The use of environmentally sustainable bio-derived solvents in solvent extraction applications—A review
Zheng Li,Kathryn H.Smith,Geoffrey W.Stevens*
Particulate Fluids Processing Centre,Department of Chemical and Biomolecular Engineering,The University of Melbourne,Melbourne,VIC 3010,Australia
1.Introduction
Solvent extraction is an effective separation technique widely used in a variety of applications,ranging from separations in analytical chemistry[1,2]to industrial processes in hydro metallurgy[1,3–6],pharmaceutical[7–9],food engineering[9–11]and waste treatment[8,12,13].Solvent extraction takes advantage of the solubility difference of a solute in two immiscible liquid phases(usually one organic and one aqueous phase)in contact with each other to attain separation.Solute A,which initially is dissolved in only one of the two phases,gradually distributes between the two liquids with the process of reaction or diffusion at the interface and eventually reaches equilibrium.Concentrations of solute A in organic and aqueous phases are[A]organd[A]aqrespectively and the distribution ratio,DA(also called the distribution coefficient),of the solute is defined as the ratio of“the total concentration of the substance in the organic phase to its total concentration in the aqueous phase,usually measured at equilibrium”[1].

Ifa second solute B is present,the corresponding distribution ratio is indicated by DBand so forth.The solutes A and B can be separated if DAand DBare different.
The organic phase solvents used in solvent extraction applications have been predominantly Volatile Organic Compounds(VOCs).The World Health Organization(WHO)defines VOCs as organic compounds with boiling points between 50 °C and 260 °C[14],including aliphatic and aromatic hydrocarbons,halogenated hydrocarbons,some esters,ethers,alcohols,aldehydes and ketones.Many of these VOCs are highly flammable(e.g.hydrocarbons),toxic(e.g.exposure to hexane causes centralnervous system effects and neurotoxic effects),and detrimental to the environment(e.g.halogenated hydrocarbons deplete ozone).Due to these health and environmental problems,the legislation upon utilization of VOCs is becoming increasingly stricter,for example REACH[15]—the Registration,Evaluation and Authorisation of Chemicals—a regulation of the European Union,is implementing stricter rules for the use of a large number of solvents resulting in their applications becoming very expensive or even prohibited as well as increasing the cost for storage and disposal of hazardous solvents..The unsteady supply and rising price of VOCs pose further problems to their utilization.The price of crude oil,which is the main raw material for VOCs,has experienced an overall increase with some fluctuations in the past two decades[16–18].Considering these environmental regulations,health concerns as well as the increasing price and unsteady supply of crude oils,the search for green solvents is imperative for sustainable development of chemical processes[19].
Numerous alternative solvents to VOCs have been developed and they are categorized into seven classes by Kerton et al.[20]including water,supercritical fluids,renewable solvents,ionic liquids and eutectic mixtures,fluorous solvents,liquid polymers and switchable solvent systems.So far there is no systematic method to determine how “green”a solventis and the greenness of a solventalso depends on how it is used.“Recognising whether a solvent is actually green”is regarded as one of the four grand challenges in the field of green solventresearch by Jessop[21].For example,ionic liquids(IL)are widely regarded as green solvents as they are stable and non-volatile.However,the IL[Bmim][BF4]was found to have larger life cycle environmental impact than several conventional solvents for their use in manufacture of cyclohexane and in a Diels-Alder reaction by life cycle assessment(LCA)[22].Nevertheless,LCA is only one method for assessing the greenness of a solvent,and other factors such as recycling,health hazards and boiling point should also be taken into consideration.The global pharmaceutical company,Glaxo Smith Kline,compiled a “Solvent Selection Guide”[23]which scores solvents using impacts such as environmental waste,environmental impact,health,process safety and LCA[24].Additionally,the“Twelve Principles of Green Chemistry”proposed by Anastas and Warner,lists the principles required to make a greener chemical,process or product[25].
Bio-derived solvents are a group of solvents that are produced in a biore finery from a range of renewable sources such as plants and algae.These solvents are of low toxicity,environmentally benign and biodegradable,and are therefore classified as green solvents.“Use of renewable feed stocks”is among the “Twelve Principles of Green Chemistry”,and Chemat et al.[26]also list the “use of bio-solvents”as one of their six principles of green extraction.It is believed thatre placement of conventional VOCs by bio-derived solvents may be faster than other alternative solvents(such as ionic liquids and CO2switchable solvents)as the technologies required for their use are similar to the present VOCs enabling easier implementation and lower costs.This paper aims to summarize the applications of bio-derived solvents in solvent extraction processes with particular emphasis given to utilization of biodiesels and terpenes.
