutes a new class of blended amine solvent systems,such as dixmixing process solution,named DMX™[38],and thermomor phicbiphasic solvents(TBSs)[39].The concept of the DMX process is that CO2absorption in such solvents is able to form two immiscible phases,a CO2-rich phase and a CO2-lean phase.Transport of only the CO2-rich phase into the stripper would result in a higher CO2stripping efficiency and a significant reduction ofreboiler heatduty.It is found that the DMX process needs only 2.3(even 2.1)GJ·t-1CO2of reboiler heat duty while the conventional30%(by mass)MEA process consumes atleast3.7 GJ·t-1CO2[40].As for TBS systems such as blended amines of N-methyl-1,3-diaminopropane(MAPA)with DEEA,CO2can be well stripped at a temperature well below the solvent boiling point.The stripping of CO2is at about 80°C,much lower than that with conventional amine solution,about120°C,enabling signifi cantreduction of energy consumption for regeneration and furthermore the utilization of a low temperature steam or even hot water to regenerate the solvent[41].
Ionic liquids(ILs),a new class of absorbents,have attracted much attention since they are non-volatile,thermally stable,non-flammable,and reusable and can be used in the process in a wide liquid range and virtually limitless tunabilities.They have been referred to as green solvents for gas separation since they appeared[42].
At the initialstage,research of CO2capture with ILs mainly focused on physicalILs,with the imidazolium-based ILs being the mostpopular ones[43].The bene fit is low energy consumption for regeneration of physical ILs since the interaction between physicalILs and CO2involves weak van der Waals forces.However,low capacity and selectivity of physical ILs to absorb CO2hinder their large-scale applications for post-combustion flue-gas.To overcome these limitations,Bates etal.[44]first introduced a functional ized or task-specific IL by functional izing imi dazolium cation with a primary amine moiety.This new task-specific IL reversibly reacts with CO2in a 2:1 stoichiometry to form an ammonium car bamate salt,which is very similar to CO2absorption by conventional primary amines.In addition,Sanchez etal.[45]have obtained functionalized ILs1-n-butyl-3-methylimidazol iumtetra fluoroborate([Ambin][BF4])and 1-n-butyl-3-methylimidazolium dicyanamide([Ambin][DCA])by modifying the[bmim]cation with an amino group,increasing CO2capacity.Another functionalized IL 3-n-butyl-3-methylimidazolium tetra fluoroborate([3Amim][BF4])also exhibits a similar trend in terms of CO2capacity.Moreover,Gurkan et al.[46]have proposed two anion-functionalized ILs,trihexyl(tetradecyl)phosphonium prolinate([P66614][Pro])and methioninate([P66614][Met]),to achieve equimolar CO2absorption with a higher efficiency.However,the reaction oftask-specific ILs with CO2can increase the already very high viscosity ofILs.By meansofmolecular simulations,Yu et al.[47]have found that the high viscosity of taskspecific ILs is due to the interaction of anions and–NH2tails of the cations via hydrogen bonding.Guto wski and Maginn[48]have also used molecular simulations to find that using mixtures of reactive task-specific ILs with nonreactive ILs wil lnot only lower viscosities,but also lower synthesis and preparation costs,such as 1-butyl-3-methylimidazolium bis(tri fluoro methanesul fonyl)imide.
In addition to solubility and selectivity,multi-aspectper formance of ILs,such as solvent cost,enthalpy of reaction,density,biodegradability and toxicity,transport properties,volume expansion and micros tructures,inevitably needs consideration before large-scale applications of ILs[49].Ramin et al.[11]have pointed out that changing the property of ILs(for example,by functiona lizing an IL with amine)can enhance their cost-ef ficacy,but ILs are still insufficient to compete with existing commercialsolvents.
3.Main Considerations for Selecting Attractive Solvents
Advanced solvent is of great important for CO2capture processes.However,an appropriate selection of an attractive and possible commercializable solvent is very complex since it involves a series of steps from experiments in laboratory to pilot plant scales,as well as an accurate process simulation.Consideration of a potential solvent should take into account several performance criteria in terms of absorption solubility,kinetics,mass transfer,regeneration,as well as its thermal and chemical stabilities,since these directly affect the capture performance and operating conditions.
3.1.Equilibrium solubility
Reliable equilibrium solubility data of CO2in a solvent is indispensable to the evaluation of the application possibility of the solvent in CO2capture.Dif ficulties with respect to the solubility measurements lie in the limitations of accuracy and precision,especially under high temperature and low CO2loading conditions.
The experimentala pparatuses for solubility measurements can be divided into static and dynamic methods[50].In the static method,the air inside the absorber cell is purged(usually to generate a very low absolute pressure by a vacuum)before starting the experiment,then,known amounts of CO2-preloaded solvent and the gas phase of CO2are fed in the absorber cell.Once the system reaches the equilibrium state,the component concentrations in both the vapor and liquid phases are sampled and analyzed.For the static method,a reliable sampling technique is required to withdraw only a small amount of samples without disturbing the equilibrium.For the dynamic method,feed gas with a certain concentration of CO2is introduced successively into the absorber cell while the equilibriumsol ubility is measured continuously.This method allows withdrawing a great amount of vapor phase samples at the outlet of the absorbercell withoutdisturbing the equilibrium for solubility measure mentat CO2partial pressure below 3 kPa.However,the operation is difficult and time-consuming(generally taking up to 4 h).Generally,the CO2concentration in the gas phase can be analyzed by an infrared CO2gas analyzer,gas chromatography or mass spectrometry;while the CO2contentin the liquid phase can be determined by the standard method given by the Association of Of ficial Analytical Chemists(AOAC)using a Chittick apparatus[51]or the method of BaCO3precipitation and titration[52].
Extensive experimentalstudies on the measurement of CO2solubility into various amines have been carried outunder various conditions.Generally,the solubility of CO2in amine solution increases as the CO2partial pressure increases but decreases as the temperature increases.The structure of an amine solvent also has a strong in fluence on the solubility of CO2.An increase of amine groups and an increase in the alkyl chain length in the amine group increase CO2equilibrium solubility,since more amine groups reactwith CO2and a larger subs tituent group holds CO2[53].The typical solubility ofCO2in various types of amines can be arranged in the following order:diamines or polyamines>tertiary amines>secondary amines>hindered amines>primary amines[54].
3.2.Speciation of CO2 in chemical solvents
Reliable qualitative and quantitative determination of species distribution in the liquid phase is crucialto estimating the characteristics of a multi-componentsolution and developing a VLE model.It can be used to explain the formation of species,to con firm the stoichiometry of overall reaction and the product stability of each chemical reaction,and to illustrate the relationship between the chemicalstructure of an amine solvent and its capacity to capture CO2[55].
Severaltechnologies have been employed to study the speciation of amine–CO2–H2O systems,including nuclear magnetic resonance(NMR)spectroscopy[56],Fourier transform infrared(FT-IR)spectroscopy[57],X-ray crystallography[58],and so on.Among them,NMR spectroscopy(using either13C or1H)has emerged to be the mostfamiliar apparatus in speciation studies to examine the speciation of various CO2-loaded amine systems such as MEA,2-amino-2-hydroxymethyl-1,3-propanediol(AHPD),DEA,DEAB and MEA–DEAB[28,56,59,60].
The NMR spectra can be used to identify the main stable intermediates and the end products since it can display specific chemical shifts with res pectto different functional groups of molecules in the solution.Besides,the content of various species can be determined by calculating their relevant peak area.It is also easier than other analytical instruments in the quantitative measurement since it does not require any calibration[61].Recently,many researchers have focused their speciation studies on combining both NMR and pHmeter.The concentrations of carbamate,bicarbonate and carbonate are tested using NMR,while the free amine and protonated amines are determined through the calculation of deprotonation constant K using a pHmeter.By this approach,a large number of amine–CO2–H2O systems can be tested.Based on summing up the experience of others,Usubharatana[62]has proposed an NMR method by combining a pH meter to evaluate speciation.This evaluation method is very effective and can be regarded as a standard approach.
However,there are stillenormous challenges for speciation evaluation by NMRsince a lot of potential substances coexistin the chemical reaction systems between amines and CO2,including carbamate(which only exists in the case ofprimary orsecondary amines),bicarbonate,carbonate,protonated amines,and so on.For example,24 species have been found in a solution of CO2-loaded aqueous diethylenetriamine(DETA),with carbamate,bicarbamate,and HCO3-/CO32-to be the main components[63].In addition,there are many fast-transferring protons among protonated amine,ions and molecules,so it is difficult to detect some of the unstable species.Furthermore,the NMR method is time-consuming and requires expensive equipment.
