Kinetic study on selective extraction of HCland H3PO4 in a micro fluidic device☆
Fang Zhao,Yangcheng Lu,KaiWang,Guangsheng Luo*
The State Key Lab ofChemicalEngineering,Department of Chemical Engineering,Tsinghua University,Beijing 100084,China
1.Introduction
KH2PO4is widely used in the agricultural,chemical,pharmaceutical and food industries.It is also used to produce other potassium salts,penicillin and sodium glutamate[1,2].Production processes for KH2PO4include the neutralization method,the direct chemical conversion method,the crystallization method,the ion exchange method and the extraction method[3,4].Herein,the extraction method[5–8]is proposed for three major advantages:using cheap KClinstead of expensive KOH,low energy consumption and high product purity.
The extraction method involves the following reaction
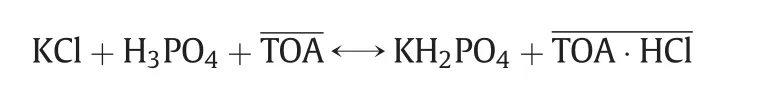
where the overbar represents the oil phase.As the extractant,trioctylamine(TOA),removes HClfrom the aqueous phase,the equilibrium shifts to the right,yielding KH2PO4.Considering that H3PO4in the aqueous phase can be simultaneously extracted by TOA,the extraction selectivity of HClto H3PO4is the basis of KH2PO4production.
Micro fluidic technology has gained widespread attention in recent years.Liquid–liquid extraction in micro fluidic devices has been studied by many researchers,forinstance,aqueous two-phase(ATP)extraction[9,10],acid extraction[11,12],and hydrometallurgy extraction[13,14].The application of micro fluidics in liquid–liquid extraction provides many advantages for extraction kinetics study,including high extraction efficiency,precise controlon the contacttime,low reagents and energy consumption,continuous processing and safety[15,16],making micro fluidic technology as an ideal platform for extraction kinetics study.
In this paper,the kinetics of the selective extraction of HCl and H3PO4has been experimentally investigated using a coaxial microchannel.Liquid–liquid system consisting of the aqueous phase containing HCl(H3PO4)and the oil phase containing TOA was selected as a model system for the extraction process in KH2PO4production.The changes of the extraction efficiency ofHCland H3PO4and the selectivity for HClalong with the residence time were investigated.These extraction kinetic data willhelp with the design and improvement of performance of this extraction method.
2.Experimental
2.1.Materials
TOA(mass fraction≥99.5%)was provided by Jinan LeqiChemical Industry Co.,Ltd,China.n-octanol(AR),KCl(AR)and NaOH(AR)were purchased from Beijing Modern Eastern Finechemical,China,H3PO4(AR,mass fraction≥85%)from Beijing Chemical Works,China,and HCl(AR,mass fraction 36–38%)from Yongfei Chemical Works,China.
Solutions were prepared using deionized water.Allchemicals were used without further puri fication.
2.2.Experimentalsetup
Aschematic diagram of the experimental setup is shown in Fig.1(a).A coaxial microchannel was used in our experiment,as shown in Fig.1(b)and(c),composed of two polymethyl methacrylate(PMMA)chips(50 mm×30 mm,microchannelsize 1.6 mm×1.6 mm),a stainless steelneedle(outer diameter 0.30 mm,inner diameter 0.16 mm)and a glass capillary(outer diameter 1.5 mm,inner diameter 1.05 mm,length about 40 mm).The outlet ofglass capillary was connected to a given length of Te flon tube,with an inner diameter of 1 mm,and an outer diameter of 1.6 mm.Oil phase samples were taken for different residence time,which was changed by the length of the Te flon tube.
