Investigation of extraction fraction in con fined impinging jet reactors for tri-butyl-phosphate extracting butyric acid process☆
Zhengming Gao ,Manting Zhao ,Yun Yu ,Zhipeng Li,Jing Han ,*
1 State Key Laboratory of Chemical Resource Engineering,School of Chemical Engineering,Beijing University of Chemical Technology,Beijing 100029,China
2 Beijing Aircraft Maintenance&Engineering Co.,LTD,Beijing 100621,China
1.Introduction
Treatment for dilute solution of organic carboxylic acid is an important part of the mass transfer and separation processes.It is very easy for the organic carboxylic acid with Lewis bases to form complexing reactions.Thus,many researchers[1–6]made plenty of studies about the treatment for dilute solution of organic carboxylic acid,such as acetic acid,oxalic acid,citric acid,and sebacic acid,by using the method of complexing.During such a process,the solute that needs to be separated in the solution comes in contact with the complexing agent firstly,and the complexes are formed after the reaction,and then transferred into the extraction agent to complete the separation.Among the previous researches,most of the complexing agents used are phosphorus oxide and amine extractants,including tributyl phosphate(TBP),trialkyl phosphine oxide(TPO)and trioctylamine.For there are alkali Lewis bases in these extractants,it is easier for them to undergo complexing reactions with organic carboxylic acid.Compared with the traditional extraction method,the complexing extraction can achieve higher extent of extraction and bigger reaction rate at low solute concentration.Besides,the complexing method has higher extraction efficiency and selectivity for dilute organic carboxylic acid solution.In addition,the used complexing agents can be refreshed by stripping the combined organic carboxylic acid with alkali agents,such as NaOH,which will make full use of the complexing agents by recycling.
The impinging jet techniques,first proposed by Elperin[7],make two or more high speed jets collide in a narrow zone.At the collision,the axialvelocity of the jets wil be converted into the fluctuating velocity,and then a collision zone characterized by very severe turbulence is formed.Because of high turbulence,the materials in the jets are brought into close contact.The shear force generated by interphase collision makes the droplets break up,leading to an increase of contacting area and enhanced surface refreshing.At the same time,the shear force also decreases the phase mass transfer resistance.For these advantages,impinging jets can strengthen the phase heat and mass transfer,and reduce the volume of the producing devices.There are many applications and researches[8]about the impinging jets in various fields,such as drying of solid particles,absorption and desorption of gas,combustion ofcoaland gas,phosphate burning,and preparation of ultra fine particles/dust.Kleingeld etal.[9]investigated the gas absorption capacity of the high strength impinging jet reactor using the sodium hydroxide–carbon dioxide system,showing that the efficiency of gas absorption was better than the traditional method.Huai et al.[10]studied the effects of the inlet air temperature,mass flow-rate ratio,initial moisture content and air velocity of the impinging stream on the drying process.Wu et al.[11]prepared ultra fine barium sulfate powder with an impinging jet reactor,and the powder showed an ellipsoidalshape with an average diameter of 100 nm.
In recent years,although impinging jets have been applied to the wastewater treatment and dilute acid extraction by some researchers,the number of related researches is limited.Tamir[12]designed a liquid–liquid impinging jet extraction device first and found that its mass transfer coefficient was much greater than the traditional devices.Dehkordi[13,14]designed a new impinging jet extraction device based on the former researches,and tested it using a weak extractant with moderately slow chemical kinetics to extract cooper ions.The experimental results indicated that the extraction and stripping rates of copper in impinging jet reactors are 7 to 8 times bigger than the traditional CSTR per unit volume of reactors.Saien et al.[15–18]made a detailed research about the in fluences of jet momentum,nozzle diameter and the inter-nozzle distance for the impinging jets on the extraction fraction E by the standard test system of toluene–acetone–water recommended by the European Federation of Chemical Engineering(EFCE).The results showed that the overall volumetric mass transfer coefficient kLa was 3–5 times bigger than that in the study made by Dehkordi[13].The values of kLa and E were very sensitive to pH,and decreased with an increase of pH in the range of 5.5 to 8.Low amounts(maximum 10-4mol·L-1)of NaCl in aqueous phase had a significant improvement for the toluene–acetone–water system,kLa and E increase about 35.6%and 22.7%,respectively.The extraction fraction E of the low inter facial tension chemical system is higher than that of the high interfacial tension chemical system under similar conditions.Compared with conventional contactors,kLa of the two impinging-jet contacting device(TIJCD)had a much better performance.Qi et al.[19]investigated the extraction fraction of the submerged circulative impinging stream reactor for acetic acid extraction,and the result proved the excellent performance of the impinging jets.Wang et al.[20]tested the extraction of phenol wastewater by TBP extractant in the submerged circulative impinging stream reactor(SCISR),and achieved a 20%promotion of the extraction fraction than the traditional reactor.Zhang et al.[21]tested the extraction effect for Cr6+using the kerosene solution of TBP in the impinging jet reactor and found a 20%increase in E compared with the traditional method.
Butyric acid(CH3CH2CH2COOH)which is easy to be dissolved in water is an important organic solvent,and it has been used to produce butyrate,medicine,spices and flavorings.Butyric acid is easy to be recognized even at a very low concentration because it has an unpleasant smelllike the spoilage cream.The aim of this study is to investigate experimentally the extraction fraction E and the overall volumetric mass transfer coefficient kLa for the extraction of butyric acid using TBP and kerosene as the organic phase.The effects of operating conditions such as the concentration of TBP and butyric acid,the impinging velocity and the impinging velocity ratio of two phases on extraction fraction were studied.And a correlation about these variables and the overall volumetric mass transfer coefficient kLa was proposed.
2.Experimental Section
2.1.Experimental setup
The experimental flowchart is shown in Figs.1 and 2.The impinging chamber is actually a 70 mm high hollow cylinder of id 7 mm.