3.3.Vapor-liquid equilibrium model
A thermodynamic vapor–liquid equilibrium(VLE)involves both chemical absorption and physical dissolution of CO2in the liquid phase.It is very challenging to find complex speciation reactions and interactions among molecules,ions and ion–molecule in the acid gas–amine systems due to the high nonideality of the liquid phase,in which many molecular and electrolyte species exist.
So far,a number of VLE models have been reported,which can be categorized into three types:non-rigorous models,activity coefficient based rigorous models,and equation of state based rigorous models.These models are presented in Table 2.Non-rigorous models such as Kent–Eisenberg[64]are usually established based on empiricalmathematical relations by fitting of experimental data. These modelsare often easy to obtain and have been successfully applied to various CO2absorption systems. However, they are restricted to the range of experimental conditions and cannot perform predictions of speciation and rigorous energy balances. Since the vapor and liquid phases are quite non-ideal, especially with a highly concentrated amine solution and high pressure, more rigorous models are needed to predict the equilibriumbehavior of the system. Among them, themost commonly used activity coefficient-based models include the Pitzer model[65],Deshmukh–Mather[66],electrolyte-NRTL(e-NRTL)[67],and extendedUNIQUAC model[68].As for the equation of state based rigorous models, its selection is less than that of activity coefficient basedmodels. The mostly used models include electrolyte equation of state(e-EOS)[69]and extended statistical associating fluid theory(SAFT)EOS[70].

Table 2 Main VLE models for CO2 absorption into amine solvents
3.4.Heatof absorption
Theheat of absorption(ΔHabs)isakey parameterinCO2capture because it is directly related to the energy requirement for solvent regeneration.TheΔHabsvalue involves both chemical reaction and physical dissolution of CO2absorption into an amine solution.
Generally,ΔHabscan be obtained by both experimental calorimetric measurements and phase-equilibrium data based onmodel estimation.Direct calorimetric measurement is an accurate, reliable and the first recommended approach to obtainingΔHabs.It is specific to operating conditions,such as temperature, pressure, heat release rate and time[71].Typically,ΔHabscan be expressed in two ways, either differentiationor integration. DifferentialΔHabsis the heat generated when an infinitesimalamount of CO2is absorbed by a solution loading with a certain amount of2,while integralΔHabscorresponds to the molar heat evolved when a certain amount of2is absorbed into pure and fresh amine solutions. The uncertainty sources in the measurement of ΔHabsmainly come fro mthe calculatedamount of2fed to the absorber cell, the content variation of gas phase2in the cell and the quantity of heat that is integrated from the heat flux curve..
If experi mental data ofΔHabsare lacking, they can be estimated bydifferentiating the Gibbs–Helmholtz equation[72],

Although this thermodynamic correlation can be used to evaluate ΔHabsfor various amines, its accuracy depends largely on using reliable and accurate solubility data. It has been reported that±(20%–30%)errorin the solubility data will cause ΔHabsvalues[71].Moreover,such calculation cannot identify the dependencyof temperature due to the linearity of plots of InPCO2vs.1/T.
Experimental studies have demonstrated that ΔHabsof differentamine functional groups can be ranked as:primary>secondary>tertiary[73].As for the solvents with multi-amine groups, their bonding strengths to different amine groups are nonequivalent, resulting in different heats of reaction. In addition,ΔHabscan vary with amine concentration,CO2partial pressure, amine type and so on. Moreover,ΔHabsvalues of tertiary amines are more greatly affected by the operating conditions than those of primary and secondary amines[74].
3.5.Reaction mechanism and kinetics of CO2absorption into amine
Reaction kinetics plays an undeniably key role in CO2absorption.Before obtaining reliable kinetic data, the reaction mechanism should be well understood.
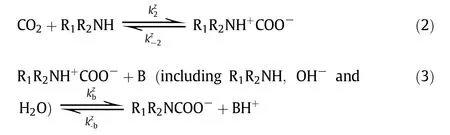
The chemical reactions of CO2with aqueous amines have been extensivelystudied in the last few decades. It is well known that the reaction of CO2with primary and secondary amines can be described by either“zwitterion mechanism”(proposed by Caplow and reintroduced by Danck werts)[75,76]or“termolecular mechanism”(proposed by Crooks and Donnellan)[77].In the zwitterion mechanism, the CO2absorption into primary or secondary amines(R1R2NH)involves a two-step reaction, first the formation of zwitterion and then the deproponation of zwitterions. In the termolecular mechanism,CO2with primary or secondary amine undergoes only one step.Regardless of how the reactions proceed, it is generally accepted that a carbamateand a protonated base are the final products. However, the reaction of CO2with tertiary amines, such asMDEA, follows the “base-catalyzed hydration” mechanism, in which the tertiary amine(R1R2R3N)doesnot react directly with CO2but acts as a base that catalyzes the hydration ofCO2.
(a)Zwitterionmechanism
(b)Termolecularmechanism
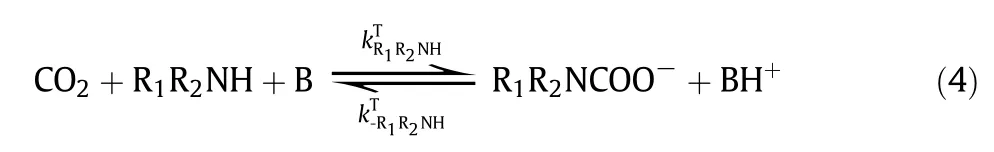
(c)Base-catalyzedhydrationmechanism

Based on the zwitterion mechanism, if the deprotonation of zwitterionsis much faster than their formation, the formation of zwitterions could be considered as the rate-determining step. In this case, theCO2absorption rate can simply be written as

When the formation of zwitterions is much faster than their deprotonation,the CO2absorption rate can be written as

According to the termolecular mechanism, the single-step reaction rate can be presented as

As for the reaction of tertiary amines with CO2,the overall absorptionrate based on the catalyzed hydration mechanism can be expressed as

As is well known, it is of significance to obtain accurate and reliablekinetic data. Several types of experimental apparatus have been used. Examples are the stirred cell reactor, wetted wall column, laminar jet absorber, wetted sphere absorber, string of disc contactor, or stoppedflow apparatus. As shown in Table 3, these apparatuses can be used to measure the kinetic data of CO2absorption into the amine solvent.However, because of the variation in the physics and chemistry as a result of contractors, the reaction rate constants obtained vary with the measurement methods, even in the absorption at similar amineconcentration and temperature[78].Therefore,a suitable experimental apparatus should be selected on the basis of the characteristics of different systems.

Table 3 CO2 absorption rate in different experimentalapparatuses
3.6.Mass transfer
Reliable estimation of mass Reliable estimation of mass transfer is of great importance to process units and operational costs.To provide con fident estimation,a deep insight into the fundamental and complex mass transfer behavior is needed.
Generally,the absorption process refers to the migration of solute from flue gas to the bulk of the liquid phase.This can be expressed by an absorption rate equation as follows.

where NAis the mass flux,aeis the effective interfacialarea,KGis the overallmass transfer coefficient,P is the absorption pressure,yA,Gis the mole concentration of A in the gas phase,H is Henry's law constant,and CA,Lis the content of A in the bulk of the liquid phase.
In addition,the overallmass transfer coefficient can be presented in terms of mass transfer resistance,in which the overallresistance(1/KG)is the sum of the gas-side(1/kG)and liquid-side(HCO2/EkoL)resistances[87].

where kGandare the gas phase and physicalliquid side mass transfer coefficients,respectively,and E is the enhancement factor.