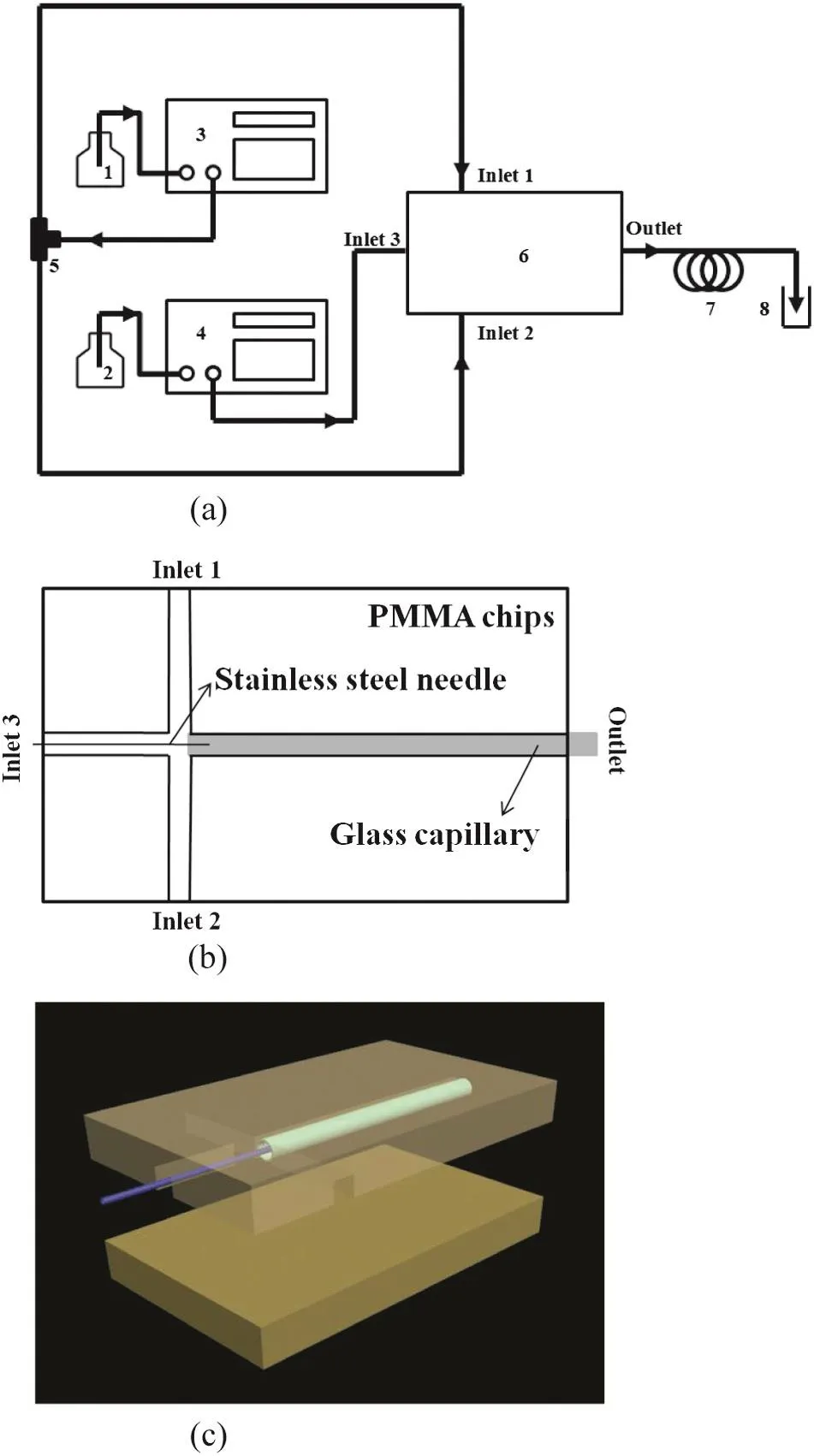
Fig.1.Schematics of experimentalsetup(a),microchanneldevices(b,c).1—oilphase feed;2—aqueous phase feed;3,4—piston pump;5—tee;6—microchannel;7—delay loop;8—collecting bottle.
The HClsolution,the H3PO4solution or H3PO4and KClsolutions of differentconcentrations were used as the aqueous phase in our experiment.And it's important to note that for extraction of the mixture solutions,KClwas always in excess of H3PO4in the initialaqueous feeds to ensure high selectivity for HCl.The extractant,used as the continuous phase,was 33.3%(by volume)TOA dissolved in n-octanoland was saturated with water before being used.Experiments were performed at room temperature in a range of 295–301 K.
2.3.Extraction experiments in shaking conical flasks
As a reference experiment,we determined the extraction kinetics of HClin conical flasks settled in a shaking thermostat water bath.