2.Application of Bio-derived Solvents in Solvent Extraction
2.1.Overview of bio-derived solvents
Bio-derived solvents are produced from biomass in a biore finery which is defined as“a facility that integrates biomass conversion processes and equipment to produce fuels,power and chemicals from biomass”[27].Biomass includes a wide range of sources including energy crops(e.g.corn),forest products(e.g.wood),aquatic biomass(e.g.microalgae)and waste materials(e.g.urban wastes).After being used,these bio-derived solvents can be biodegraded(Fig.1).Chemists are striving to build a bio-platform for biore finery to replace the convention alpetroleumre finery to overcome those environmental and societal challenges.Twelve bio-sourced platform chemicals that can be produced either biologically or chemically from natural carbohydrates feed stocks have been identified by the U.S.Department of Energy as building bricks for a biore finery[27].Searching for new bio-derived solvents attracted intensive study in recent years and numerous new solvents have been discovered.However,not all of them can be used in solvent extraction as there are some specific requirements for solvents to be used for this application.
2.1.1.Potentialbio-derived solvents for solvent extraction
To replace VOCs in a solvent extraction process,ideally the new solventneeds to have low toxicity,good solute selectivity,high capacity for desired solute,non-flammability,inertness to equipment materials,sufficiently high interfacialtension,minimal viscosity and reasonable cost.Moreover,the solubility of the solvent in water should be sufficiently low so as to minimize solvent losses during extraction.Admittedly there are some other solvents that are insoluble in many organic solvents can be used in solvent extraction besides water,such as sulfolane and propylene carbonate,whereas they are much less popular than water.Despite some studies investigating the physicochemical properties of newly derived solvents[28–33],information is far from comprehensive.From this point of view,solubility of a solvent in water can be used as a prerequisite to evaluate suitability of solvents for applications in solvent extraction.A collection of bio-derived solvents and their solubilities in water are listed in Table 1,which shows that biodiesels,terpenes and 2,5-dimethylfuran(DMF)are insoluble in water and thus have the potential to be used in solvent extraction processes.
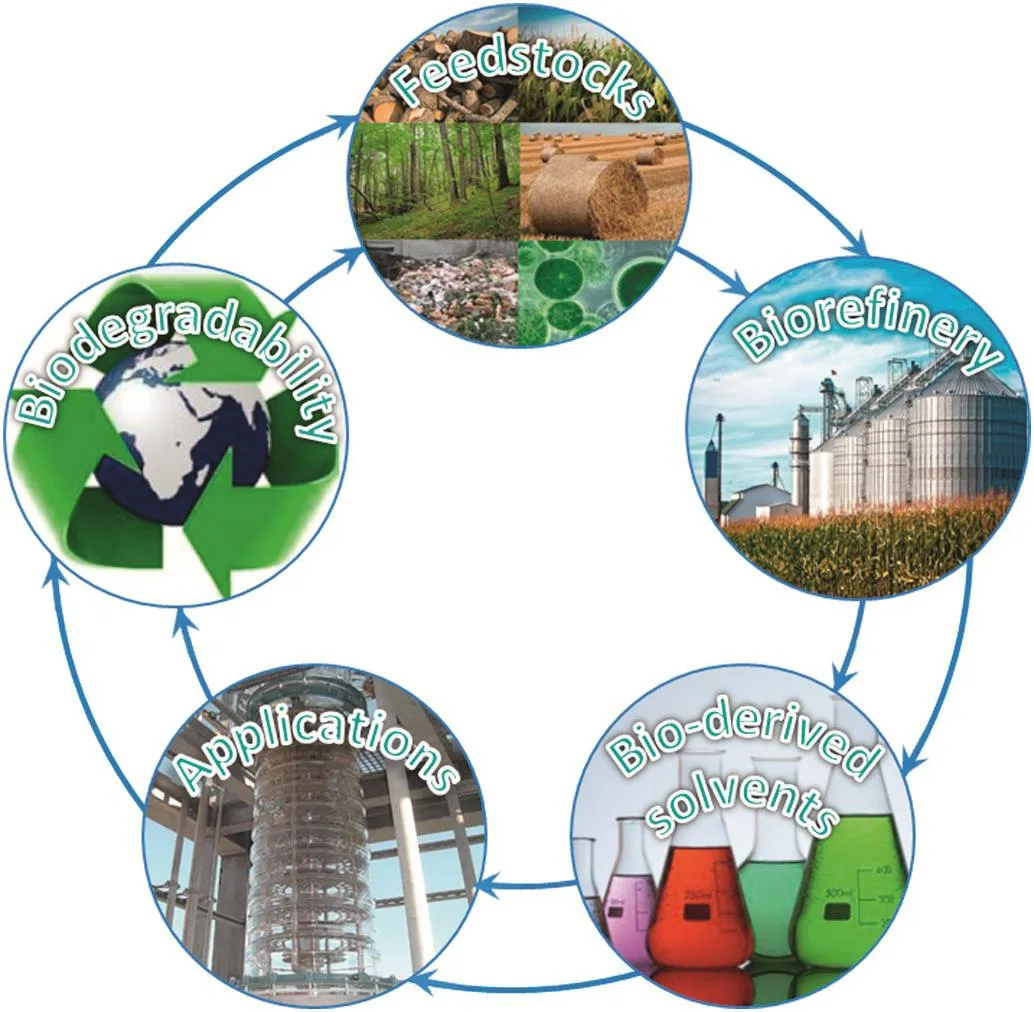
Fig.1.Life cycle ofbio-derived solvents.