The calculation of the overallmass transfer depends significantly on the accurate estimation of the associated basic parameters,i.e.,kG,,aeand E.Unfortunately,their estimation is very complicated since they are affected simultaneously by many factors including the characteristics of both gas and liquid phases[88],the performance of the absorber[89,90]and also the operating condition[91].More specifically,an increase of amine concentration and temperature as well as a decrease of CO2lean loading willincrease the enhancement factor and eventually increase the mass transfer(but high amine concentration results in a high viscosity of solution,leading to a poor mass transfer);an increase of liquid flow rate yields better mass transfer performance because it willimprove the liquid side mass transfer coefficients and the effective interfacialarea[90];also,an introduction of low concentration surfactants such as n-octyltrimethylammonium bromide(OTABr),sodium dodecylbenzene sulfonate(SDBS)and Tween 80 was found to increase the effective inter facialarea and decrease the liquid phase mass transfer coefficientfor CO2absorption into aqueous solutions in a bubble column[92],furthermore,a 90°successive packing arrangement in a packed column provides the highest comparable efficiency[93].In most cases,the mass transfer resistance of CO2absorption into amine solution resides mostly in the liquid phase,since amine solvents are so reactive that they can absorb CO2within a shortdistance near the gas–liquid interface,consequently,the effective area becomes a principalmass transfer parameter to CO2absorption[81,94].For simplicity of calculation,many researchers determine the partof these parameters by setting assumed conditions and using a special absorber.For example,the liquid side mass transfer EkLocan be obtained by using a lab-scale inter facial area measurable absorber(such as wetted wall column and laminar jet)and pure CO2gas,so that the effects of aeand kGcan be ignored.However,the mass transfer data obtained from these absorbers and operating conditions sometimes vary significantly with those of the real CO2capture process,in which large-scale and effective gas–liquid separators are required and their performance is quite different.
In recentdecades,packed columns are increasingly enjoying market share of industrial-scale gas separation due to their high performance in terms of hydrodynamics and mass transfer,especially with the emergence and rapid development of structured packings.Numerous empirical correl ations and the oretical models have been proposed to predict the performance of various packings,such as the correlations proposed by Onda et al.[95]and De Brito et al.[96]for random and structured packings,respectively.With regard to the CO2absorption in packing columns,Kohland Riesenfeld[97]have proposed a widely used mass transfer correlation for a MEA–CO2system in columns packed with random packings.In addition,Aroonwilas and Tonti wach wuthikul[98]have proposed a correlation for CO2absorption into AMP using Sulzer EX type structured packing.However,these correlations vary greatly with systems,operating conditions and accuracy since they are affected by many parameters associated with gas and liquid phase substances and packings.In addition,these correlations have been developed based on differentmass transfer theories, including the double-film theory,penetration theory and surface renewal theory.
To improve the reliability and accuracy of mass transfer models,a number of computer-based programs have been employed to develop more rigorous and comprehensive mathematical models for CO2absorption into reactive solvents with random and structured packings.Aroonwilas and Tontiwachwuthikul[99]proposed a comprehensive model to estimate the mass transfer ofsheet-metalstructured packings using aqueous NaOH according to the liquid distribution feature.Based on Pandya's algorithm[100],Aboudheir etal.[101]developed a rigorous mathematicalmodelthat considers the effects of heattransfer to simulate the absorption of CO2in aqueous AMP solutions in a column with structured packing.Khan et al.[102]developed a rate-based process model for CO2–MEA absorption in a packed column with the fast second-order kinetics,mass transfer resistances,and heat effects.Hanley and Chen[103]developed a procedure via a combination of dimensional analysis and concurrent fitting of binary height equivalent to a theoretical plate(HETP)data and CO2absorption data by means of Aspen Rate Based Distillation v7.2.These studies have come to a preliminary conclusion that the rate-based models offer process engineers a more rigorous and reliable basis for assessing column performance than the than the traditional equilibrium-stage approach.Furthermore,Fu et al.[104]have applied applied artificial neural networks,which are widely used to settle engineering problems that are difficult for conventional simulators or mechanistic correlations,to predict the overall mass flux of CO2absorption into aqueous MEA in various types of packed column.
Anyway,to use these models for industrial CO2capture applications,extensive experimental data obtained from pilot plant tests are essentially required to build con fidence and minimize predictive uncertainty.
3.7.Regeneration
The reboiler heat duty(Qreg)for solvent regeneration,which accounts for more than two-thirds of the total operational costs,is an extremely important parameter for evaluating solvent performance.Therefore,a comprehensive investigation of Qregrequirement for amine regeneration is crucialto evaluating both the technicaland economic performance of the amine-based CO2capture process.
In the industrialCO2capture process,Qregis provided by heattransfer from an external higher-temperature energy source,such as low pressure steam or hot oil.It mainly consists of three parts:(1)absorption heat(qabs)for CO2-stripping reaction,(2)sensible heat(qsen)for elevating the temperature of the solution,and(3)vaporization heat(qvap)for evaporating liquid water to vapor for CO2stripping.Generally,a solvent with high absorption efficiency and low qabsis the primary consideration to reduce Qreg.The blending of various primary(or secondary)amines with tertiary amines such as MEA–MDEA has been con firmed to be able to significantly reduce the regeneration energy compared to the traditional MEA process[105].In addition,polya mine,such as DETA,with two primary and one secondary amine groups,presents significant advantages for both mass transfer and regeneration performance compared with MEA[31,33].The load of solid acid catalysts(such as HZSM-5 andγ-Al2O3,a Brϕnsted and a Lewis solid acid catalyst,respectively)into stripperrepresents another promising approach to facilitate CO2stripping[106].Its core ideas are that(i)the solid acid catalysts enable effective solvent regeneration at a drastic reduction of operating temperature from 120–140 °C to 90–95 °C,thereby significantly reducing qsenand qvapand(ii)solid catalysts are fixed in the stripper without entrance into the absorber in case the solvent loses its absorption performance.Laboratory scale tests showed that heat duty for 30%(by mass)MEA regeneration with catalystsγ-Al2O3and HZSM-5 consumed about 69%and 63%ofthat without catalyst,respectively[107].
In addition to the solvent,optimization of operating conditions and process con figuration is also bene ficialto reducing energy consumption.However,the estimation of regeneration energy is very complex since there are many operating parameters and units in the process with mutualin fluences.For example,an increase of CO2loading cyclic capacity(Δa=arich-alean)requires less solution flow rate to keep the same CO2absorption capacity.This implies that less qsenis required to raise the solvent temperature.However,a low CO2lean loading requires a huge qvapto evaporate liquid water to reach a low equilibrium CO2partial pressure;on the other hand,a high CO2rich loading wil be at the cost of larger packed height of the absorber.Recently,advanced commercial process simulators,such as Aspen plus and ProMax,are available to simulate process,so as to find key trade-offs of operating conditions and optimize the energy exchange network[108].In the past decade,extensive studies on developing innovative con figurations have been reported,including absorption flash stripping[109],absorption multi-pressure with splitstripperas well as absorption internalexchange stripper process[110],split flow-based heat pump distillation[111],and vapor recompression[112],etc.These innovative con figurations have made great improvement,but they need to be validated by pilot plant tests prior to commercial applications.
3.8.Degradation
Solventde gradation is an undesirable operating problem caused by prolonged exposure of amine solvent to CO2,O2,SOxand fly ash in the flue gas.It introduces unwanted byproducts,such as heat stable salts(HSSs),heavy hydrocarbons and particulates,into the system.These byproducts either reduce the ability of the solvent to absorb CO2or to strip CO2under typical regeneration conditions.
Generally,degradation can be divided into three types,including carbamate polymerization(for primary and secondary amines),oxidative degradation and thermal degradation,depending on the products,mechanisms and conditions[50].Among them,thermal degradation only takes place when temperature is higher than 200°C and is not considered for CO2capture with amine solvents[50].For this reason,only carbamate polymerization and oxidative degradation wil be discussed in this section.
Carbamate polymerization is an important type of degradation and produces high molecular weight polymers.It occurs in the presence of CO2at high temperature(insignificant at less than 100°C),primarily in the stripper,thermal reclaiming unit,and the hot end of the lean/rich heatexchanger.The widely accepted mechanism for carbamate polymerization of MEA was first proposed by Polderman et al.[113].The carbamate polymerization is reversible and requires carbamate species to produce oxazolidone.Then oxazolidone reacts with its parent amine to form ethylenediamine.Finally,the substituted ethylenediamine is condensed to a substituted piperazine.The use ofvacuum stripping to reduce the temperature in the stripperwould probably eliminate carbamate polymerization.In addition,the rate ofcarbamate polymerization is affected by the CO2loading and amine concentration,implying that the degradation rate can be reduced by lowering amine concentration and CO2loading[114].
