Selective extraction of In(III),Ga(III)and Zn(II)using a novelextractant with phenylphosphinic acid
Yuji Sasaki,NaokiMatsuo,Tatsuya Oshima,YoshinariBaba*
Department of Applied Chemistry,Faculty ofEngineering,University ofMiyazaki,Gakuen-KibanadaiNishi,Miyazaki889-2192,Japan
1.Introduction
In recentyears,the demand for In(III)and Ga(III)hasincreased more and more in the field ofelectronic devices.In particular,In(III)is used as an In(III)-tin oxide(ITO)in liquid crystaldisplays,and Ga(III)is used in semiconductor materials such as GaAs.In addition,these metals are used in solar panels and emerged as important strategic metals since they are essential elements for the electronic industries.However,there is no ore containing just In(III)and Ga(III),and their metals are mainly contained in zinc ores.Therefore,In(III)and Ga(III)have been generally recovered as by-products in the zinc re finery residues.However,it is difficult to separate In(III)and Ga(III)from zinc re finery residues since these metals show similar chemical properties.Thus,it is desired to develop a selective separation technique for separating In(III)and Ga(III)from a large amountofzinc.
Solventextraction is one of the major separation techniques for metal ions.A large number of extractants for In(III)and Ga(III)have been reported,including phosphoric acids[1–7],phosphinic acids[8–10],oximes[11],carboxylacids[12],and amines[13].In addition,the synergistic extraction of In(III)and Ga(III)has been reported using trialkyl amine,3,5-dichlorophenol and primary amine as the synergists[14–16].However,there are some problems,forexample,the extraction equilibrium time using oxime series was very long,and the solubility of the carboxylacid series and amine series in the aqueous phase was high.On the other hand,the phosphoric acid series extractants have low solubility in the aqueous phase and resistance properties for the irhydrolysis.Therefore,the phosphoric acid series ofextractants are suitable for separation of In(III)and Ga(III).In particular,di-(2-ethylhexyl)phosphoric acid(D2EHPA)and 2-ethylhexylphosphoric acid mono 2-ethylhexylester(PC-88A)have been used as selective extractants of In(III)and Ga(III).However,it is difficult to perform the selective and mutualseparation of these metals from the zinc re finery residue containing lots of zinc by one step extraction using industrial extractants such as D2EHPA and PC-88A.Untilnow,an extractant that can selectively separate In(III)and Ga(III)from lots of Zn(II)has notbeen developed.
In this study,we have developed a new extractant with a tertiary amino group and phenylphosphinic acid group so as to perform the mutualseparation of In(III),Ga(III)and Zn(II)by one step extraction.The presentextractantis expected to exhibit different extraction selectivities from those of commercial phosphorus acid extractants such as D2EHPA and PC-88A.In particular it is expected that the amine moiety could bring about an anion exchange in a low pH region while the phosphinic acid moiety causes a cation exchange and also chelating formation with the nitrogen atom of the amine moiety.The extraction equilibria of In(III),Ga(III)and Zn(II)were examined to determine their extracted species and extraction equilibrium constants.
2.Experimental Section
2.1.Apparatus
The infrared spectrum ofDEAPP prepared in this study was obtained by a Fourier transform infrared spectrometer(JASCO,Co.,FT-IR 4200,Tokyo,Japan).The1H NMR(400 MHz)spectrum of the extractant in CDCl3was recorded with a nuclear magnetic resonance spectrometer modelAV400 M(Bruker Co.,Rheinstetten,Germany).The pHof aqueous solutions was measured using a pHmeter(HM-30S,DKK-TOACo.,Tokyo,Japan).A flame atomic absorption spectrophotometer(FAAS)model Analyst 100(Perkin-Elmer Co.,CT,USA)was used for the determination of metalconcentrations using an air–acetylene flame.
2.2.Materials and reagents
Metal nitrates of analyticalgrade(Wako Pure Chemical Ind.Ltd.)were used to prepare testsolutions of the metalions.Phenylphosphinic acid(Tokyo Kasei Kogyo Co.),di-(2-ethylhexyl)amine(Wako Pure Chemical Ind.Ltd.)and paraformaldehyde(Sigma-Aldrich Co.LLC.)were used as raw materials to prepare DEAPP.Allother reagents and solvents were of analyticalgrade and used without further puri fication.Allaqueous solutions were prepared with distilled and deionized water.
2.3.Synthesis of[N,N-di-(2-ethylhexyl)amino]methyl phenyl phosphinicacid(DEAPP)
DEAPP was synthesized by the Mannich reaction using phenylphosphinic acid and di-(2-ethylhexyl)amine.Di-(2-ethylhexyl)amine and phenylphosphinic acid in ethanol and hydrochloric acid were stirred and re fluxed at 90°C for 1 h.Then,paraformaldehyde was added dropwise to the solution,and then re fluxed with stirring for 24 h according to the scheme in Fig.1.The ethanolwas evaporated and then the product wasd is solved in chloroform.The chloroformsolution was washed with distilled water for removal of unreacted raw material,and dried over anhydrous magnesium sulfate.After filtration,the chloroform was evaporated in vacuo.The product was obtained with 85%yield and its purity was over 97%.The following properties of DEAPP were identified as follows:1H NMR(400 MHz,CDCl3,25°C)0.86(12H,m,C–),1.23(16H,m,C––C),1.51(9H,m,–(CH2)3),2.82(4H,d, –CH2–N–()2),3.12(2H,s,–N–(CH2)2),7.35(3H,t,–Ph),and 7.82(2H,d,–Ph).