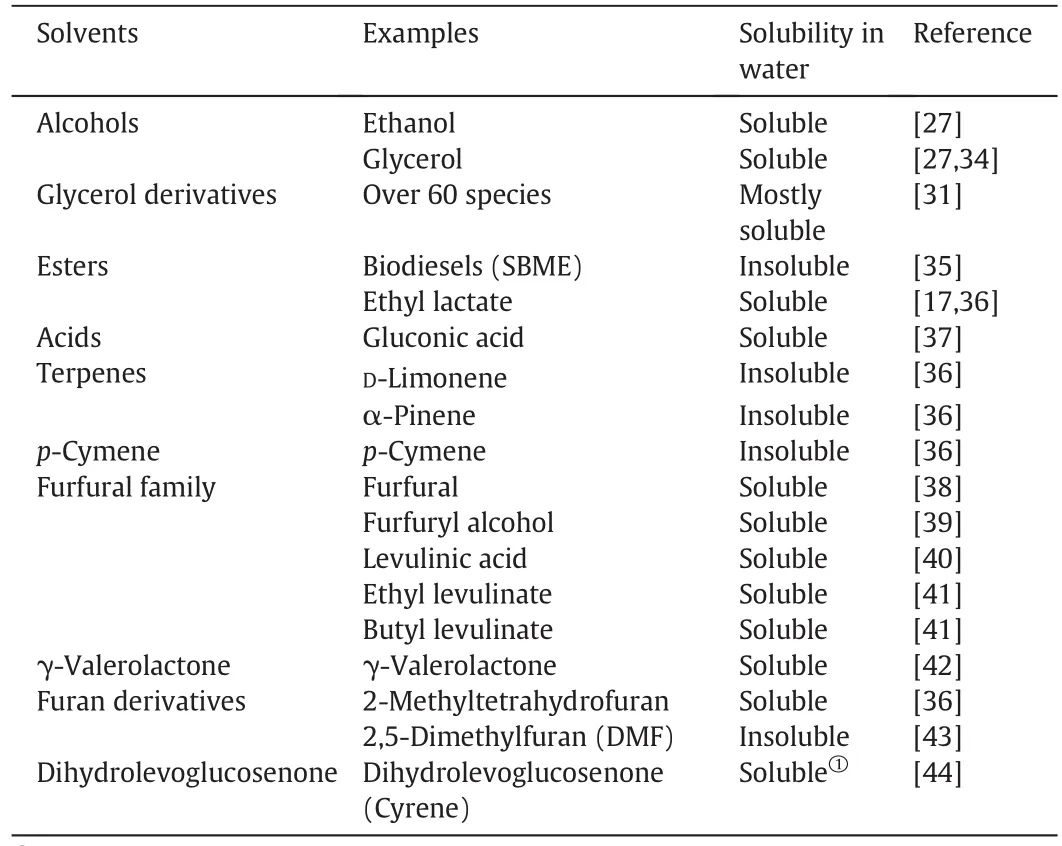
Table 1 Solubilities ofbio-derived solvents in water
The physical properties of the potential bio-derived solvents and hexane are given in Table 2.Viscosities of these bio-derived solvents are quite low,making them easy to handle in solventextraction applications.The density of solvents used in solvent extraction is recommended to be about0.8 g·ml-1to aid phase separations[45].The densities ofthese bio-derived solvent are lower than 0.9 g·ml-1,although higher than 0.8 g·ml-1.Except for DMF,they allhave very low vapor pressure at room temperature,preventing solvent loss by evaporation.In terms of the risk of fire,soybean oilmethylester(SBME)is the safest solvent due to its high flash point.Overallt hese bio-derived solvents are good candidate solvents for solvent extraction.Indeed biodiesels and terpenes have been investigated for suitability in solvent extraction as discussed in the following sections.