In comparison with carbamate polymerization,oxidative degradation is a more noticeable degradation,which has been proven in a recentpilot plantstudy on MEA degradation[115].Oxidative degradation takes place readily when the amounts of O2and other oxidative contaminants,such as NOxand SOx,are high in flue gas.Characterization results have shown that the main oxidative degradation products of MEA include volatile substances,methylamine,ammonia,aldehydes as well as carboxylic acids[116].The degradation behaviorcan be explained by eitherthe electron transfer mechanism[114]or the hydrogen abstraction mechanism[117].In the electron transfer mechanism,the nitrogen atom loses an electron(abstracted by the oxidant)to become an N-centered radicalcation.With regard to the hydrogen abstraction mechanism,the oxidant abstracts a hydrogen atom among N,α-C,orβ-C atom[118].The oxidative degradation rate depends strongly on temperature,CO2loading,O2concentration,MEA concentration,and dissolved metal concentration[119].
In order to avoid degradation,solvents with high degradation resistances should be considered for CO2absorption.Studies have shown that tertiary amines possess the most excellent oxidation resistance,followed by secondary amines and then by primary amines.Recently,a significant research eff ortis underway on the detection of intermediate and final products and the exploration of a degradation reaction pathway.Only after thatcan we better understand the degradation process and develop an inhi bitorto eliminate impurity-induced degradation[120].
3.9.Solvent reclaiming
Since degradation extensively exists in amine-based CO2capture,a reclamation process is required to separate degradation products and HSSs from the parent amine solvent.To ensure effective operations of CO2capture,the concentration of HSSs in solution should be kept preferably below 10%of that of amine(e.g.,no more than 3%HSSs in 30%MEA)[121].
So far,many solutions have been considered for solventreclamation,including solventpurging and feeding,filtration based on mechanics or activated carbon,thermal reclamation,on line neutralization,ionexchange reclaimer and electrodialysis reclaimer[122].Among these reclamation methods,solventpurging/feeding is an early used method.It involves purging part of contaminated solvent and feeding in fresh solvent.This method is solvent inefficient,involves high disposalcost,and is not environmentally friendly.In addition,mechanical filtration will only be effective when suspended solids or colloidalparticles and corrosion products are to be removed,while activated carbon filtration is able to remove impurities that cause foaming[123].However,mechanicaland activated carbon filtrations can hardly remove HSSs and degradation products.As to thermalreclamation,it puri fies an amine solventby evaporating amine and water fromthe high-boiling degradation products as well as the suspended solids,suffering greatly from its intensive energy consumption[124].
Presently,the commonly used solventreclaiming methods are on line neutralization and ion-exchange,by adding a strong base(such as NaOH or Na2CO3)into the solution to recover the absorption ability of the solvent.These two methods are similar but there are a few differences in the neutralization mechanisms.Online neutralization involves an interaction between amine and a strong base,i.e.liquid–liquid phases,whereas ion-exchange involves interaction between amine and hydroxidecontained strong base resin,i.e.liquid–solid phases[125].Normally,a small amount of NaOH/Na2CO3,which should slightly exceed that of HSSs,is added in the system.The reclamation processes are as follows.
The addition of NaOH:
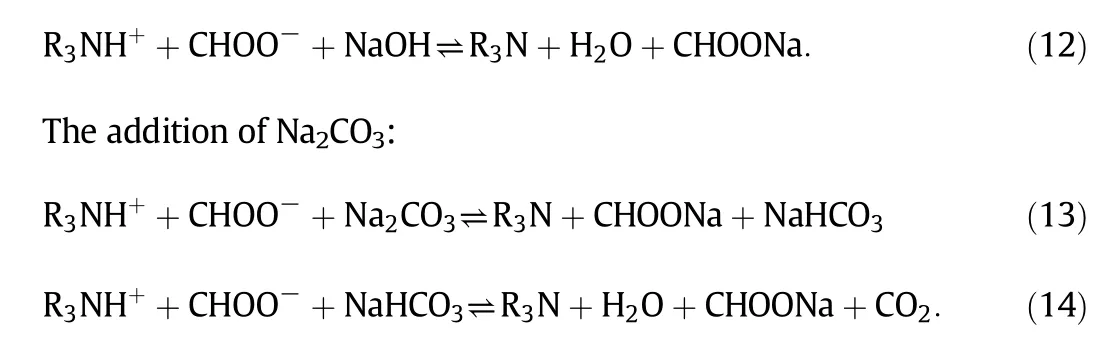
The use of on line neutralization and an ion-exchanger,which convert the degradation products to usefulamines,is attractive as a reclamation method.They not only increase absorption efficiency,decrease fouling,but also reduce equipment corrosion.However,they cause additionalproblems such as salt precipitation,and higher viscosity and density[123,126].To improve the operationalperformance,integration of on line neutralization or ion-exchange with an electrodialysis reclaimer has been employed to remove acid anions and sodium cations fromsolution[125,127].Thermalreclamation has also been considered,evaporating effective amine fromits degradation products.This method is mostly suitable for amines thatare highly volatile butchemically stable under distillation conditions.However,thermalreclamation may increase solvent degradation and consume more energy since it exposes the amine solvent to a high temperature.
Although numerous methods can be applied to recycle amines,degradation prevention is the best way to maintain solvent ability.The prime consideration is to select degradative-resistant amine solvents.Fortunately,the introduction of a low concentration of chemical additives or inhibitors into the solution can reduce degradation rate and even eliminate the possibility of degradation.Recently,many laboratories around the world,such as University of Regina(Canada)and the US Naval Research Laboratory,have proposed a lot of inhibitors to protect solvent degradation[128].Based on the experimental tests,the degradation of MEA can be reduced by over 98%in the presence of CO2and H2S[122].
3.10.Corrosion
Corrosion is another undesired operating problem in the CO2capture processusing amines.Itseverely damages equipment,reducing operation efficiency and increasing maintenance expenses.
Corrosion occurs on the interface between the metal and electrolyte solution through two electrochemical reactions,,an oxidation and a reduction,with the loss of electrons frommetals and the reception of electrons from metals to oxidizing agents[129].Corrosion will deteriorate the accumulation of HSSs,which are acidic,electroconductive,and metal–chelate(i.e.,oxalate,malonate,formate).In orderto mitigate corrosion,it is necessary to comprehensively understand the effects of amine types,HSSs,operating parameters and impurities on corrosion behavior.According to the experimentalresults from laboratory and pilotplants,the degree of corrosiveness of several common CO2saturated amines is ranked as:MEA>AMP>DEA>MDEA,because MEA yields the largest cathodic and very high anodic(smaller than that of AMP)current densities throughout the active region[130].Many studies have shown that the corrosiveness of both single and blended amines wil be increased with the increase ofsolution temperature,solution velocity and contents of amine,HSSs,CO2and O2[130–132].
To minimize damage of plant units,inhibitors are usually added to the amine solvent to prevent corrosion.Generally,inorganic inhibitors,composed by various cations(such as sodium and copper)and anions(such as metavanadate,VO3-,sulfite,SO32-and carbonate,CO32-),are more popular than those oforganic ones,since they can form a passive film to preventthe units from being further corroded[133,134].Among these inhibitors,the vanadium-containing compounds,especially sodium metavanadate(NaVO3),are the most popular ones for CO2capture in MEA-treating plants,since they have less toxicity compared to other compounds such as chromate[135].It should be noted that,heavy metal-based corrosion inhibitors are generally not used for minimizing the equipment corrosion since they may tremendously accelerate the degradation of solvent[136].In comparison,inorganic corrosion inhibitors are less environmentally friendly than organic inhibitors due to their toxicity.Organic inhibitors normally form a protective film to prevent corrosion by means of adsorption onto the metal surface.Due to the nature of adsorption,the stability of the film is greatly affected by many factors,including system temperature,inhibitor concentration and the strength of adsorption bond.The most widely used organic inhibitors for corrosion in the CO2capture process are the nitrogen based surfactant inhibitor,such as imidazolines and their precursors[137].
4.Concluding Remarks and Future Prospects
This paper has described the applicability and progress in the use of amine-based solvent for post-combustion CO2capture and reviewed the current types and emerging trends of chemical sol vents.Moreover,considerations for evaluation to selection of a candidate solvent have been discussed in detailin terms of reaction mechanism,absorption solubility,VEL model,kinetics,mass transfer,regeneration,as well as solvent management.