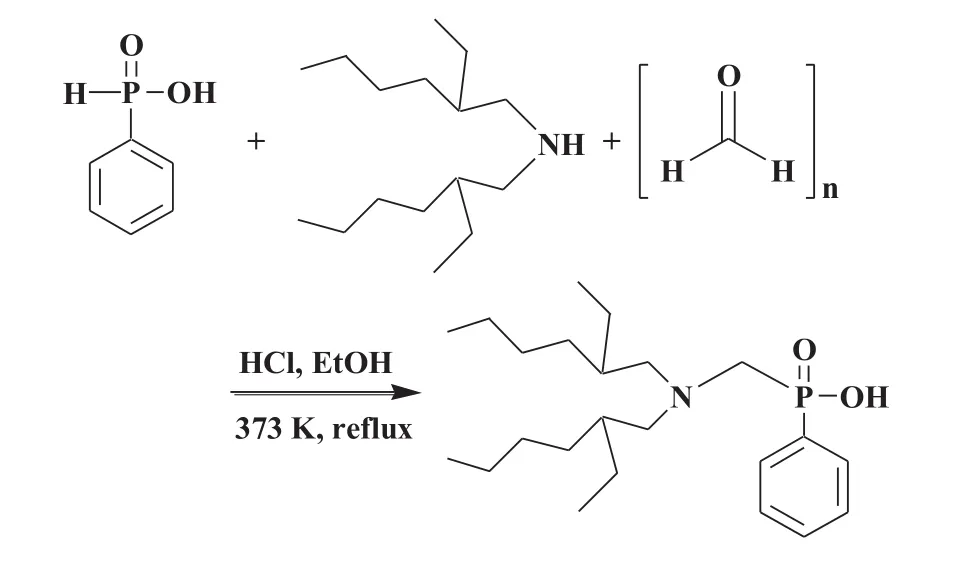
Fig.1.Synthetic reaction scheme for DEAPP.
2.4.Liquid-liquid extraction tests
To evaluate metalextractability of DEAPP,liquid–liquid extraction was carried outusing 1.0 mol·L-1aqueous ammonium nitrate solution containing 1 mmol·L-1metalions.The pH was adjusted with concentrated HNO3or NH3.Toluene was used for the organic phase containing DEAPP.In a 50 cm3Erlenmeyer flask,equalvolumes(10 cm3)of the aqueous phase and the organic phase were shaken mechanically for 24 h at 303 K.After phase separation,the pH of the aqueous solution was measured with a pH meter and was determined by the acid–base titration in lower pH region than 0.5.The metal concentration in the aqueous solution was determined with atomic absorption spectrophotometry.The metalion concentration in the organic phase was calculated from the mass balance between the aqueous and organic phases.Extraction percentage(E,%)and distribution ratio(D)of metal were calculated according to Eqs.(1)and(2),respectively.
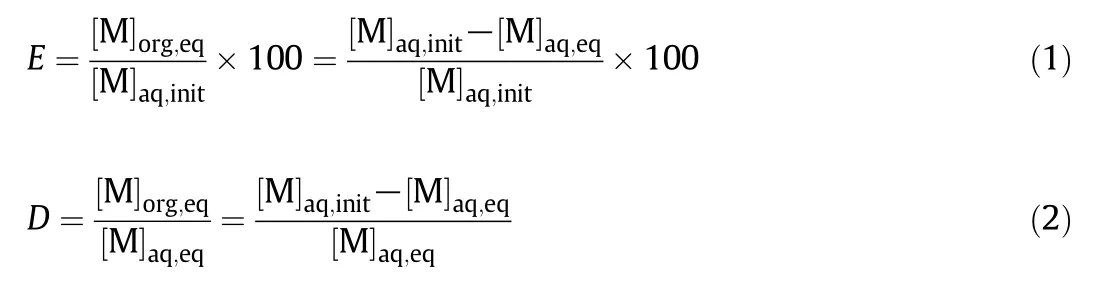
where[M]aq,initrepresents the initialconcentration of metalion in the aqueous phase.[M]aq,eqand[M]org,eqare the total concentrations of metalion in the aqueous and organic phases atequilibrium,respectively.
3.Results and Discussion
3.1.Extraction equilibria of In(III),Ga(III)and Zn(II)from 1 mol·L-1 aqueous ammonium nitrate solution
3.1.1.Effect ofcontact time
The extraction percentages of In(III),Ga(III)and Zn(II)with DEAPP were measured at different time intervals at initialpH values of0,1.0 and 1.0,respectively.The experimental result is shown in Fig.2.This result shows that the extraction equilibria of In(III),Ga(III)and Zn(II)were attained within 10,20 and 60 min,respectively.Therefore,a contact time for extraction equilibrium was carried out over 60 min in allsubsequent experiments.