The initialaqueous and oilphase were 1.97 mol·L-1HClsolution and the extractant[33.3%(by volume)TOA dissolved in n-octanol],respectively.The oil/water phase ratio was 3:1.Water bath was kept at 298.2 K(uncertainty of±0.1 K)with a shaking frequency of 120 r·min-1.Oilphase samples were taken at different shaking times.
2.4.Analysis
To acquire the kinetic data,for a certain residence time,the oilphase sample with a mass of about 0.22 g was taken and then stripped by a 0.2 mol·L-1NaOH solution with a mass of about 5 g.After complete stripping,the aqueous was analyzed for Cl-ortotalPO43-concentration by ion chromatography(IC),from which the total HClor total H3PO4concentration in the oilsample could be obtained.
The residence time in this work was referred as the time for the fluid flowing through the Te flon tube.As long as the extraction was notcompleted at this residence time,the acid concentration of the collected oil phase sample would be higher than the acid concentration of the oil phase at the outlet of the tube,due to the mass transfer in the collector.We found that for residence time above 5 s,such an in fluence on the acid concentration determination was less than 5%.
For measurement errors,uncertainties in acid concentrations of the oil phases were evaluated using the standard deviation of repeated measurements.The relative uncertainties in total HClconcentrations and total H3PO4concentrations of the oilphase are both within±4%.Considering the errors of the sampling and the measurement,the relative uncertainties of the measured acid concentrations of the oilphases were from-4%to 9%.
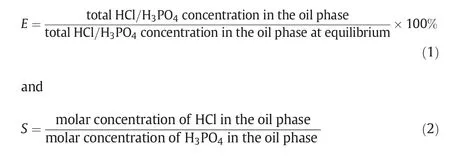
Using the acid concentrations of the oilphases,we can calculate the extraction efficiency(E)of HClor H3PO4,and the selectivity(S)for HCl,which are defined respectively as
3.Results and Discussion
3.1.Hydrodynamics of the liquid-liquid flow in the microchannel
Jetting flow occurred in the coaxial microchannel under our experimental conditions..The widening jet broke into drops(Fig.2)with an average size in a range of 0.67–0.70 mm for the different aqueous feeds in our experiment.
3.2.Comparison of the extraction in shaking conical flasks and the micro fluidic device
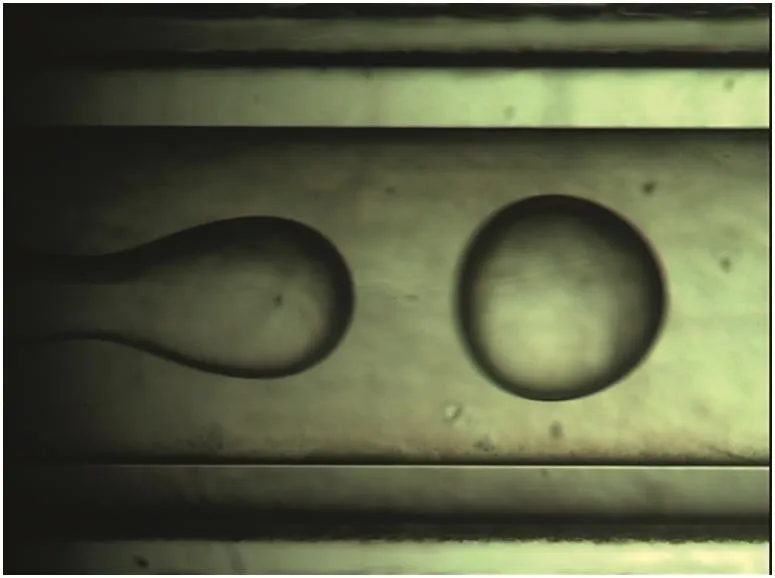
Fig.2.A typicalphoto of the drop forming in our microchannel.Initialaqueous phase:C(H3PO4)=1.71 mol·L-1,C(KCl)=1.89 mol·L-1,1 ml·min-1;initialoilphase:water saturated extractant with 33.3%(by volume)TOA,3 ml·min-1.
For extraction kinetics in shaking conical flasks,the extraction efficiency of HClwas calculated using Eq.(1)and plotted against the shaking time as shown in Fig.3(a).Meanwhile,the extraction of a 1.74 mol·L-1HCl solution was carried out in the micro fluidic device for comparison,and the results are shown in Fig.3(b).We can see that,under similar extraction conditions,HCl extraction was almost completed in 1 min in the micro fluidic device,while the extraction efficiency was only about60%at30 min in shaking conical flasks.We can conclude that fast extraction kinetics can be realized in micro fluidic devices.