The current amine-based CO2absorption process represents the most promising technology for CO2capture,which will keep attracting attention in the near future.However,they still face great challenges especially the high energy consumption associated with solvent regeneration and solvent management.Moreover,most of the amine-based absorption studies to date have focused mainly on the separation of CO2from a CO2/N2mixture.However,there exist other minor components,such as O2,CO,SOx,and NOx,in a real exhaust gas,which have a significant impact on absorption performance,energy requirements for regeneration,and long-term stability of the solvents.Therefore,screening,designing and synthesizing a novel absorbent for special gas composition separation are becoming indispensable and will continue inde finitely for application in CO2capture studies.
With respect to next-generation absorbents for CO2capture,the key scientific challenges should lie in the development of advanced absorbents that require lower energy for regeneration as well as possess chemical and thermal robustness capable of withstanding the longterm presence of impurity components in the flue gas.In our opinion,efforts can be made towards developing improved absorbents in the following three areas,(i)molecular structural variations on absorbents,(ii)CO2absorption/desorption with catalysis,and(iii)simulation of the absorption process with amines.To better explore novel solvents,advanced characterization methods,such as NMR and FTIR spectroscopy,are essential to be employed to determine the characteristics of solvents and the interaction between solvents and CO2or other flue gas constituents.In parallel with experimental studies,computational modeling techniques are recommended to predict the performance of absorbents.Such techniques will enable a clear understanding of the properties of solvent molecule absorbed CO2,a development of related thermodynamic and transport models to predict the performance of solvent,and a large scale screening of new absorbents.Clearly,a combined experimental and modeling approach will direct the development of absorbents towards higher performance and lower operational costs for CO2capture.
Fortunately,tremendous progress in improving the performance of amine-based absorbents has already been seen in the past decade,increasing the potential application of amine-based solvents in industrial CO2capture.In this perspective,the time is ripe for us to offer attractive solutions for CO2capture.
[1]CO2Emission From Fuel Combustion—Highlights,2013 Edition,International Energy Agency.Imprimerie Centrale,Luxembourg,2013.
[2]R.S.Haszeldine,Carbon capture and storage:how green can black be?Science 325(5948)(2009)1647–1652.
[3]Q.Schiermeier,J.Tollefson,T.Scully,A.Witze,O.Morton,Electricity without carbon,Nature 454(7206)(2008)816–823.
[4]Energy Technology Perspectives,International Energy Agency,Paris,2008.
[5]K.Z.House,C.F.Harvey,M.J.Aziz,D.P.Schrag,The energy penalty of postcombustion CO2capture&storage and its implications for retro fitting the US installed base,Energy Environ.Sci.2(2)(2009)193–205.
[6]E.Favre,Membrane processes and postcombustion carbon dioxide capture:challenges and prospects,Chem.Eng.J.171(3)(2011)782–793.
[7]A.B.Rao,E.S.Rubin,A technical,economic,and environmental assessment of amine-based CO2capture technology for power plant greenhouse gas control,Environ.Sci.Technol.36(20)(2002)4467–4475.
[8]G.T.Rochelle,Amine scrubbing for CO2capture,Science 325(5948)(2009)1652–1654.
[9]H.Yang,S.Fan,X.Lang,Y.Wang,J.Nie,Economic comparison ofthree gas separation technologies for CO2capture from power plant flue gas,Chin.J.Chem.Eng.19(4)(2011)615–620.
[10]D.M.D'Alessandro,B.Smit,J.R.Long,Carbon dioxide capture:prospects for new materials,Angew.Chem.Int.Ed.49(35)(2010)6058–6082.
[11]M.Ramdin,T.W.de Loos,T.J.H.Vlugt,State-of-the-art of CO2capture with ionic liquids,Ind.Eng.Chem.Res.51(24)(2012)8149–8177.
[12]K.Sumida,D.L.Rogow,J.A.Mason,T.M.McDonald,E.D.Bloch,Z.R.Herm,T.H.Bae,J.R.Long,Carbon dioxide capture in metal–organic frameworks,Chem.Rev.112(2)(2012)724–781.
[13]L.Wei,Y.Jing,Z.Gao,Y.Wang,Development of a pentaethylenehexaminemodified solid support adsorbent for CO2capture from model flue gas,Chin.J.Chem.Eng.23(2)(2015)366–371.
[14]M.Pera-Titus,Porous inorganic membranes for CO2capture:present and prospects,Chem.Rev.114(2)(2014)1413–1492.
[15]A.S.Bhown,B.C.Freeman,Analysis and status of post-combustion carbon dioxide capture technologies,Environ.Sci.Technol.45(20)(2011)8624–8632.
[16]D.Aaron,C.Tsouris,Separation of CO2from flue gas:A review,Sep.Sci.Technol.40(1-3)(2005)321–348.
[17]A.L.Kohl,R.Nielsen,Gas Puri fication,Gulf Professional Publishing,1997.
[18]B.T.Zhao,Y.X.Su,W.W.Tao,L.L.Li,Y.C.Peng,Post-combustion CO2capture by aqueous ammonia:A state-of-the-art review,Int.J.Greenhouse Gas Control9(2012)355–371.
[19]N.Yang,H.Yu,L.Li,D.Xu,W.Han,P.Feron,Aqueous ammonia(NH3)based post combustion CO2capture:A review,Oil Gas Sci.Technol.-Rev.IFP Energies Nouv.(2013)http://dx.doi.org/10.2516/ogst/2013160.
[20]P.D.Vaidya,E.Y.Kenig,CO2–alkanolamine reaction kinetics:A review of recent studies,Chem.Eng.Technol.30(11)(2007)1467–1474.
[21]T.Sema,A.Naami,K.Fu,M.Edali,H.Liu,H.Shi,Z.Liang,R.Idem,P.Tonti wach wuthikul,Comprehensive mass transfer and reaction kinetics studies of CO2absorption into aqueous solutions of blended MDEA–MEA,Chem.Eng.J.209(2012)501–512.
[22]B.Mandal,S.S.Bandyopadhyay,Simultaneous absorption of CO2and H2S into aqueous blends of N-methyldiethanolamine and diethanolamine,Environ.Sci.Technol.40(19)(2006)6076–6084.
[23]A.Samanta,S.S.Bandyopadhyay,Absorption of carbon dioxide into aqueous solutions of piperazine activated 2-amino-2-methyl-1-propanol,Chem.Eng.Sci.64(6)(2009)1185–1194.
[24]E.Chen,Carbon Dioxide Absorption into Piperazine Promoted Potassium Carbonate Using Structured Packing(Ph.D.Thesis)The University of Texas at Austin,2007.
[25]S.Ma'mun,J.P.Jakobsen,H.F.Svendsen,O.Juliussen,Experimental and modeling study of the solubility of carbon dioxide in aqueous 30 mass%2-((2-aminoethyl)amino)ethanolsolution,Ind.Eng.Chem.Res.45(8)(2006)2505–2512.
[26]Z.Xu,S.Wang,C.Chen,Kinetics study on CO2absorption with aqueous solutions of 1,4-butane diamine,2-(diethylamino)-ethanol,and their mixtures,Ind.Eng.Chem.Res.52(29)(2013)9790–9802.
[27]T.Sema,A.Naami,K.Fu,G.Chen,Z.Liang,R.Idem,P.Tontiwachwuthikul,Comprehensive mass transfer and reaction kinetics studies of a novelreactive 4-diethylamino-2-butanolsolvent for capturing CO2,Chem.Eng.Sci.100(2013)183–194.
[28]H.Shi,T.Sema,A.Naami,Z.Liang,R.Idem,P.Tontiwachwuthikul,13C NMR spectroscopy of a novelamine species in the DEAB–CO2–H2O system:VLE model,Ind.Eng.Chem.Res.51(25)(2012)8608–8615.
[29]T.Sema,A.Naami,Z.Liang,R.Idem,P.Tontiwachwuthikul,H.Shi,P.Wattanaphan,A.Henni,Analysis of reaction kinetics of CO2absorption into a novelreactive 4-diethylamino-2-butanolsolvent,Chem.Eng.Sci.81(2012)251–259.
[30]A.Hartono,E.F.da Silva,H.F.Svendsen,Kinetics of carbon dioxide absorption in aqueous solution of diethylenetriamine(DETA),Chem.Eng.Sci.64(14)(2009)3205–3213.
[31]X.Zhang,K.Fu,Z.Liang,W.Rongwong,Z.Yang,R.Idem,P.Tontiwachwuthikul,Experimental studies of regeneration heat duty for CO2desorption from diethylenetriamine(DETA)solution in a stripper column packed with Dixon ring random packing,Fuel136(2014)261–267.