At first,the butyric acid solution of1%(v/v)and TBP kerosene solution of 10%(v/v)were prepared in two separate Plexiglas tanks.Then,the solution would be pumped into the impinging chamber,and the velocity of the impinging jet was adjusted through the ball valves.After two streams collide in the impinging zone with the expected impinging velocity,the solution mixture exits from the lower outlet.During the testing,samples were taken when the flow was stabilized and sealed by the plastic wrap to allow the solution to stratify and clarify.The upper layer was the organic extraction phase,and the lower layer was the raffinate.

Fig.1.Experimental flowchart.1.tank;2.solution pumps;3.valve;4.flowmeters;5.impinging zone;6.solution export and 7.sample cup.
2.2.Chemicals
The chemicals used in this study were deionized water,aviation kerosene,tri-butyl-phosphate,butyric acid,NaOH standard solution(0.01 mol·L-1),and HClstandard solution(0.01 mol·L-1).Allreagents are from local markets and of AR grade.
2.3.Physico-chemical properties
According to the density of the pure reagents,the density of the solutions used in the experiment were calculated and shown in Table 1.
A number of experiments were conducted to determine the distribution coefficient of butyric acid.Six initial concentrations of butyric acid solution were 0.5%,1%,2%,3%,5%,and 10%(v/v),and the initial concentration of TBP was 10%(v/v).The equilibrium concentration of butyric acid in the aqueous phase was analyzed by acid–base titration,while thatin the organic phase was calculated by mass-balance.The distribution relationship between the aqueous phase and organic phase of butyric acid is plotted in Fig.3,and the slope of the line is the distribution coefficient.
When the concentration of the butyric acid Caqis less than 1%(v/v),the fitted formula is

The distribution coefficient is 2.5978 in the interval.
On the other hand,the slope of the line is relatively small when Caqis more than 1%(v/v),and the formula is

The distribution coefficient is 1.3279 in the interval.
2.4.Measurements
Five milliliters of raf finate was sampled with a pipette when samples were strati fied.The concentration of butyric acid in the samples was analyzed by acid–base titration.Then,the extraction fraction E and overall volumetric mass transfer coefficient kLa were calculated using the data obtained before.
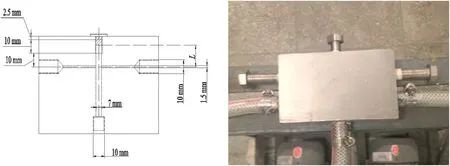
Fig.2.Impinging chamber.
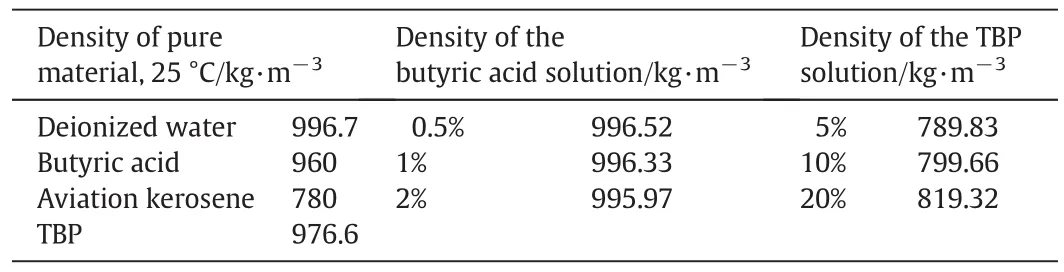
Table 1 Density of the solutions
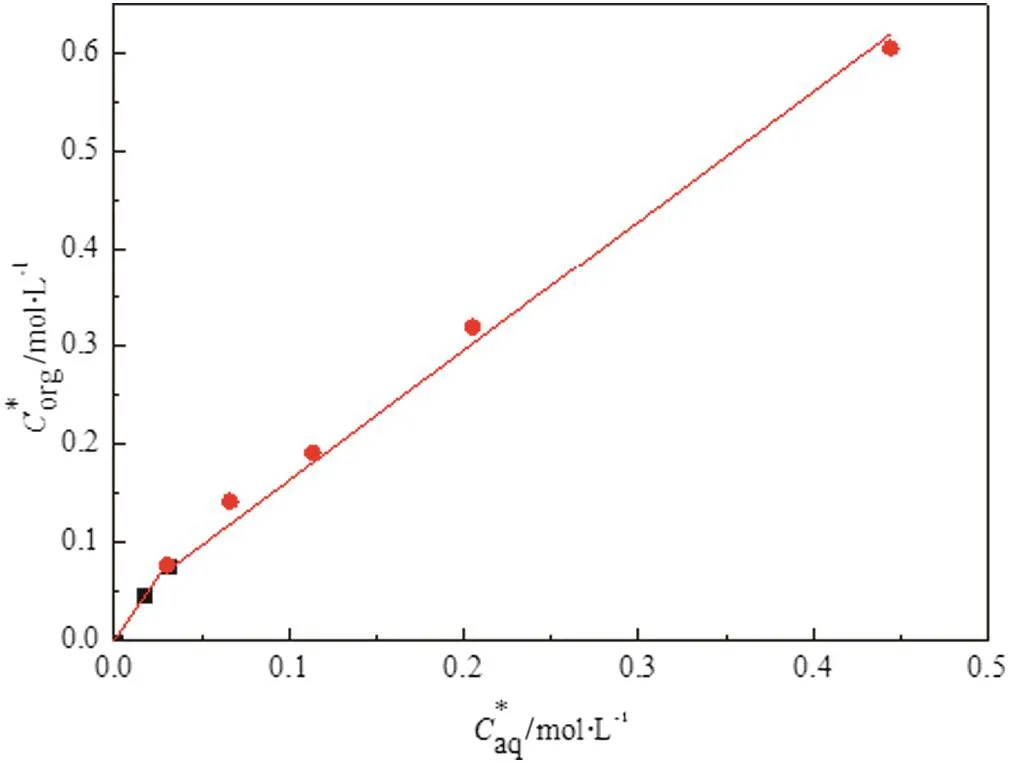
Fig.3.Distribution coefficient ofbutyric acid between organic and aqueous phases.