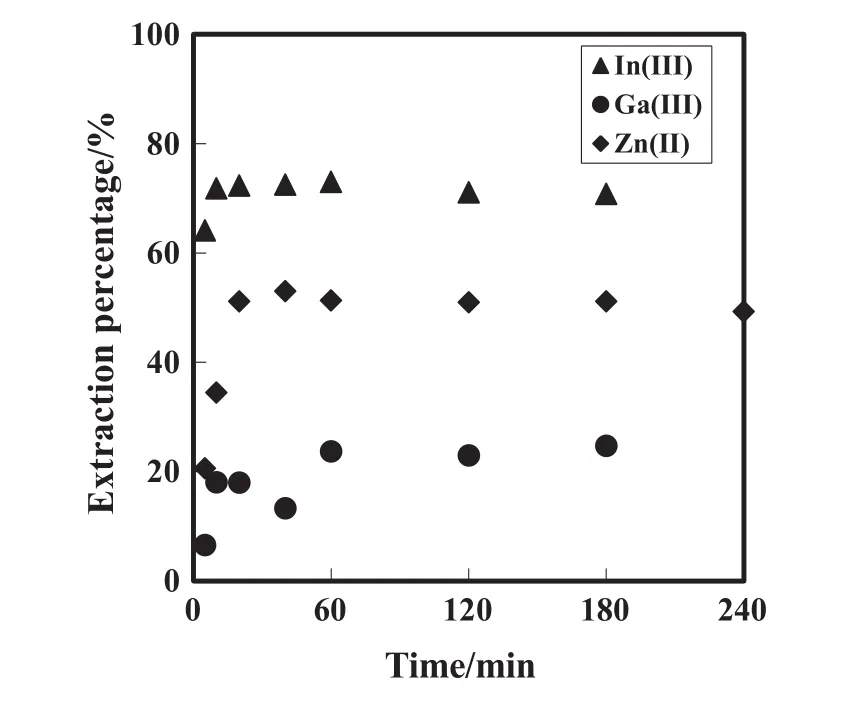
Fig.2.Effect of contact time on the extraction percentage of In(III),Ga(III)and Zn(II).=50 mmol·L-1,[metal ion]init=1 mmol·L-1,pHinit=0,1.0 and 1.0 for In(III),Ga(III)and Zn(II),respectively.
3.1.2.Extraction selectivity of metals with DEAPP
The extraction selectivity ofDEAPP was examined using various metal ions such as In(III),Ga(III),Zn(II),Co(II),Cd(II),Cu(II)and Ni(II).Fig.3 shows the effect of equilibrium pH on the extraction percentage of metalions from 1 mol·L-1aqueous ammonium nitrate solution with DEAPP.As seen from Fig.3,the order of extraction for In(III),Ga(III),Zn(II)and Cu(II)with DEAPP was In(III)>Ga(III)>Zn(II)>Cu(II).This result indicated that the selective recovery for In(III)and Ga(III)from zinc re finery residue and solar panels can be performed.In addition,DEAPP exhibited selective separation of Cd(II)/Zn(II)and Cd(II)/Ni(II).The extraction behavior of DEAPP for In(III),Ga(III)and Zn(II)is different from those of commercial extractants such as PC-88A and D2EHPA.This suggested that the different extraction behaviors of In(III),Ga(III)and Zn(II)were due to the steric hindrance of the phenyl group or the presence of the amine group on DEAPP.It was found that the selective separation and recovery of In(III)and Ga(III)from zinc re finery residues are possible with DEAPP.
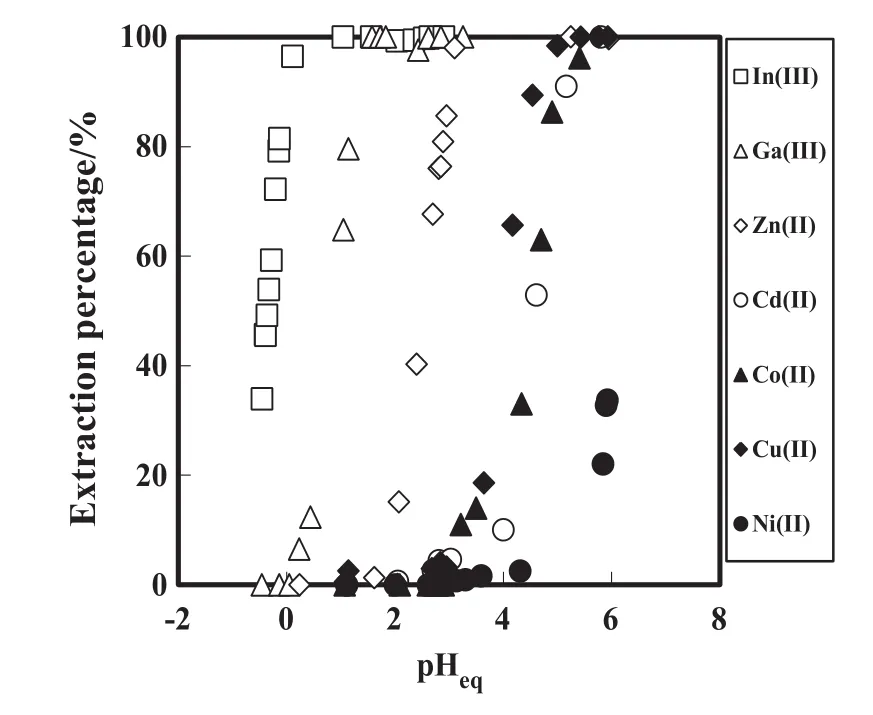
Fig.3.EffectofpH on the extraction percentage ofmetalions from 1 Maqueous ammonium nitrate solution.=50 mmol·L-1,[metalion]init=1 mmol·L-1.