[32]K.Fu,G.Chen,T.Sema,X.Zhang,Z.Liang,R.Idem,P.Tontiwachwuthikul,Experimentalstudy on mass transfer and prediction using artificial neural network for CO2absorption into aqueous DETA,Chem.Eng.Sci.100(2013)195–202.
[33]K.Fu,T.Sema,Z.Liang,H.Liu,Y.Na,H.Shi,R.Idem,P.Tontiwachwuthikul,Investigation of mass-transfer performance for CO2absorption into diethylenetriamine(DETA)in a randomly packed column,Ind.Eng.Chem.Res.51(37)(2012)12058–12064.
[34]K.Robinson,A.McCluskey,M.I.Attalla,The effect molecular structuralvariations has on the CO2absorption characteristics of heterocyclic amines,Recent Advances in Post-combustion CO2Capture Chemistry,1097,American Chemical Society 2012,pp.1–27.
[35]A.K.Chakraborty,G.Astarita,K.B.Bischoff,CO2absorption in aqueous solutions of hindered amines,Chem.Eng.Sci.41(4)(1986)997–1003.
[36]P.Singh,J.P.M.Niederer,G.F.Versteeg,Structure and activity relationships for amine based CO2absorbents— I,Int.J.Greenhouse Gas Control1(1)(2007)5–10.
[37]P.Singh,J.P.M.Niederer,G.F.Versteeg,Structure and activity relationships for amine-based CO2absorbents— II,Chem.Eng.Res.Des.87(2)(2009)135–144.
[38]P.L.Carrette,R.Cadours,P.Boucot,P.Mougin,M.Prigent,A.Gibert,M.Jacquin,New solventfor CO2with lowenergy ofregeneration,10th Meeting of the IEAInternational Post-Combustion CO2Capture Network,24–25th May 2007,Lyon,France,2007.
[39]J.Zhang,O.Nwani,Y.Tan,D.W.Agar,Carbon dioxide absorption into biphasic amine solvent with solvent loss reduction,Chem.Eng.Res.Des.89(8)(2011)1190–1196.
[40]L.Raynal,P.Alix,P.-A.Bouillon,A.Gomez,M.l.F.de Nailly,M.Jacquin,J.Kittel,A.di Lella,P.Mougin,J.Trapy,The DMX™process:an originalsolution for lowering the cost of post-combustion carbon capture,Energy Procedia 4(2011)779–786.
[41]D.Agar,Y.Tan,X.Zhang,CO2removal processes by means of absorption using thermomorphic biphasic aqueous amine solutions.Patent WO/2008/015217,(2008).
[42]L.A.Blanchard,D.Hancu,E.J.Beckman,J.F.Brennecke,Green processing using ionic liquids and CO2,Nature 399(6731)(1999)28–29.
[43]C.Cadena,J.L.Anthony,J.K.Shah,T.I.Morrow,J.F.Brennecke,E.J.Maginn,Why is CO2so soluble in imidazolium-based ionic liquids?J.Am.Chem.Soc.126(16)(2004)5300–5308.
[44]E.D.Bates,R.D.Mayton,I.Ntai,J.H.Davis,CO2capture by a task-specific ionic liquid,J.Am.Chem.Soc.124(6)(2002)926–927.
[45]L.M.G.Sanchez,G.W.Meindersma,A.B.de Haan,Solvent properties of functionalized ionic liquids for CO2absorption,Chem.Eng.Res.Des.85(A1)(2007)31–39.
[46]B.E.Gurkan,J.C.de la Fuente,E.M.Mindrup,L.E.Ficke,B.F.Goodrich,E.A.Price,W.F.Schneider,J.F.Brennecke,Equimolar CO2absorption by anion-functionalized ionic liquids,J.Am.Chem.Soc.132(7)(2010)2116–2117.
[47]G.R.Yu,S.J.Zhang,G.H.Zhou,X.M.Liu,X.C.Chen,Structure,interaction and property of amino-functionalized imidazolium ILs by molecular dynamics simulation and ab initio calculation,AICHE J.53(12)(2007)3210–3221.
[48]K.E.Gutowski,E.J.Maginn,Amine-functionalized task-specific ionic liquids:A mechanistic explanation for the dramatic increase in viscosity upon complexation with CO2from molecular simulation,J.Am.Chem.Soc.130(44)(2008)14690–14704.
[49]J.Xu,S.Wang,W.Yu,Q.Xu,W.Wang,J.Yin,Molecular dynamics simulation for the binary mixtures of high pressure carbon dioxide and ionic liquids,Chin.J.Chem.Eng.22(2)(2014)153–163.
[50]G.T.Rochelle,S.Bishnoi,S.Chi,H.Dang,J.Santos,Research Needs for CO2Capture from Flue Gas by Aqueous Absorption/Stripping,US Department of Energy,Pittsburgh,PA,USA,2001.
[51]W.Horwitz,Association of Of ficial Analytical Chemists(AOAC)Methods,12th ed.George Banta Company,Menasha,WI,1975.
[52]F.Y.Jou,A.E.Mather,F.D.Otto,The solubility of CO2in a 30 mass percent monoethanolamine solution,Can.J.Chem.Eng.73(1)(1995)140–147.
[53]I.Attalla Moetaz,Recent advances in post-combustion CO2capture chemistry,Am.Chem.Soc.1097(2012).
[54]A.V.Rayer,K.Z.Sumon,T.Sema,A.Henni,R.O.Idem,P.Tontiwachwuthikul,Part5c:solvent chemistry:solubility of CO2in reactive solvents for post-combustion CO2,Carbon Manag.3(5)(2012)467–484.
[55]Q.Yang,M.Bown,A.Ali,D.Winkler,G.Puxty,M.Attalla,A carbon-13 NMRstudy of carbon dioxide absorption and desorption with aqueous amine solutions,Energy Procedia 1(2009)955–962.
[56]J.Y.Park,S.J.Yoon,H.Lee,Effect of steric hindrance on carbon dioxide absorption into new amine solutions:thermodynamic and spectroscopic verification through solubility and NMR analysis,Environ.Sci.Technol.37(8)(2003)1670–1675.
[57]G.Richner,G.Puxty,Assessing the chemicalspeciation during CO2absorption by aqueous amines using in situ FTIR,Ind.Eng.Chem.Res.51(44)(2012)14317–14324.
[58]Y.S.Choi,J.Im,J.K.Jeong,S.Y.Hong,H.G.Jang,M.Cheong,J.S.Lee,H.S.Kim,CO2absorption and desorption in an aqueous solution of heavily hindered alkanolamine:structural elucidation ofCO2-containing species,Environ.Sci.Technol.48(7)(2014)4163–4170.
[59]A.F.Ciftja,A.Hartono,H.F.Svendsen,13C NMR as a method species determination in CO2absorbent systems,Int.J.Greenhouse Gas Control16(2013)224–232.
[60]H.Shi,A.Naami,R.O.Idem,P.Tontiwachwuthikul,1D NMR analysis of a quaternary MEA–DEAB–CO2–H2O amine system:Liquid phase speciation and vapor–liquid equilibria at CO2absorption and solvent regeneration conditions,Ind.Eng.Chem.Res.53(2014)8577–8591.
[61]W.Böttinger,M.Maiwald,H.Hasse,Online NMR spectroscopic study of species distribution in MEA–H2O–CO2and DEA–H2O–CO2,Fluid Phase Equilib.263(2)(2008)131–143.
[62]P.Usubharatana,A Study of Monoethanolamine–Methanol Hybrid Solvents for Carbon Dioxide Capture by Absorption(Ph.D.Thesis)The University of Regina,2009.
[63]A.Hartono,E.F.da Silva,H.Grasdalen,H.F.Svendsen,Qualitative determination of species in DETA–H2O–CO2system using13C NMR spectra,Ind.Eng.Chem.Res.46(1)(2007)249–254.
[64]R.Kent,B.Eisenberg,Better data for amine treating,Hydrocarb.Process.55(2)(1976)87–90.
[65]K.S.Pitzer,Thermodynamics of electrolytes.I.Theoreticalbasis and generalequations,J.Phys.Chem.77(2)(1973)268–277.
[66]R.Deshmukh,A.Mather,A mathematicalmodel for equilibrium solubility of hydrogen sul fide and carbon dioxide in aqueous alkanolamine solutions,Chem.Eng.Sci.36(2)(1981)355–362.