Extraction fraction is defined as follows:

where[∑n]organd[∑n]aqrepresent the respective overall mole quantities of the solute in the aqueous and organic phases after extraction.
2.5.Jet momentum and mass transfer coefficient
The jet momentum is normally given by

where F,ρ,Q and diare the jet momentum(N),solution density(g·m-3),volumetric flow rate(m3·s-1),and the nozzle diameter(m),respectively.The jet momentum used in the experiments is shown in Table 2.
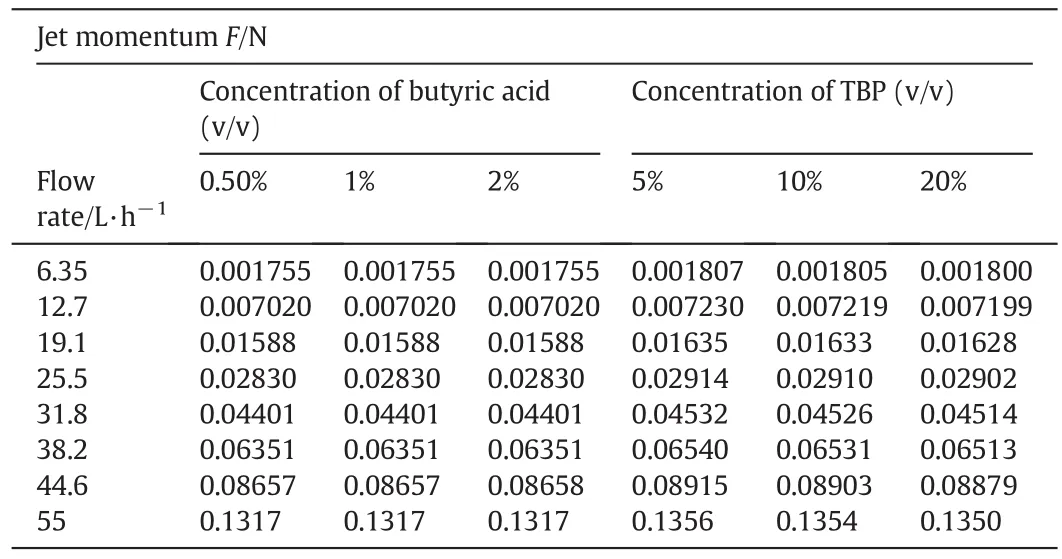
Table 2 Jet momentum under experimental conditions
The overall volumetric mass transfer coefficient kLa is used to quantify the efficiency of mass transfer processes and it is defined by
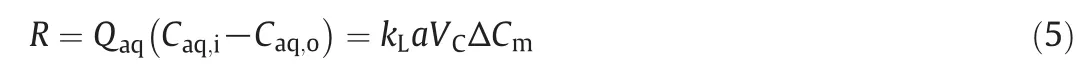
where R,Qaq,Vc,Caq,i,Caq,oand kLa are the extraction rate,volumetric flow rate of the aqueous phase,contacting device volume,initial concentration of butyric acid in the inleta queous phase,and concentration ofbutyric acid in the outleta queous phase,respectively.ΔCm,an appropriate mean concentration driving force,sometimes can be calculated as ΔCln,and is described as
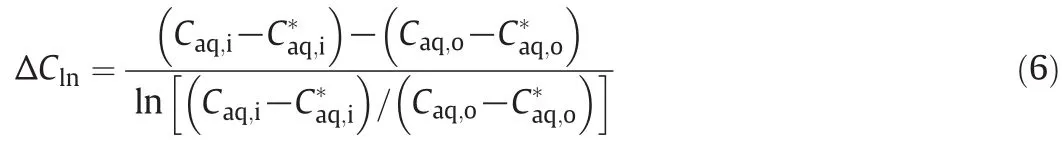
where Caq,i*and Caq,o*are the concentration of butyric acid of the inlet aqueous phase and the concentration of butyric acid of the outlet aqueous phase in equilibrium with the actual inlet and outlet concentration of the butyric acid of the organic phase.
3.Results and Discussion
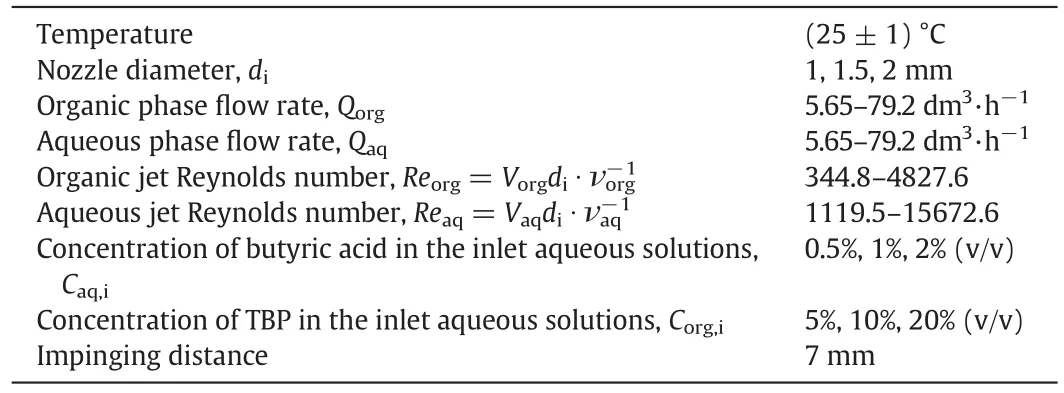
The ranges of operating conditions are as follows:
3.1.Selection of concentration of butyric acid and TBP
The butyric acid and TBP concentrations are important parameters to extraction fraction in CIJR.Fig.4 shows that the extraction efficiency of butyric acid is above 60%when the concentration of TBP is bigger than 10%(v/v),but the further increase of the extraction efficiency slows down with the increase of the concentration of TBP.Hence,the proper concentration of TBP is 10%(v/v).Fig.5 indicates that a good extraction efficiency of butyric acid is achieved when its concentration is 0.5%,1%and 2%(v/v),and a big decrease happens when the concentration is above 2%.Considering that the realistic concentration of the butyric acid waste water is around 1%(v/v),the concentration of butyric acid is chosen as 1%(v/v).Subsequently,the experiments are conducted with the fixed inlet concentrations of butyric acid(1%)and TBP(10%).