3.1.3.Selective extraction of In(III),Ga(III)and Zn(II)with DEAPP
The extraction selectivities from the mixture solution of Ga(III)/In(III)and Zn(II)/Ga(III)using DEAPP are shown in Fig.4.The selective extraction of In(III)from the Ga(III)/In(III)mixture was performed with DEAPP in the presence of a 100-fold excess of Ga(III).Under these conditions Ga(III)was not extracted at all.In addition,Ga(III)was selectively extracted with DEAPP in the presence of a 130-fold excess of Zn(II).These results were considered to be due to the steric hindrance by the phenyl moiety in DEAPP.The commercial extractant was not able to separate In(III),Ga(III)and Zn(II).Therefore DEAPP is the most suitable extractant for mutual separation of In(III),Ga(III)and Zn(II)and expected to separate In(III)and Ga(III)from zinc re finery residues.
3.2.Extraction equilibria ofIn(III),Ga(III)and Zn(II)with DEAPP
3.2.1.Effect of equilibrium pH
Fig.5 shows the effect of the equilibrium pHon the distribution ratio ofIn(III),Ga(III)and Zn(II)with DEAPP.As seen from Fig.5,the slopes of these straight lines indicate the number of protons that have been exchanged with their metals during extraction.The plots of lg D versus pH in the In(III),Ga(III)and Zn(II)extraction were linear with slopes of 3,2 and 2 for In(III),Ga(III)and Zn(II),respectively.In addition,there is in fluence of another anion on the extraction reaction of Ga(III)because the charge neutralization is necessary for the distribution of the metal–extractant complex to the organic phase.Therefore,the effect ofnitrate anion participating in the extraction of Ga(III)with DEAPP was studied.