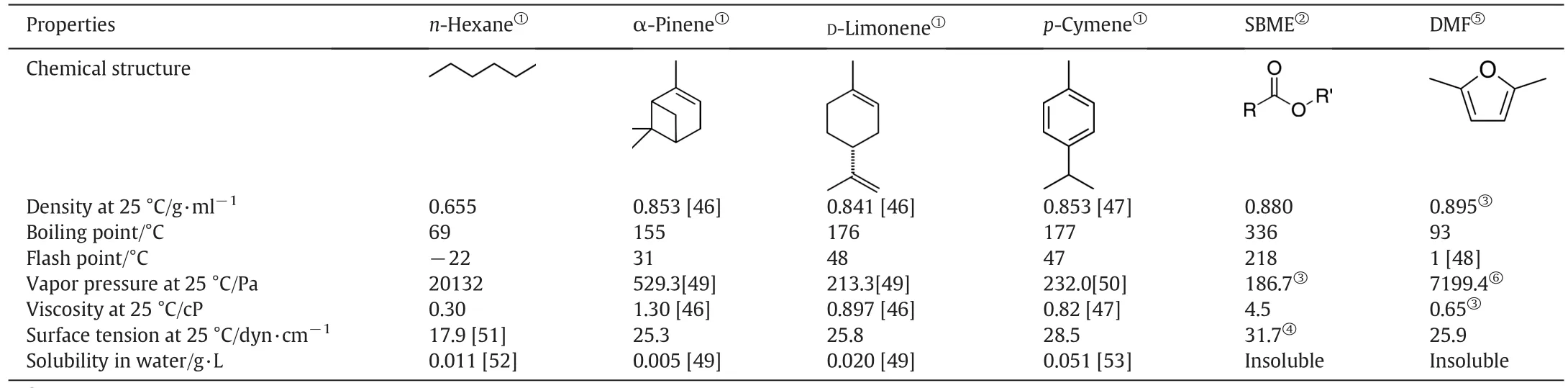
Table 2 Properties ofn-hexane and potentialbio-derived solvents for solvent extraction
2.1.2.Hansen solubility parameters
The Hansen three dimensional solubility parameters of these selected potentialbio-derived solvents for applications of solvent extraction and some widely used VOCs are given in Table 3.The principle of the Hansen three dimensional solubility parameters can be expressed in the following equation[58]:

where δTis the total Hansen solubility parameter and δd,δpand δhare the solubility parameters of the compound due to dispersion,polar and hydrogen bonding,respectively.The theory is based on the idea that “like dissolves like”.The closer the solubility parameters of the solute is to that of the solvent,the greater the solubility of the solute in the solvent.The relative energy difference(RED)can be used to determine whether a solute and a solvent are miscible.REDnumbers smaller than 1 indicate the solute and the solvent are miscible,REDnumbersequalto or close to 1 is a boundary condition and higher REDnumbers mean low affinity of the two components[58].
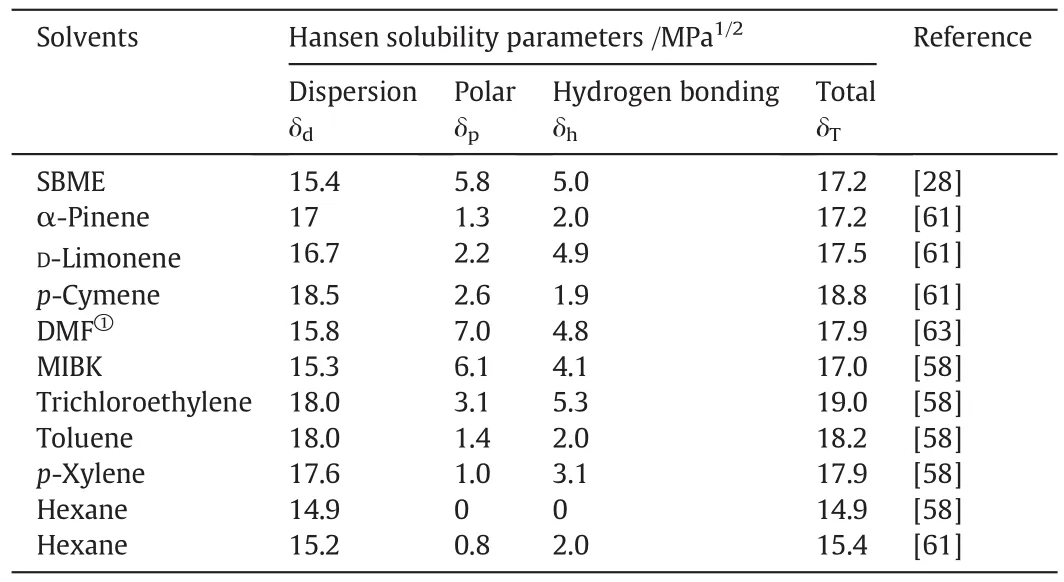
Table 3 Hansen parameters for potentialbio-derived solvents and some VOCs

where R0is the radius of the Hansen sphere,which can be determined by optimizing the REDvalues of the solvent with a group of solutes whose Hansen solubility parameters are known to let the Hansen sphere contain the miscible solutes and excludes the immiscible solutes[58,59].Rais the distance between the solvent(subscript“1”)and the solute(subscript“2”)in Hansen solubility parameters and can be defined as[58]:
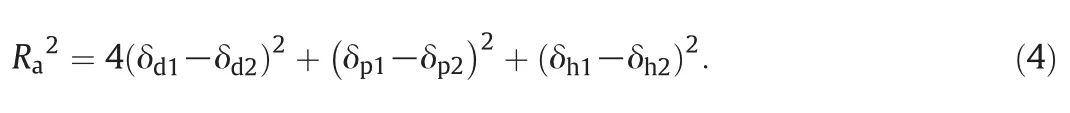