[67]C.C.Chen,H.Britt,J.Boston,L.Evans,Localcomposition model for excess Gibbs energy of electrolyte systems.Part I:single solvent,single completely dissociated electrolyte systems,AICHE J.28(4)(1982)588–596.
[68]K.Thomsen,P.Rasmussen,Modeling of vapor–liquid–solid equilibrium in gas–aqueous electrolyte systems,Chem.Eng.Sci.54(12)(1999)1787–1802.
[69]W.Fürst,H.Renon,Representation of excess properties of electrolyte solutions using a new equation of state,AICHE J.39(2)(1993)335–343.
[70]J.Button,K.Gubbins,SAFT prediction ofvapour–liquid equilibria of mixtures containing carbon dioxide and aqueous monoethanolamine or diethanolamine,Fluid Phase Equilib.158(1999)175–181.
[71]I.Kim,H.F.Svendsen,Heat of absorption of carbon dioxide(CO2)in monoethanolamine(MEA)and 2-(aminoethyl)ethanolamine(AEEA)solutions,Ind.Eng.Chem.Res.46(17)(2007)5803–5809.
[72]A.Sherwood,J.Prausnitz,The heat ofsolution of gases at high pressure,AICHE J.8(4)(1962)519–521.
[73]I.Kim,H.F.Svendsen,Comparative study of the heats of absorption of postcombustion CO2absorbents,Int.J.Greenhouse Gas Control5(3)(2011)390–395.
[74]M.W.Arshad,P.L.Fosbol,N.von Solms,H.F.Svendsen,K.Thomsen,Heat of absorption of CO2in phase change solvents:2-(diethylamino)ethanoland 3-(methylamino)propylamine,J.Chem.Eng.Data 58(7)(2013)1974–1988.
[75]M.Caplow,Kinetics of carbamate formation and breakdown,J.Am.Chem.Soc.90(24)(1968)6795–6803.
[76]P.Danckwerts,The reaction of CO2with ethanolamines,Chem.Eng.Sci.34(4)(1979)443–446.
[77]J.E.Crooks,J.P.Donnellan,Kinetics and mechanism of the reaction between carbon dioxide and amines in aqueous solution,J.Chem.Soc.Perkin Trans.2(4)(1989)331–333.
[78]A.Aboudheir,P.Tontiwachwuthikul,A.Chakma,R.Idem,Kinetics of the reactive absorption of carbon dioxide in high CO2-loaded,concentrated aqueous monoethanolamine solutions,Chem.Eng.Sci.58(23–24)(2003)5195–5210.
[79]H.Kierzkowska-Pawlak,A.Chacuk,M.Siemieniec,Reaction kinetics of CO2in aqueous 2-(2-aminoethylamino)ethanol solutions using a stirred cellreactor,Int.J.Greenhouse Gas Control24(2014)106–114.
[80]X.Luo,A.Hartono,H.F.Svendsen,Comparative kinetics of carbon dioxide absorption in unloaded aqueous monoethanolamine solutions using wetted wall and string of discs columns,Chem.Eng.Sci.82(2012)31–43.
[81]X.Luo,A.Hartono,S.Hussain,H.F.Svendsen,Mass transfer and kinetics ofcarbon dioxide absorption into loaded aqueous monoethanolamine solutions,Chem.Eng.Sci.123(2015)57–69.
[82]D.J.Seo,W.H.Hong,Effect of piperazine on the kinetics of carbon dioxide with aqueous solutions of 2-amino-2-methyl-1-propanol,Ind.Eng.Chem.Res.39(6)(2000)2062–2067.
[83]T.Sema,A.Naami,Z.W.Liang,R.Idem,H.Ibrahim,P.Tontiwachwuthikul,1D absorption kinetics modeling ofCO2–DEAB–H2Osystem,Int.J.Greenhouse Gas Control 12(2013)390–398.
[84]M.Edali,R.Idem,A.Aboudheir,1D and 2D absorption-rate/kinetic modeling and simulation of carbon dioxide absorption into mixed aqueous solutions of MDEA and PZ in a laminar jet apparatus,Int.J.Greenhouse Gas Control 4(2)(2010)143–151.
[85]H.Liu,T.Sema,Z.Liang,K.Fu,R.Idem,Y.Na,P.Tontiwachwuthikul,CO2absorption kinetics of 4-diethylamine-2-butanol solvent using stopped-flow technique,Sep.Purif.Technol.136(2014)81–87.
[86]J.G.M.Monteiro,S.Hussain,S.Majeed,H.Mba,E.O.Hartono,A.Knuutila,H.Svendsen,H.F.,Kinetics of CO2absorption by aqueous 3-(methylamino)propylamine solutions:experimental results and modeling,AICHE J.60(11)(2014)3792–3803.
[87]R.H.Perry,D.Green,J.Maloney,Perry's Handbook of Chemical Engineering,7th ed.McGraw-Hill Book Company,New York,1997.
[88]G.Zarca,I.Ortiz,A.Urtiaga,Recovery ofcarbon monoxide from flue gases by reactive absorption in ionic liquid imidazolium chlorocuprate(I):mass transfer coefficients,Chin.J.Chem.Eng.23(5)(2015)769–774.
[89]H.Jin,S.Yang,G.He,D.Liu,Z.Tong,J.Zhu,Gas–liquid mass transfer characteristics in a gas–liquid–solid bubble column under elevated pressure and temperature,Chin.J.Chem.Eng.22(9)(2014)955–961.
[90]W.Yang,X.Yu,J.Mi,W.Wang,J.Chen,Mass transfer performance of structured packings in a CO2absorption tower,Chin.J.Chem.Eng.23(1)(2015)42–49.
[91]Z.Y.Yu,B.T.Zhao,S.S.He,Mass transfer performance of enhanced CO2absorption in swirling flow field,CIESC J.66(2015)1012–1018.
[92]X.Jia,W.Hu,X.Yuan,K.Yu,Effect ofsurfactant type on interfacialarea and liquid mass transfer for CO2absorption in a bubble column,Chin.J.Chem.Eng.23(3)(2015)476–481.
[93]A.Aroonwilas,P.Tontiwachwuthikul,A.Chakma,Effects of operating and design parameters on CO2absorption in columns with structured packings,Sep.Purif.Technol.24(3)(2001)403–411.
[94]R.E.Tsai,A.F.Seibert,R.B.Eldridge,G.T.Rochelle,A dimensionless model for predicting the mass-transfer area of structured packing,AICHE J.57(5)(2011)1173–1184.
[95]K.Onda,H.Takeuchi,Y.Okumoto,Mass transfer coefficients between gas and liquid phases in packed columns,J.Chem.Eng.Jpn 1(1)(1968)56–62.
[96]M.H.De Brito,U.Von Stockar,A.M.Bangerter,P.Bomio,M.Laso,Effective masstransfer area in a pilot plant column equipped with structured packings and with ceramic rings,Ind.Eng.Chem.Res.33(3)(1994)647–656.
[97]A.L.Kohl,F.C.Riesenfeld,Gas Puri fication,4th ed.Gulf Publishing,Houston,TX,1985.
[98]A.Aroonwilas,P.Tontiwachwuthikul,Mass transfer coefficients and correlation for CO2absorption into 2-amino-2-methyl-1-propanol(AMP)using structured packing,Ind.Eng.Chem.Res.37(2)(1998)569–575.
[99]A.Aroonwilas,P.Tontiwachwuthikul,Mechanistic model for prediction of structured packing mass transfer performance in CO2absorption with chemicalreactions,Chem.Eng.Sci.55(18)(2000)3651–3663.
[100]J.Pandya,Adiabatic gas absorption and stripping with chemicalreaction in packed towers,Chem.Eng.Commun.19(4-6)(1983)343–361.
[101]A.Aboudheir,P.Tontiwachwuthikul,R.Idem,Rigorous model for predicting the behavior of CO2absorption into AMP in packed-bed absorption columns,Ind.Eng.Chem.Res.45(8)(2006)2553–2557.
[102]F.M.Khan,V.Krishnamoorthi,T.Mahmud,Modelling reactive absorption of CO2in packed columns for post-combustion carbon capture applications,Chem.Eng.Res.Des.89(9)(2011)1600–1608.
[103]B.Hanley,C.C.Chen,New mass-transfer correlations for packed towers,AICHE J.58(1)(2012)132–152.