Fig.3.The time pro files of HClextraction efficiency in shaking conical flask(a)and in the micro fluidic device(b).
3.3.Extraction of the HClsolution and the H3PO4 solution
Extraction of the HClsolution and the H3PO4solution was investigated respectively,with the acid concentrations in the initialaqueous feeds both being about 1.75 mol·L-1.The results are shown in Fig.4.We can see that HCl was extracted little faster than H3PO4with the same acid concentrations in the initialaqueous feeds.Such a difference may resultfromthe mass transfer resistance orthe binding capacity between the acid and TOA,since the sizes of the droplets generated in our microchannel,as well as the specific area of mass transfer,didn't differ much for differentaqueous feeds.The viscosity ofHClextraction system is usually smaller than that of H3PO4extraction system,leading to smaller mass transfer resistance in HClextraction.
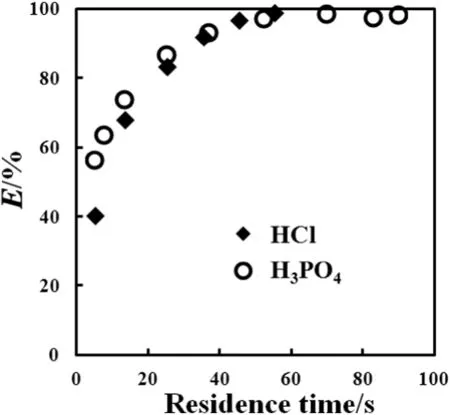
Fig.4.Comparison of extraction of the HClsolution and the H3PO4 solution.
3.4.Extraction of the mixture solutions of H3PO4 and KCl
In this part,the extraction efficiency of both HCl and H3PO4and the selectivity for HCl(calculated using Eq.(2))at different residence time were examined.According to whether the initial TOA was in excess of H3PO4or not,two scenarios were discussed separately.
3.4.1.Initial TOA being in excess of H3PO4
When the initialTOA was in excess of H3PO4,the extraction of H3PO4was enhanced atlater stage of the extraction process(Fig.5(b)),leading to the decrease of the selectivity for HCl(Fig.5(c)).When HClextraction approached equilibrium,the mass transfer of HClwould slow down due to remarkably decreased driven force,and even overwhelmed by H3PO4extraction.
From Fig.5(a)and(c),we can also see that when the initial H3PO4concentration was increased with fixed value of C(KCl):C(H3PO4),HCl extraction became slower and the selectivity for HCl increased.
3.4.2.Initial H3PO4 being in excess of TOA
When the initial H3PO4was in excess of TOA,the extraction of H3PO4was inhibited soon after a short period(Fig.6(b)),leading to the further increase of the selectivity for HCl(Fig.6(c)).We suppose that the decrease of H3PO4occurs after the loading of TOA approaches saturation,determined by two probable mechanisms.One is that ion exchange occurs between the TOA–H3PO4complex in the oil phase and Cl-in the aqueous phase.The second is that the equilibrium
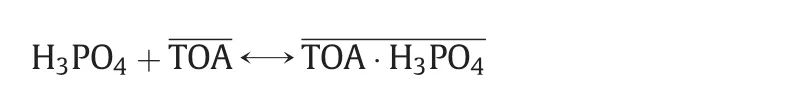
shifts to the left and the released TOA is preferred to combine with HCl.