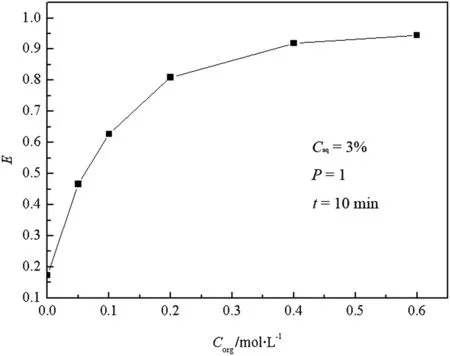
Fig.4.Relationship between E and C org.
3.2.Effect of the impinging velocity and impinging velocity ratio
The relationship of E and kLa with impinging velocity V and jet momentum F of the organic phase is shown in Figs.6–8.In these experiments,the inner diameter diwas 1.5 mm,and the upper space of the impinging zone L was 3 mm.
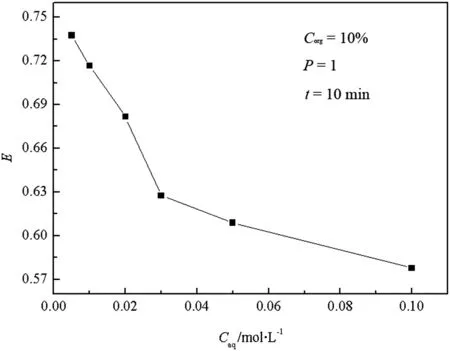
Fig.5.Relationship between E and C aq.
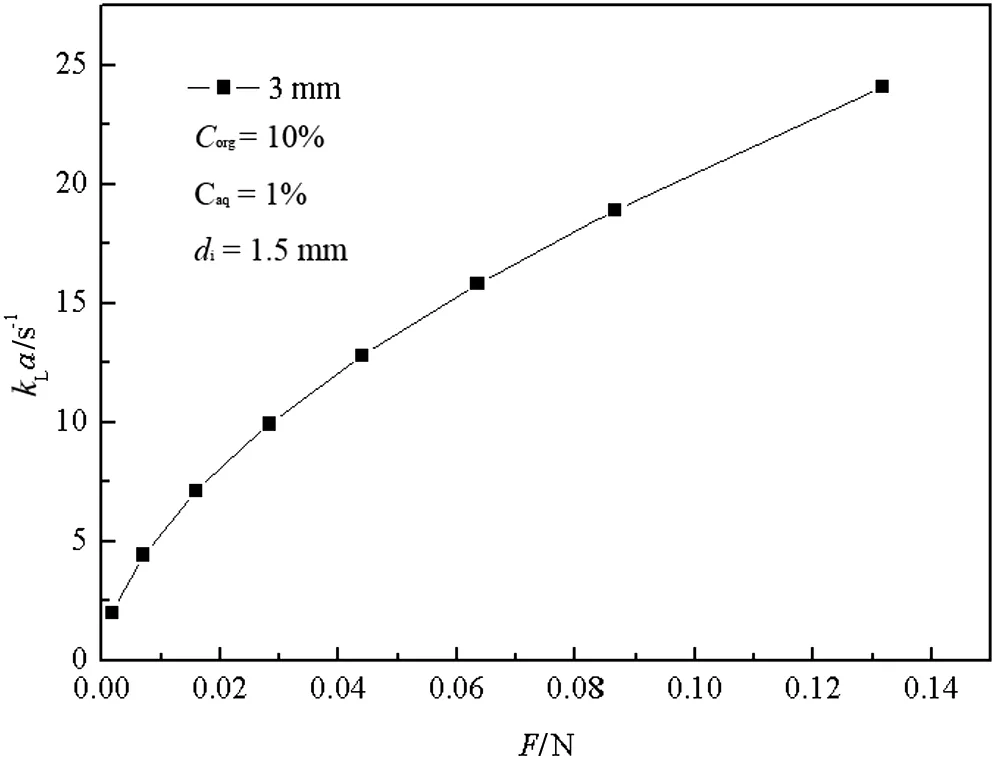
Fig.6.Relationship between k L a and F of organic phase.

Fig.7.Relationship between E and V org.