Fig.5.Effect of pHeq on distribution ratio of In(III),Ga(III)and Zn(II).=50 mmol·L-1,[metalion]init=1 mmol·L-1.
3.2.2.Effect of nitrateion
Fig.6 shows the effect of nitrate ion on the distribution ratio of In(III),Ga(III)and Zn(II)with DEAPP.The plots oflg D–n pH(n:number of hydrogen ion that takes part in the extraction obtained from Fig.5)versus lg[]were straight lines with slopes of0,1 and 0 respectively,indicating that one nitrate ion is incorporated in the formation of the Ga(III)–extractantcomplex.
3.2.3.Effect of extractant concentration
Fig.7 shows the effect of the dimer concentrationon the distribution ratio of In(III),Ga(III)and Zn(II)with DEAPP(=HR).The distribution ratios of these metals increase with increasing concentration of the DEAPP.
The plots oflg D–n pH(n=3,3 and 2 for In(III),Ga(III)and Zn(II),respectively)versusgive lines with a slope of 1.5 in the extraction of Ga(III)and Zn(II)with DEAPP,indicating that 3 molecules of the extractant are involved in the complex formation with Ga(III)and Zn(II).On the other hand,the plot of lg D–2pH versusfor In(III)indicates a straight line with a slope of2,indicating that 4 molecules of the extractantare involved in the complex formation with In(III).
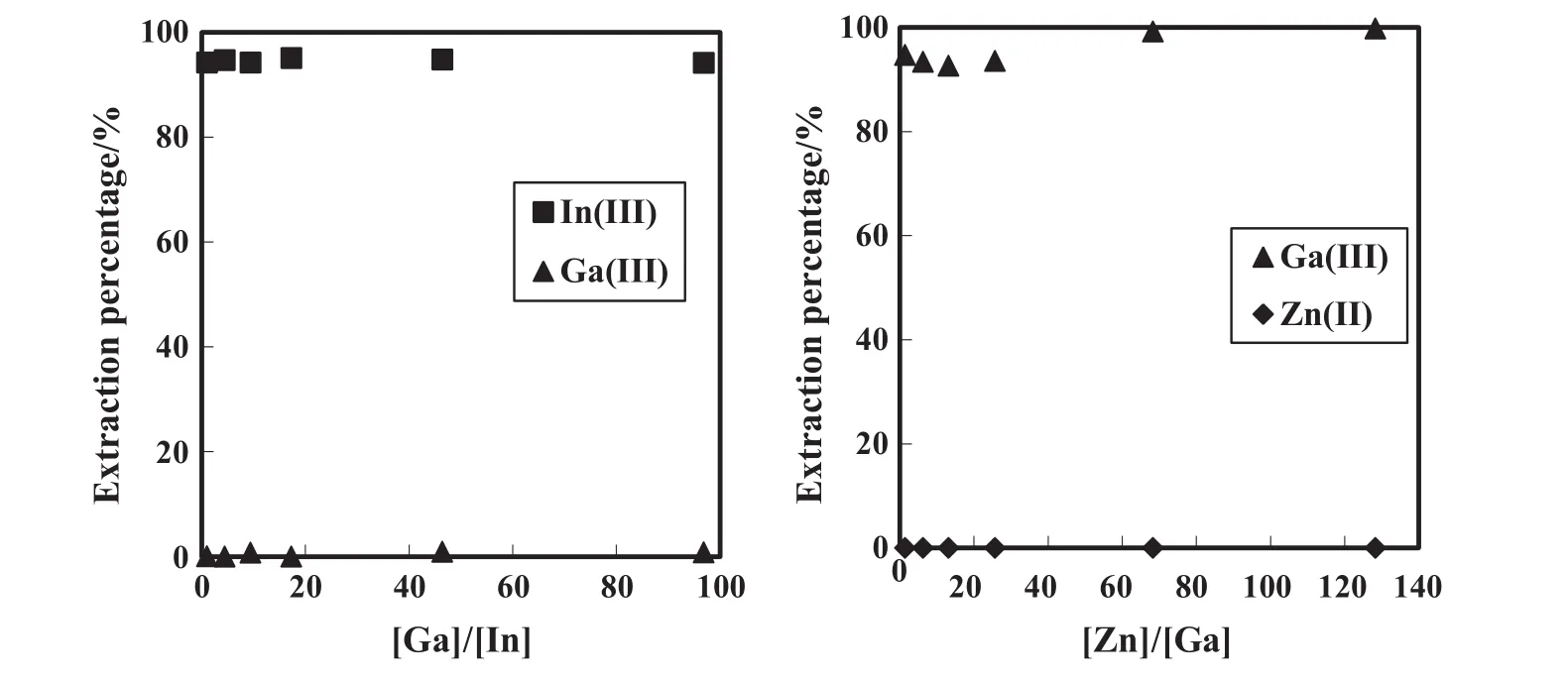
Fig.4.Selective extraction of In(III)and Ga(III)from Ga(III)/In(III)or Zn(II)/Ga(III)mixture solution.=50 mmol·L-1,[In(III)]init=1 mmol·L-1,[Ga(III)]init=1 or 1–100 mmol·L-1,[Zn(II)]init=1–100 mmol·L-1.
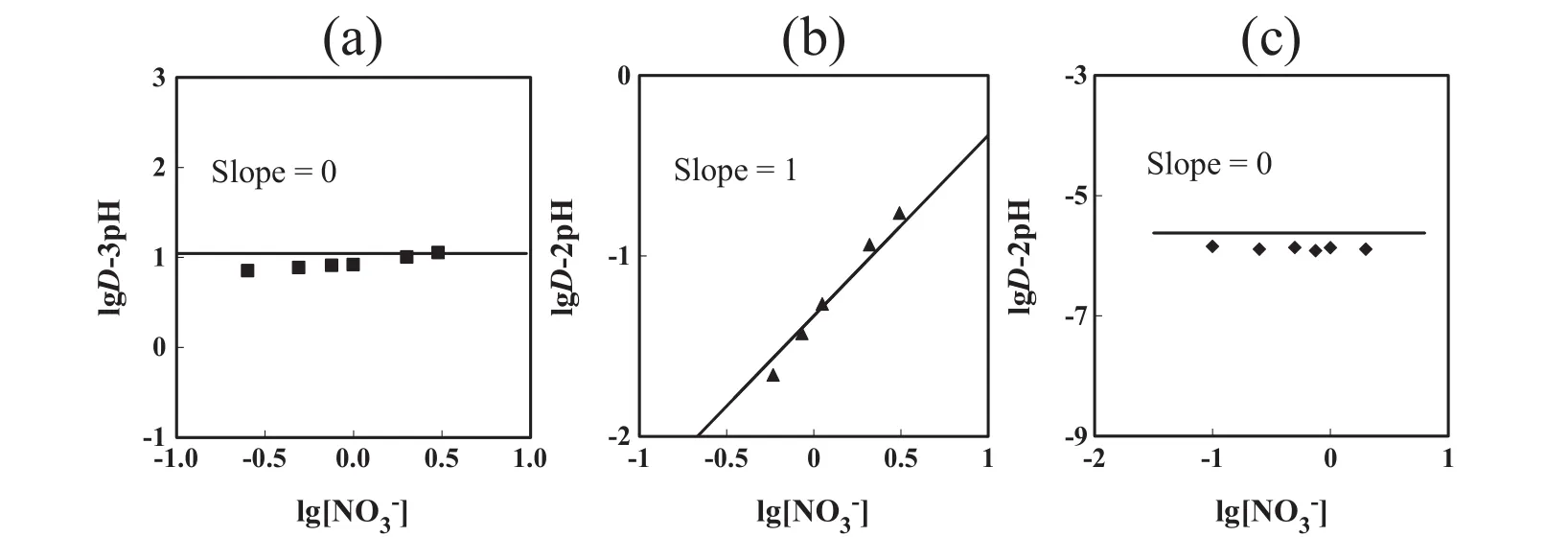
Fig.6.Effect of nitrate ion on the distribution ratio of(a)In(III),(b)Ga(III)and(c)Zn(II).=50 mmol·L-1,[metalion]init=1 mmol·L-1,pHeq=0,1 and 2 for In(III),Ga(III)and Zn(II),respectively.