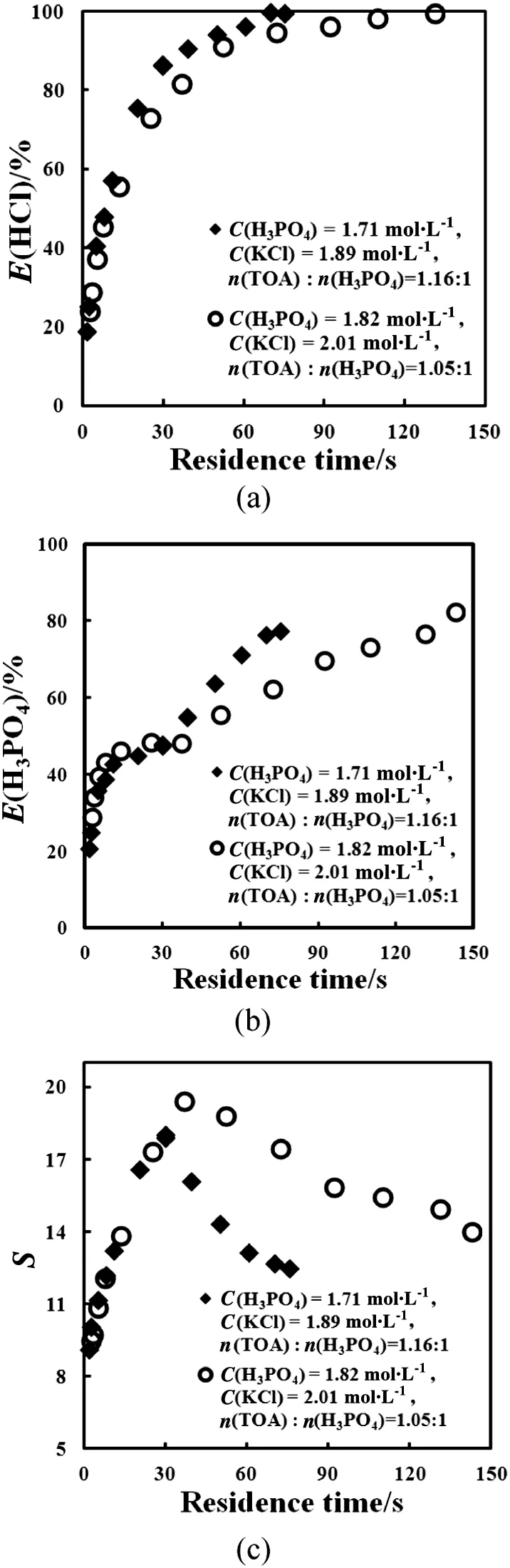
Fig.5.The time pro files of H3PO4 extraction efficiency(a),HClextraction efficiency(b),and selectivity(c)for H3PO4 and KClsolution extraction with the initialTOA in excess of H3PO4.
As observed from Fig.6(c),higher selectivity for HCl could be achieved when the initial H3PO4concentration was increased with fixed value of C(KCl):C(H3PO4),or when the value of C(KCl):C(H3PO4)was increased with fixed H3PO4concentration of the initial aqueous phase.
It's noteworthy that the changes of the selectivity for HClalong with the residence time were sensitive to the initial composition of the aqueous feed.Itimplies thatwe may have flexible methods to control the selectivity in micro fluidic devices,by changing the residence time,as well as the initial composition of the aqueous feed.

Fig.6.The time pro files of H3PO4 extraction efficiency(a),HClextraction efficiency(b),and selectivity(c)for H3PO4 and KClsolution extraction with the initial H3PO4 in excess of TOA.
4.Conclusions
In this work,towards designing and optimizing the KH2PO4production by the extraction method,kinetics of the selective extraction ofHCl and H3PO4was studied in a micro fluidic device with a coaxial microchannel,using the extractantof33.3%(by volume)TOA dissolved in n-octanol.
Extraction equilibrium could be achieved quickly in the micro fluidic device(less than 3 min in our experiments).HCl extraction seemed faster than H3PO4extraction due to smaller mass transfer resistance and much stronger reaction between HCland TOA.
For extraction of the H3PO4and KClsolutions,when TOA was in excess of H3PO4in the initialfeeds,the extraction of H3PO4was promoted atlaterstage of the extraction process.As a result,the selectivity for HCl increased firstand then decreased.When H3PO4was in excess of TOAin the initialfeeds,the extraction ofH3PO4was inhibited soon aftera short period,leading to the decrease of H3PO4extraction efficiency and the further increase of the selectivity for HCl.
The diverse changes of selectivity for HClalong with the residence time indicate that a dynamic control of selectivity in micro fluidic devices may be importantand accessible forimproving the KH2PO4conversion efficiency in the extraction method.
In the future study,we will focus on the mechanism for the changes of selectivity,and a model for the kinetics of the selective extraction of HCland H3PO4.
[1]L.Fan,J.G.Zhao,The current status and prospect of potassium dihydrogen phosphate in China,Phosphate Compd.Fertil.21(3)(2006)34–37.