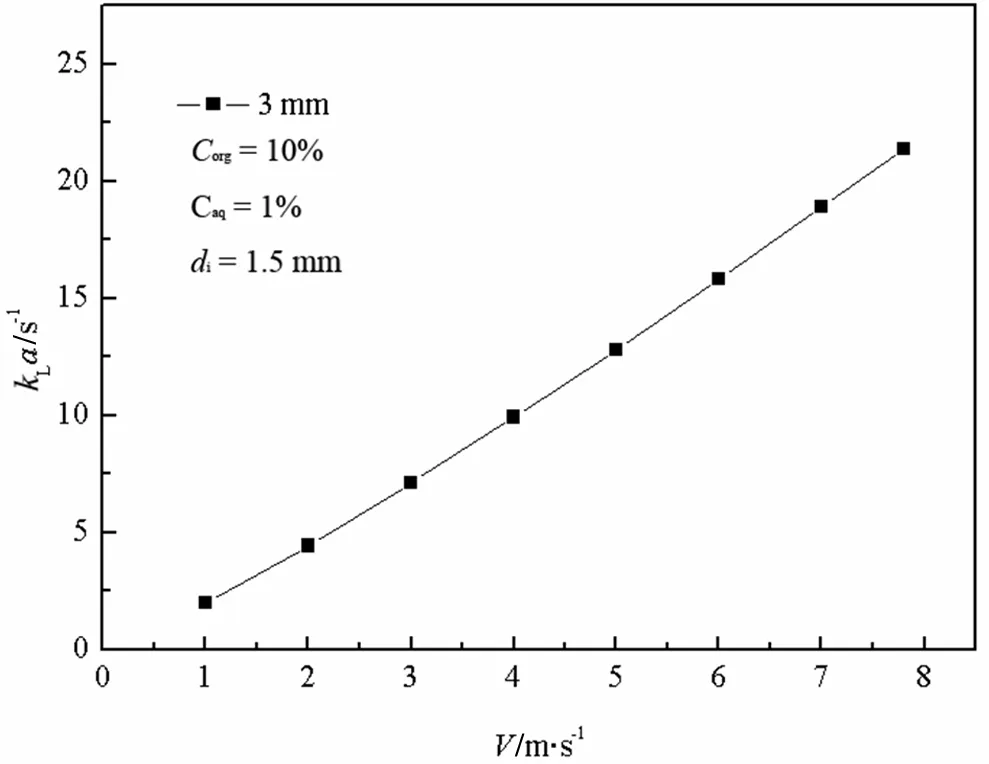
Fig.8.Relationship between k L a and V org.
Fig.6 shows the relationship between the overall volumetric mass transfer coefficient kLa and jetmomentum F.It can be seen thata nearly linear increase of kLa is visible by increasing F from 0.01 to 0.13 N.This behavior is the consequence of the increase of the interaction and mixing between fluids.Figs.7 and 8 demonstrate that E and kLa ascend along with an increase ofimpinging velocity V.As the impinging velocity increases from 1 to 7.8 m·s-1,E increases from60.7%to 70%,and kLa also increases from 2 to 25 s-1.Strong collision and a high impactforce in the impinging zone willincrease the velocity pulsation and shearing force.In addition,the surface renewalrate also becomes bigger,leading to an increase in the eddy diffusivity and interfacialmass transfer area.The residence time of liquid willdecrease with an increase ofimpinging velocity.Therefore,E and kLa increase with an increase of impinging velocity.
The effects of the impinging velocity ratio of the two phases Vorg/Vaqata fixed aqueous phase velocity of4 m·s-1(Fig.9)and a fixed organic phase velocity of4 m·s-1(Fig.10)on E were investigated separately.
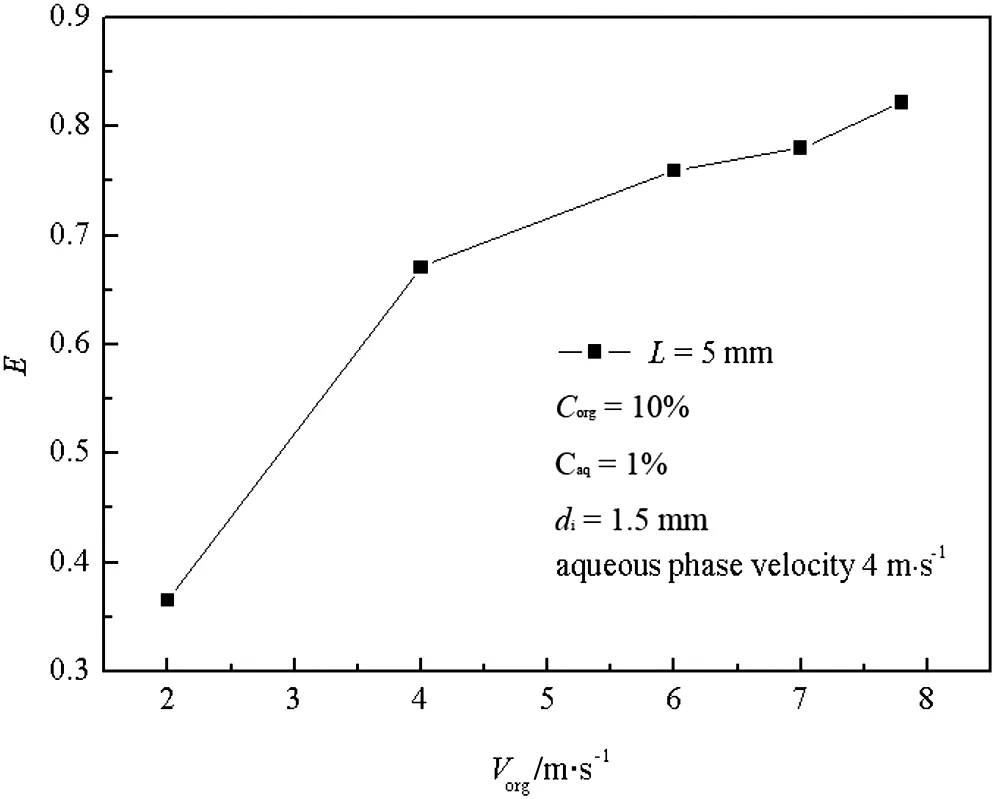
Fig.9.Relationship between E and V org.
Fig.9 shows that under a fixed flow rate of the aqueous phase,there is a nearly linear increasing relationship between E and Vorg.The increase is because the larger flow rate of the organic phase adds more TBP in the impinging zone,and enhances the mass transfer driving force,and it is also because a higher liquid relative velocity between two impinging jets leads to enhanced interaction between liquid elements in the impinging zone.Fig.10 shows that when the flow rate of the organic phase is fixed,the extraction efficiency decreases sharply from 0.82 to 0.47.The main reason is that although the increased flow rate of butyric acid adds more energy input,the increased burden of extraction overwhelms the advantage of enhanced level of turbulent contact.