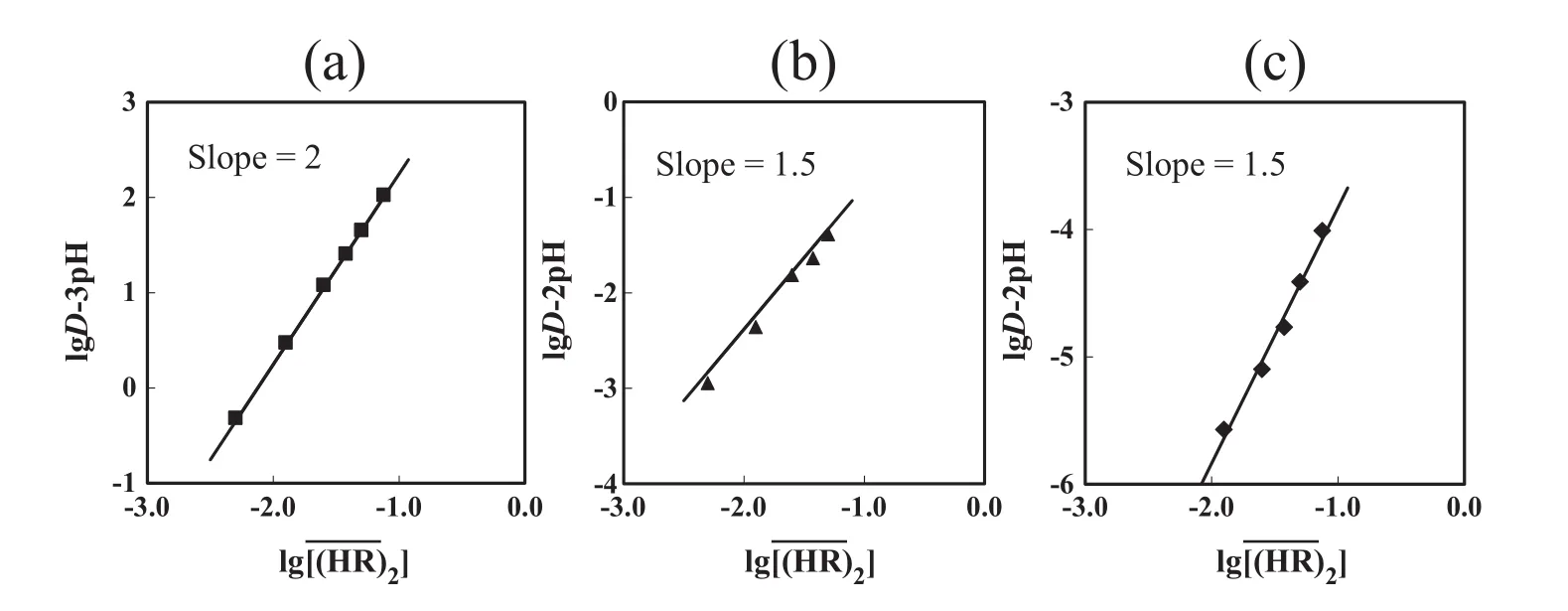
Fig.7.Effect of DEAPP concentration on the distribution ratio of(a)In(III),(b)Ga(III)and(c)Zn(II).=10–150 mmol·L-1,[metalion]init=1 mmol·L-1,pHeq=0,1 and 2 for In(III),Ga(III)and Zn(II),respectively.
3.2.4.Extraction equilibria for In(III),Ga(III)and Zn(II)with DEAPP
Based on the experimental results mentioned above,the extraction equilibrium of In(III)with DEAPP can be expressed as follows:
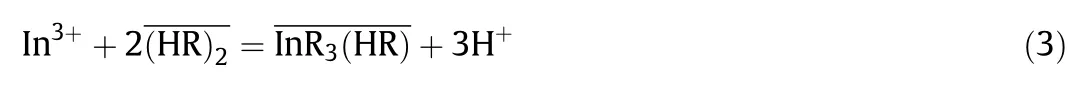
where the bars denote the species in the organic phase.
The extraction equilibrium constant Kex,Inis given by Eq.(4):

Also,the distribution ratio of In(III)between the organic and aqueous phases is defined as

The mass balance equation of the extractantis given by the following equations:
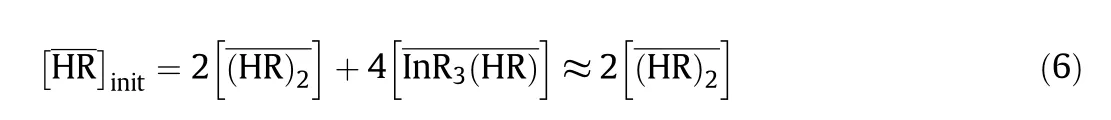
whereinitindicates the initial analytical concentration of DEAPP in the organic phase.
By a combination of Eqs.(4)–(6),we obtained the following equation in its logarithmic form:
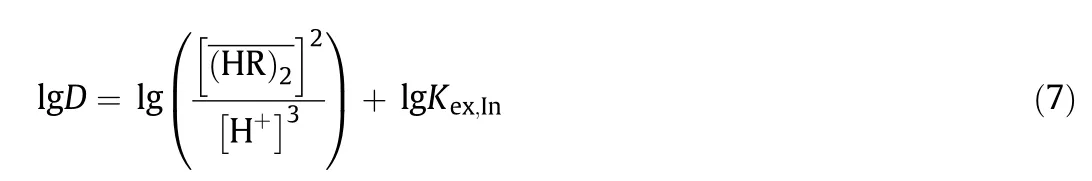
Rearrangement of Eq.(7)gives the following equation.