[2]J.M.Wang,Production situation and marketanalysis of potassium dihydrogen phosphate,Chem.Enterp.Manag.(11)(2010)31–37.
[3]H.Q.Chen,Preparation method of potassium dihydrogen phosphate,Sichuan Chem.Ind.16(5)(2013)16–19.
[4]J.H.Luo,J.Li,Y.H.Wang,Y.Jin,Development trend of potassium dihydrogen phosphate production method,Phosphate Compd.Fertil.27(3)(2012)8–9.
[5]C.X.You,Z.F.Yang,G.Z.Wang,J.H.Zhou,N.Sun,Preparation of potassium dihydrogen phosphate with raf finate from phosphoric acid puri fication by extraction method,Inorg.Chem.Ind.46(1)(2014)56–58.
[6]C.L.Jiang,S.C.Zhen,Y.L.Zheng,Z.J.Wang,Preparation of potassium dihydrogen phosphate by triotylamine-isoamyl alcoholorganic solvent extraction,Inorg.Chem.Ind.46(2)(2014)44–46.
[7]Y.Sun,J.Li,K.Zhou,Y.Q.Zhang,Study on preparation of potassium dihydrogen phosphate from wet-process phosphoric acid by solvent extraction method,Inorg.Chem.Ind.45(4)(2013)34–37.
[8]E.Rubin,E.Szpruch,A.Orell,Production of KH2PO4from KCland H3PO4in an organic liquid medium,Ind.Eng.Chem.Process.Des.Dev.17(4)(1978)460–468.
[9]Y.S.Huang,T.Meng,T.Guo,W.Li,W.L.Yan,X.R.Li,S.Wang,Z.P.Tong,Aqueous twophase extraction for bovine serum albumin(BSA)with co-laminar flow in a simple coaxialcapillary micro fluidic device,Micro fluid.Nano fluid.16(3)(2014)483–491.
[10]Y.C.Lu,Y.Xia,G.S.Luo,Phase separation of parallellaminar flow for aqueous two phase systems in branched microchannel,Micro fluid.Nano fluid.10(5)(2011)1079–1086.
[11]N.Sen,M.Darekar,K.K.Singh,S.Mukhopadhyay,K.T.Shenoy,S.K.Ghosh,Solvent extraction and stripping studies in microchannels with TBP nitric acid system,Solvent Extr.Ion Exch.32(3)(2014)281–300.
[12]A.Woitalka,S.Kuhn,K.F.Jensen,Scalability of mass transfer in liquid–liquid flow,Chem.Eng.Sci.116(2014)1–8.
[13]S.H.Ju,P.Peng,Y.Q.Wei,L.Xu,S.H.Guo,L.B.Zhang,L.H.Zhang,L.Q.Dai,Solvent extraction of In3+with microreactor from leachant containing Fe2+and Zn2+,Green Process.Synth.3(1)(2014)63–68.
[14]H.Hiroyasu,M.Tokeshi,M.Harada,T.Kitamori,Y.Ikeda,Development of the innovative nuclide separation system for high-levelradioactive waste using microchannelchip,Prog.Nucl.Energy 47(2005)439–447.
[15]E.Kamio,Y.Seike,H.Yoshizawa,T.Ono,Modeling of extraction behavior of docosahexaenoic acid ethylester by utilizing slug flow prepared by microreactor,AIChE 56(8)(2010)2163–2172.
[16]J.W.Swarts,Effective Use of Enzyme Microreactors—Thermal,Kinetic,and Ethical Guidelines(Ph.D.Thesis)Wageningen University,the Netherlands,2009.
 Chinese Journal of Chemical Engineering2016年2期
Chinese Journal of Chemical Engineering2016年2期
- Chinese Journal of Chemical Engineering的其它文章
- Experimental evaluation and modeling of liquid jet penetration to estimate droplet size in a three-phase riser reactor
- Analysis of drop deformation dynamics in turbulent flow
- Review on current advances,future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents☆
- Photorheologically reversible micelle composed ofpolymerizable cationic surfactant and 4-phenylazo benzoic acid☆
- Experimental study on the effects of big particles physical characteristics on the hydraulic transport inside a horizontal pipe
- Relationship between breakthrough curve and adsorption isotherm of Ca(II)imprinted chitosan microspheres for metaladsorption☆