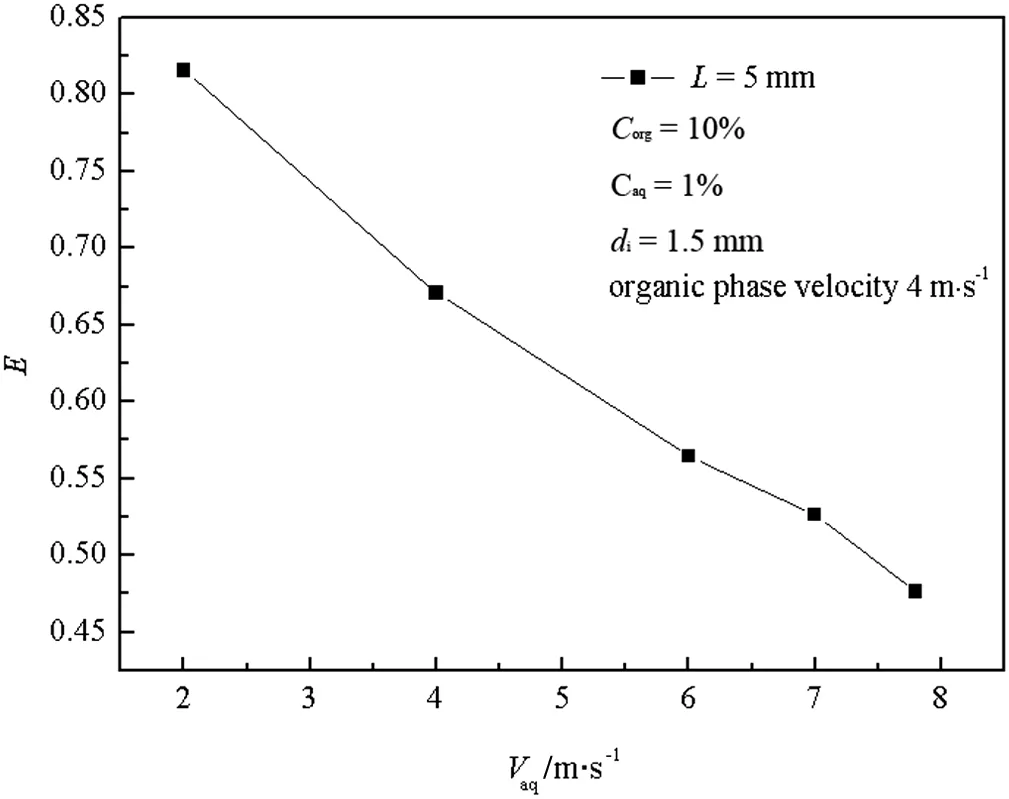
Fig.10.Relationship between E and V aq.
3.3.Effect of the distance L
The effect of L on extraction fraction is shown in Fig.11.It demonstrates that the increase of the upper space of the impinging zone L has negligible impacton E.As can be observed,when the impinging velocity V is 6 m·s-1,the extraction efficiency achieves a maximum value at L=5 mm,probably because the turbulent momentum reaches the biggest when L is 5 mm.However,the change of E is relatively small as L increases from 3.0 to 11.0 mm.For impinging velocity of 4 m·s-1,there is basically no change of the extraction efficiency with L.Maybe all L values in the experimental conditions can provide sufficient space to allow a fully developed turbulence.

Fig.11.Relationship between E and L.
3.4.Effectof the inner diameter
Figs.12 and 13 demonstrate that atany fixed impinging velocity,E decreases with the increase of the inner diameter from 1 to 2 mm,while kLa increases linearly.Fora constantim pinging velocity,a smaller inner diameter(less liquid flow rate)allows two phases to have more contacting time,so the mixing is more adequate.However,kLa is affected greatly by the flow volume of two phases,so kLa increases with the increase of the inner diameter di.

Fig.12.Relationship between E and the d i.

Fig.13.Relationship between k L a and d i.
3.5.Effect of the concentration of TBP and butyric acid
The effects of the concentration of TBP Corgand the butyric acid Caqon the extraction efficiency E are shown in Figs.14 and 15.The inner diameter diwas 1.5 mm,and the upper space of the impinging zone L was 3 mm.
Fig.14 demonstrates when the impinging velocity V keeps constant,E changes from 0.55 to 0.77(v/v)with the increase of the concentration of TBP from 5%to 20%(v/v).This can be explained on the one hand by that the increasing concentration of TBP enhances the mass transfer driving force of the extraction processes,which increases the overall volumetric mass transfer coefficient.On the other hand,the decrease of the surface tension caused by the increasing concentration of TBP also leads to an increase of the viscosity of the system.Generally speaking,the decrease of the surface tension causes a better mass transfer performance;however,the increase of the viscosity does the opposite.The impinging mass transfer process is mainly affected by the convective masstransfer,notby the molecularmass trans fermainly controlled by the viscosity.Therefore,the mass transfer performance is still improved.Fig.14 also shows that E ascends with the increase of the impinging velocity V under a certain concentration of TBP.

Fig.14.Effect of the concentration of TBP.