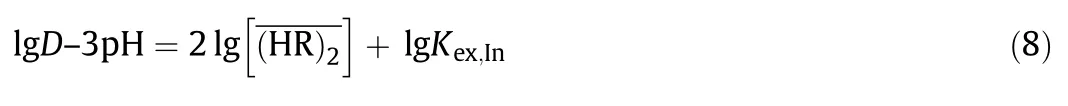
For the extraction equilibrium of Ga(III),the following equations also were obtained by the similar method as described above.
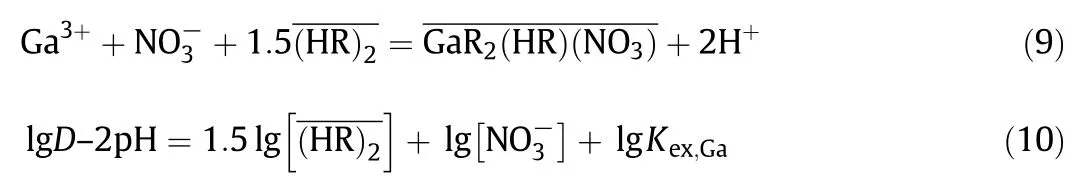
In a similar manner,the following equation was obtained for the extraction equilibrium of Zn(II).
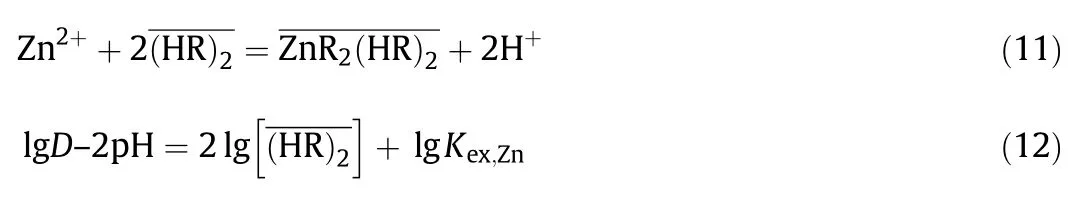
The experimentalresults of In(III),Ga(III)and Zn(II)shown in Fig.7 agree with Eqs.(8),(10)and(12).The extraction equilibrium constants of In(III),Ga(III)and Zn(II)using DEAPP were determined to be Kex,M=1.7 × 104[dm3·mol-1],4.17[(dm3·mol-1)0.5],and-1.55 × 10-2,respectively from their interceptions with the ordinate in Fig.7.
3.3.Back extraction of In(III),Ga(III)and Zn(II)
The back extraction of In(III),Ga(III)and Zn(II)from the loaded organic phase was examined using HCl,HNO3and EDTA by varying their concentrations.Back extraction percentage(BE,%)was calculated according to Eq.(13).

where[M]org,initrepresents the initial concentration of metal ion in the organic phase.
Table 1 summarizes the back extraction percentage with back extraction agents.As shown in Table 1,hydrochloric acid or the chelating agent EDTA effectively stripped Zn(II)from the organic phase.A high back extraction percentage of In(III)was achieved using 5 MHNO3,while the back extraction of Ga(III)was not effective using all back extraction reagents.Therefore,further examination of the back extraction reagents is necessary.
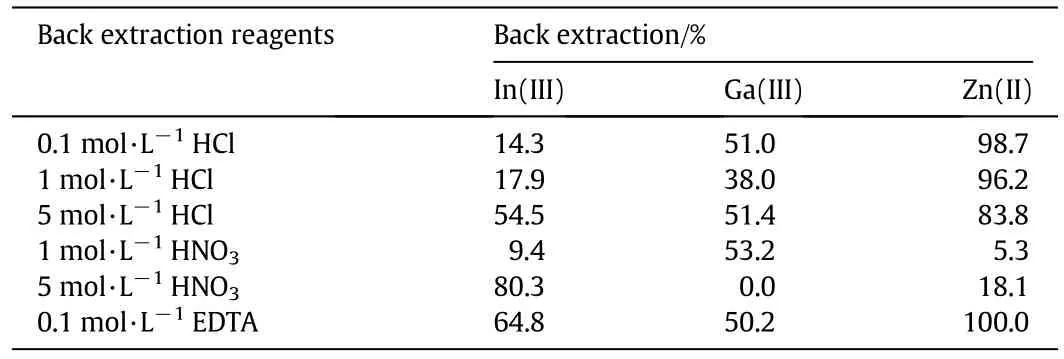
Table 1 Back extraction reagents and back extraction percentages in back extraction of In(III),Ga(III)and Zn(II)
4.Conclusions
The newly synthesized extractant,[N,N-di(2-ethylhexyl)amino]-methyl phenyl phosphinic acid(=DEAPP)was found to be efficient for the mutualextraction of In(III),Ga(III)and Zn(II)ions.The extraction equilibria of In(III),Ga(III)and Zn(II)with DEAPP were attained within 60 min.The mutual separation ability of DEAPP for the extraction of In(III),Ga(III)and Zn(II)was higher than that of commercial phosphorous acidic extractants..This is probably due to the steric hindrance by the phenyl moiety and chelating effect by the amino moiety and phosphinic acid in DEAPP.The high selectivity of In(III)and Ga(III)over Zn(II)with DEAPP was con firmed by extracting selectively and mutually In(III),Ga(III)and Zn(II)ions from their mixture solution.The stoichiometries in the extraction reactions of In(III),Ga(III)and Zn(II)with DEAPP were determined by slope analysis based on the experimented results.Moreover,the back extraction of In(III)and Zn(II)from the organic phase was achieved using 5 mol·L-1HNO3and 1 mol·L-1HCl,respectively,although further study on the back extraction of Ga(III)is necessary.