[104]K.Fu,G.Chen,Z.Liang,T.Sema,R.Idem,P.Tontiwachwuthikul,Analysis of mass transfer performance of monoethanolamine-based CO2absorption in a packed column using arti ficial neural networks,Ind.Eng.Chem.Res.53(11)(2014)4413–4423.
[105]R.Idem,M.Wilson,P.Tontiwachwuthikul,A.Chakma,A.Veawab,A.Aroonwilas,D.Gelowitz,Pilot plant studies of the CO2capture performance of aqueous MEA and mixed MEA/MDEA solvents at the University of Regina CO2capture technology development plant and the Boundary Dam CO2capture demonstration,Ind.Eng.Chem.Res.45(8)(2006)2414–2420.
[106]R.Idem,H.Shi,D.Gelowitz,P.Tontiwachwuthikul,Catalytic method and apparatus for separating a gas component from an incoming gas stream.WO Patent 2011/12013821,(2011).
[107]H.C.Shi,A.Naami,R.Idem,P.Tontiwachwuthikul,Catalytic and non catalytic solvent regeneration during absorption-based CO2capture with single and blended reactive amine solvents,Int.J.Greenhouse Gas Control26(2014)39–50.
[108]L.X.Kang,Y.Z.Liu,Step-by step retro fit ofheat exchanger network with heat pump installation and multi-objective optimization strategies,CIESC J.65(2014)3976–3983.
[109]C.Alie,L.Backham,E.Croiset,P.L.Douglas,Simulation of CO2capture using MEA scrubbing:a flow sheet decomposition method,Energy Convers.Manag.46(3)(2005)475–487.
[110]B.A.Oyenekan,G.T.Rochelle,Alternative stripper con figurations for CO2capture by aqueous amines,AICHE J.53(12)(2007)3144–3154.
[111]H.Gao,L.Zhou,Z.Liang,R.O.Idem,K.Fu,T.Sema,P.Tontiwachwuthikul,Comparative studies of heat duty and total equivalent work of a new heat pump distillation with split flow process,conventional split flow process,and conventional baseline process for CO2capture using monoethanolamine,Int.J.Greenhouse Gas Control24(2014)87–97.
[112]Z.Liang,H.Gao,W.Rongwong,Y.Na,Comparative studies of stripper overhead vapor integration-based con figurations for post-combustion CO2capture,Int.J.Greenhouse Gas Control34(2015)75–84.
[113]L.Polderman,C.Dillon,A.Steele,Why monoethanolamine solution breaks down in gas-treating service,Oil Gas J.54(2)(1955)180–183.
[114]S.Chi,G.T.Rochelle,Oxidative degradation of monoe thanol amine,Ind.Eng.Chem.Res.41(17)(2002)4178–4186.
[115]H.Lepaumier,E.F.da Silva,A.Einbu,A.Grimstvedt,J.N.Knudsen,K.Zahlsen,H.F.Svendsen,Comparison of MEA degradation in pilot-scale with lab-scale experiments,Energy Procedia 4(2011)1652–1659.
[116]H.Lepaumier,D.Picq,P.-L.Carrette,New amines for CO2capture.II.Oxidative degradation mechanisms,Ind.Eng.Chem.Res.48(20)(2009)9068–9075.
[117]C.Gouedard,D.Picq,F.Launay,P.L.Carrette,Amine degradation in CO2capture.I.A review,Int.J.Greenhouse Gas Control10(2012)244–270.
[118]T.Wang,K.J.Jens,Oxidative degradation of aqueous 2-amino-2-methyl-1-propanol solvent for postcombustion CO2capture,Ind.Eng.Chem.Res.51(18)(2012)6529–6536.
[119]A.K.Voice,G.T.Rochelle,Products and process variables in oxidation of monoethanolamine for CO2capture,Int.J.Greenhouse Gas Control12(2013)472–477.
[120]G.Léonard,A.Voice,D.Toye,G.Heyen,In fluence of dissolved metals and oxidative degradation inhibitors on the oxidative and the rmalde gradation of monoethanolamine in postcombustion CO2capture,Ind.Eng.Chem.Res.53(47)(2014)18121–18129.
[121]T.Bacon,Amine solution quality controlthrough design,operation and correction,Proceedings—1987 Gas Conditioning Conference,The University of Oklahoma,Norman,Oklahoma,1987,1987.
[122]W.ElMoudir,T.Supap,C.Saiwan,R.Idem,P.Tontiwachwuthikul,Part 6:solvent recycling and reclaiming issues,Carbon Manag.3(5)(2012)485–509.
[123]A.L.Cummings,G.D.Smith,D.K.Nelsen,Advances in amine reclaiming—why there's no excuse to operate a dirty amine system,Laurance Reid Gas Conditioning Conference,2007,2007.
[124]T.L.Wang,J.Hovland,K.J.Jens,Amine reclaiming technologies in post-combustion carbon dioxide capture,J.Environ.Sci.(China)27(2015)276–289.
[125]A.L.Cummings,G.D.Smith,D.K.Nelsen,Advances in amine reclaiming—why there's no excuse to operate a dirty amine system,Laurence Reid Gas Conditioning Conference,Dickinson TX,USA,2007,2007.
[126]N.Verma,A.Verma,Amine system problems arising from heat stable salts and solutions to improve system performance,Fuel Process.Technol.90(4)(2009)483–489.
[127]I.Raphael,S.Teeradet,S.Chintana,E.Walid,T.Paitoon,Solvent recycling and reclaiming in CO2capture processes,Recent Progress and New Developments in Post-combustion Carbon-capture Technology with Reactive Solvents,Future Science Ltd.2013,pp.142–160.
[128]R.Idem,P.Tontiwachwuthikul,C.Saiwan,T.Supap,P.Pitipuech,Method for inhibiting amine degradation during CO2capture from a gas stream.In Google Patents:2012.
[129]M.G.Fontana,Corrosion Engineering,Tata McGraw-Hill Education,2005.
[130]A.Veawab,P.Tontiwachwuthikul,A.Chakma,Corrosion behavior ofcarbon steelin the CO2absorption process using aqueous amine solutions,Ind.Eng.Chem.Res.38(10)(1999)3917–3924.
[131]W.Tanthapanichakoon,A.Veawab,B.McGarvey,Electrochemicalinvestigation on the effect ofheat-stable salts on corrosion in CO2capture plants using aqueous solution of MEA,Ind.Eng.Chem.Res.45(8)(2006)2586–2593.
[132]I.R.Soosaiprakasam,A.Veawab,Corrosion and polarization behavior of carbon steel in MEA-based CO2capture process,Int.J.Greenhouse Gas Control 2(4)(2008)553–562.
[133]A.Veawab,P.Tontiwachwuthikul,S.D.Bhole,Studies of corrosion and corrosion control in a CO2–2-amino-2-methyl-1-propanol(AMP)environment,Ind.Eng.Chem.Res.36(1)(1997)264–269.
[134]A.Bello,R.O.Idem,Comprehensive study of the kinetics of the oxidative degradation of CO2loaded and concentrated aqueous monoethanolamine(MEA)with and without sodium metavanadate during CO2absorption from flue gases,Ind.Eng.Chem.Res.45(8)(2006)2569–2579.
[135]M.Antonijevic,M.Petrovic,Copper corrosion inhibitors.A review,Int.J.Electrochem.Sci.3(1)(2008)1–28.
[136]G.S.Goff,G.T.Rochelle,Monoethanolamine degradation:O2mass transfer effects under CO2capture conditions,Ind.Eng.Chem.Res.43(20)(2004)6400–6408.
[137]V.Jovancicevic,S.Ramachandran,P.Prince,Inhibition of carbon dioxide corrosion of mild steel by imidazolines and their precursors,Corrosion 55(5)(1999)449–455.
 Chinese Journal of Chemical Engineering2016年2期
Chinese Journal of Chemical Engineering2016年2期
- Chinese Journal of Chemical Engineering的其它文章
- Experimental evaluation and modeling of liquid jet penetration to estimate droplet size in a three-phase riser reactor
- CFD based extraction column design—Chances and challenges
- Analysis of drop deformation dynamics in turbulent flow
- Photorheologically reversible micelle composed ofpolymerizable cationic surfactant and 4-phenylazo benzoic acid☆
- Experimental study on the effects of big particles physical characteristics on the hydraulic transport inside a horizontal pipe
- Relationship between breakthrough curve and adsorption isotherm of Ca(II)imprinted chitosan microspheres for metaladsorption☆