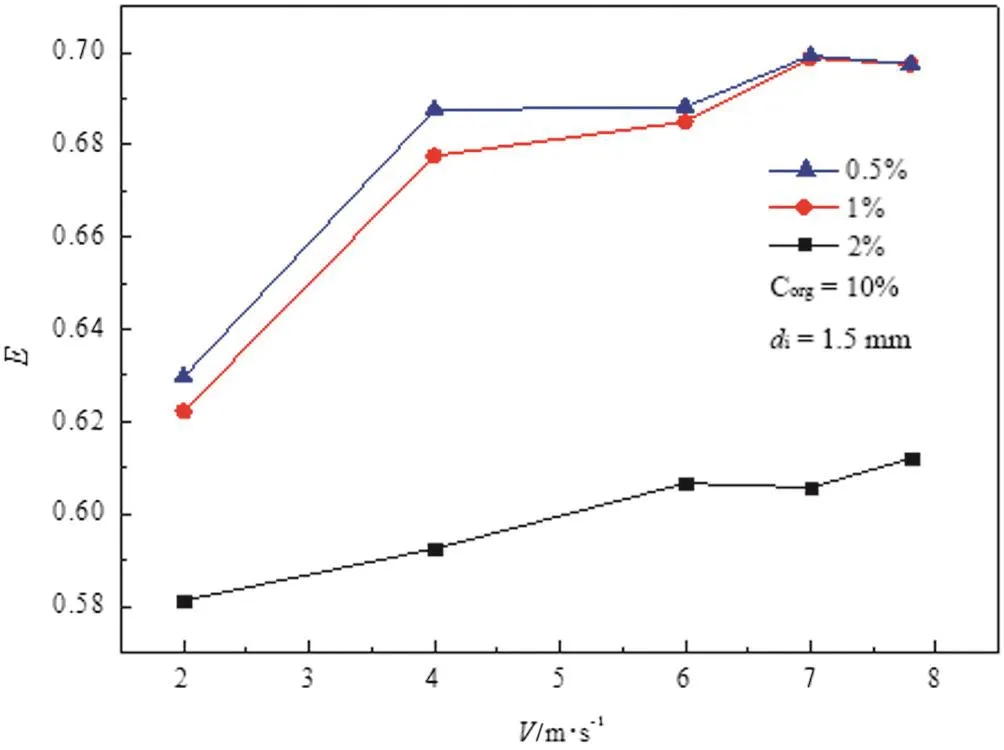
Fig.15.Effect of the concentration ofbutyric acid.
Fig.15 demonstrates a positive correlation between E and the impinging velocity V at a fixed concentration of butyric acid.Otherwise,the extraction efficiency descends with the increase of the concentration of butyric acid ata fixed V.However,E is ratherclose atlow concentration of butyric acid of0.5%and 1%.
3.6.Fitting of the experimental data
The present investigation indicated that the concentration of TBP Corg,the inner diameter diand the total flow rate of the two phases Q significantly affect E and kLa.Ignoring the variables thathad a negligible effect,a correlation of kLa to these in fluencing variables was made.The result is given as

where kLa is the overallvolumetric mass transfer coefficient,s-1;diis the inner diameter,m;RTBPis the volume fraction of TBP;Q is the total flow of the two phases,m3·h-1;VCis the volume of the impinging zone,and its value is 9.228×10-7m3.
Fig.16 is the parity plot for this correlation,which can predict the overall mass transfer coefficient satisfactorily.The average relative error is 16%,and the maximum relative deviation ofmostdata(82%)between the experimentaland predicted values of kLa does not exceed±25%.
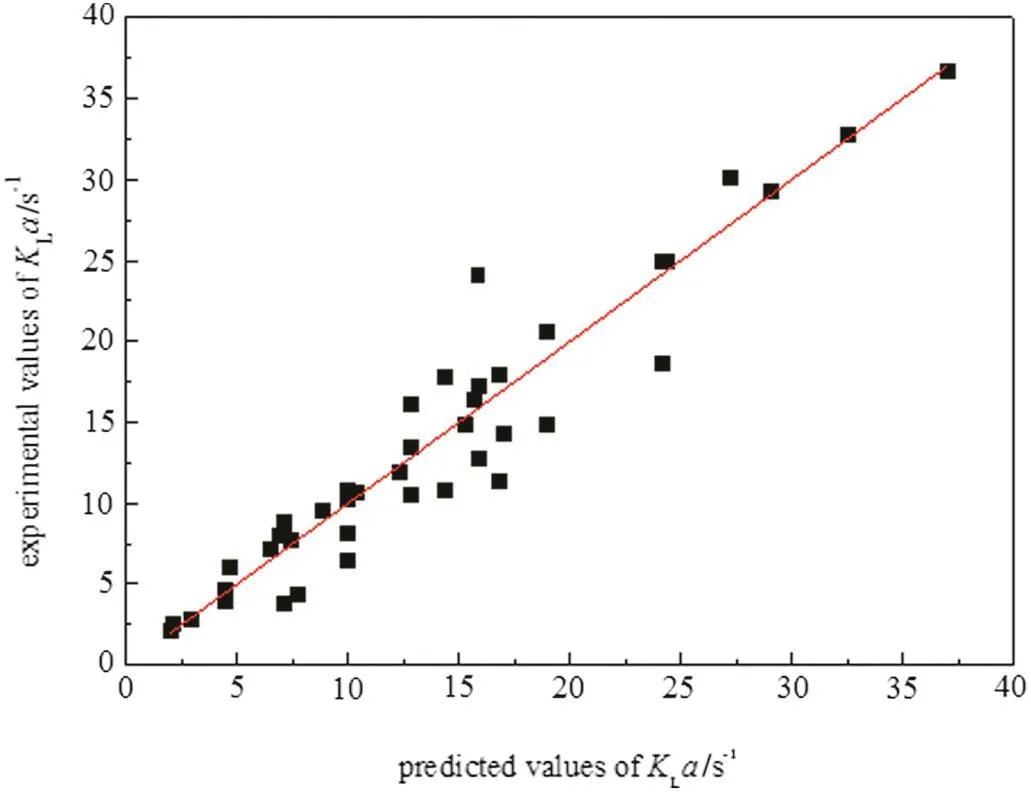
Fig.16.Relationship between the experimental data and the predicted data of k L a.