Acknowledgments
This work was supported by The Environment Research and Technology Development Fund(3K133005).The authors thank The Ministry of Environment(3K133005),Japan for providing Grant for Research and Technology Development on Waste Management.
[1]J.S.Liu,H.Chen,X.Y.Chen,Z.L.Guo,Y.C.Hu,C.P.Liu,Y.Z.Sun,Extraction and separation of In(III),Ga(III)and Zn(II)from sulfate solution using extraction resin,Hydrometallurgy 82(2006)137–143.
[2]J.Jayachandran,P.Dhadke,Solvent extraction separation of gallium(III)with 2-ethylhexyl phosphonic acid mono 2-ethylhexyl ester(PC-88A),Hydrometallurgy 50(1998)117–124.
[3]S.Nishihama,T.Hirai,I.Komasawa,Separation and recovery gallium and indium from simulated zinc residue by liquid–liquid extraction,Ind.Eng.Chem.Res.38(1999)1032–1039.
[4]S.Nishihama,A.Hino,T.Hirai,I.Komasawa,Extraction and separation ofgallium and indiumfromaqueous chloride solution using severalorgano phosphorus compounds as extractants,J.Chem.Eng.Jpn 31(1998)818–827.
[5]S.Virolainen,D.Ibana,E.Paatero,Recovery of indium from indium tin oxide by solvent extraction,Hydrometallurgy 107(2011)56–61.
[6]J.S.Liu,H.Chen,Z.L.Guo,Y.C.Hu,Selective separation of In(III),Ga(III),and Zn(II)from dilute solution using solvent-impregnated resin containing Di(2-ethylhexyl)phosphoric acid,J.Appl.Polym.Sci.100(2006)253–259.
[7]M.S.Lee,J.G.Ahn,E.C.Lee,Solvent extraction separation of indium and gallium from sulphate solutions using D2EHPA,Hydrometallurgy 63(2002)269–276.
[8]B.Gupta,N.Mudhar,S.N.Tandon,Extraction and separation ofgallium using Cyanex 301:its recovery from Bayer's liquor,Ind.Eng.Chem.Res.44(2005)1922–1927.
[9]B.Gupta,N.Mudhar,I.Singh,Separations and recovery of indium and gallium using bis(2,4,4-trimethylpentyl)phosphinic acid(Cyanex 272),Sep.Purif.Technol.57(2007)294–303.
[10]B.Gupta,A.Deep,P.Malik,Liquid–liquid extraction and recovery of indium using Cyanex 923,Anal.Chim.Acta 513(2004)463–471.
[11]F.J.Alguacil,Solvent extraction of indium(III)by LIX 973N,Hydrometallurgy 51(1999)97–102.
[12]X.Zhang,G.Yin,Z.Hu,Extraction and separation of gallium,indium and thallium with several carboxylic acids from chloride media,Talanta 59(2003)905–912.
[13]I.S.EL-Yamani,E.I.Shabana,Studies on extraction of indium(III)chloride complexes by long-chain amines,J.Radioanal.Nucl.Chem.88(1985)217–223.
[14]S.Fan,Q.Jia,N.Song,R.Su,W.Liao,Synergistic extraction study of indium from chloride medium by mixtures of sec-nonylphenoxy acetic acid and trialkylamine,Sep.Purif.Technol.75(2010)76–80.
[15]H.Imura,A.Oshiro,K.Ohashi,Synergistic extraction ofgallium(III)and indium(III)with 2,4-pentanedione and 3,5-dichlorophenolon the basis ofouter-sphere complexation,Anal.Sci.14(1998)1093–1098.
[16]H.Ma,Y.Lei,Q.Jia,W.Liao,L.Lin,An extraction study of gallium,indium,and zinc with mixtures of sec-octylphenoxyacetic acid and primary amine N1923,Sep.Purif.Technol.80(2011)351–355.
 Chinese Journal of Chemical Engineering2016年2期
Chinese Journal of Chemical Engineering2016年2期
- Chinese Journal of Chemical Engineering的其它文章
- Experimental evaluation and modeling of liquid jet penetration to estimate droplet size in a three-phase riser reactor
- Analysis of drop deformation dynamics in turbulent flow
- Review on current advances,future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents☆
- Photorheologically reversible micelle composed ofpolymerizable cationic surfactant and 4-phenylazo benzoic acid☆
- Experimental study on the effects of big particles physical characteristics on the hydraulic transport inside a horizontal pipe
- Relationship between breakthrough curve and adsorption isotherm of Ca(II)imprinted chitosan microspheres for metaladsorption☆