4.Conclusions
The mass transferfraction E and overall volumetric mass transfercoefficient kLa of TBP extracting butyric acid processes in CIJR are determined.The effects of several variables including operating conditions and reactor structure on E and kLa have been studied.It is found that:
(1)When the volume fraction ofTBP is bigger than 10%(v/v)and the volume fraction of butyric acid is 0.5%,1%and 2%(v/v),the extraction fraction E is relatively good.10%(v/v)TBP and 1%(v/v)butyric acid are proper experimental concentrations.
(2)E and kLa increase to an asymptotic level with the increasing impinging velocity.
(3)Generally,the extraction efficiency is worse at higher concentration of butyric acid or lower concentration of TBP.
(4)The increase of the flow ratio of organic over aqueous phase results in improved extraction efficiency.
(5)As the inner diameter increases from 1.0 to 2.0 mm,the extraction efficiency becomes worse.The upper distance of the impinging zone L has a negligible effect on the mass transfer process.
(6)A correlation of kLa is established with good prediction accuracy.
Nomenclature

Subscripts

[1]C.J.King,Handbook ofSeparation Process Technology,John Wiley&Sons,New York,1987 760–774.
[2]K.Thomas,M.Gerd,Distribution ofoxalic acid between water and organic solutions of tri-n-octylamine,Ind.Eng.Chem.Res.35(1996)1722–1735.
[3]F.Poposka,Kinetics,mechanism and mathematical modeling of extraction of citric acid with isodecanol/n-paraffins solutions of trioctylamine,Chem.Eng.Sci.53(1998)3227–3237.
[4]V.Bizek,J.Horacek,M.Kousova,A.Heyberger,J.Prochazka,Mathematical modelof extraction of citric acid with amine,Chem.Eng.Sci.47(1992)1433–1440.
[5]A.T.Janet,A.S.Kertes,C.J.King,Extraction ofcarboxylic acids with amine extractants,Ind.Eng.Chem.Res.29(1990)1319–1326.
[6]H.Xu,Q.Zhou,J.F.Wang,Treatment ofsebacic acid industrial wastewater by extraction process using castor oil acid as extractant,Chin.J.Chem.Eng.21(2013)967–973.
[7]I.T.Elperin,Heat and mass transfer in opposing currents,J.Eng.Phys.6(1961)62–68.
[8]L.M.Tang,M.Hao,Application development ofimpinging jets in the petrochemical industry,Chem.Dev.28(2009)35–37(in Chinese).
[9]A.W.Kleingeld,L.Lorenzen,F.G.Botes,The development and modeling of highintensity impinging streams jet reactors for effective mass transfer in heterogeneous systems,Chem.Eng.Sci.54(1999)4991–4995.
[10]X.L.Huai,D.Y.Liu,K.Shigeru,Experimental and theoretical analysis of the impinging stream drying,Chin.J.Chem.Eng.11(2003)42–48.
[11]G.Wu,H.Hong,S.Zhu,Researches about the production of ultra-fine barium sulfate in a impinging jets reactor,J.Inorg.Mater.21(2006)1079–1084.
[12]A.Tamir,Impinging-stream Reactors:Fundamentals and Applications,Elsevier Science B.V.,Amsterdam,1994.
[13]A.M.Dehkordi,A noveltwo-impinging-jets reactor for copper extraction and stripping processes,Chem.Eng.J.87(2002)227–238.
[14]A.M.Dehkordi,Experimental investigation of an air-operated-two impingingstreams reactor for copper extraction processes,Ind.Eng.Chem.Res.41(2002)2512–2520.
[15]J.Saien,S.A.E.Zonouzian,A.M.Dehkordi,Investigation of a two impinging-jets contacting device for liquid–liquid extraction processes,Chem.Eng.Sci.61(2006)3942–3950.
[16]J.Saien,V.Moradi,Low interfacialtension liquid–liquid extraction with impingingjets contacting method:in fluencing parameters and relationship,J.Ind.Eng.Chem.18(2012)1293–1300.
[17]J.Saien,S.A.Ojaghi,Effect of aqueous phase pH on liquid–liquid extraction with impinging-jets contacting technique,J.Ind.Eng.Chem.16(2010)1001–1005.
[18]J.Saien,S.Asadabadi,Salting-outeffect of NaClon the rate of mass transfer of liquid–liquid extraction in a two impinging-jets contacting device,J.Taiwan Inst.Chem.Eng.41(2010)295–301.
[19]G.S.Qi,Y.Z.Liu,W.Z.Jiao,Experimental investigation of the separation of dilute acetic acid solution in the impinging jets-rotating packed bed reactor,Model.Chem.11(2008)65–70(in Chinese).
[20]T.L.Wang,C.P.Bao,Y.B.Zhu,Y.Wu,Experimental investigation of the extraction of phenolwastewater in the impinging jets reactor,Chem.Environ.9(2007)39–41(in Chinese).
[21]L.J.Zhang,Z.H.Liu,W.D.Zhuang,W.Yin,Researches about the treatment of the electroplating wastewater in an impinging jets reactor,Chem.Environ.26(2006)99–102(in Chinese).
 Chinese Journal of Chemical Engineering2016年2期
Chinese Journal of Chemical Engineering2016年2期
- Chinese Journal of Chemical Engineering的其它文章
- Relationship between breakthrough curve and adsorption isotherm of Ca(II)imprinted chitosan microspheres for metaladsorption☆
- Experimental study on the effects of big particles physical characteristics on the hydraulic transport inside a horizontal pipe
- Experimental evaluation and modeling of liquid jet penetration to estimate droplet size in a three-phase riser reactor
- Photorheologically reversible micelle composed ofpolymerizable cationic surfactant and 4-phenylazo benzoic acid☆
- Review on current advances,future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents☆
- Analysis of drop deformation dynamics in turbulent flow