Either Raorthe REDnumbers can be used to rank solutes with respect to a specific solvent.Srinivas etal.[28]ranked a range of solutes according to the REDnumbers with respect to SBME and found that Methyl IsobutylKetone(MIBK)has the highest affinity,followed by trichloroethylene and toluene.Therefore these three VOCs,particularly MIBK,have the potential to be replaced by SBME.The Hansen solubility parameters of DMF are also close to thatof MIBK,whereas it is less preferable compared with SBME due to its high vaporpressure(7199.4 Pa at 25 °C).α-Pinene,D-limonene and p-cymene have been explored for the possibility ofreplacing hexane in the extraction ofnatural products such as edible oil[10]and lipids[60–62].According to the Hansen solubility parameters obtained by Chemat et al.[61],α-pinene and hexane are alike,however,they are quite different if the parameters given by Hansen[58]are used.In factα-Pinene and toluene are very alike,as indicated by the Hansen solubility parameters.Xylene has the potentialto be replaced by any ofα-pinene,D-limonene and p-cymene.
2.2.Biodiesels
2.2.1.Production and properties ofbiodiesels
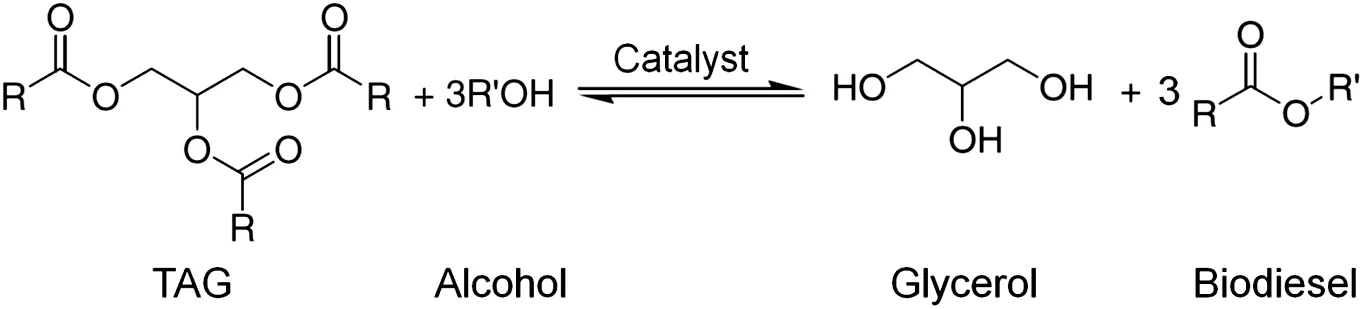
Fig.2.Production of biodieselby transesteri fication reaction:three Rs in triacylglycerol(TAG)can be different;alcoholis normally methanol.
Biodieselis defined by the American Society for Testing and Materials(ASTM)as“a fuel composed of monoalkyl esters of long-chain fatty acids derived from renewable vegetable oils or animal fats,designated as B100”[64,65].Vegetable oils and animal fats are principally composed of triacylglycerols(TAG),which could react with alcohols in the presence of catalysts through a chemical process known as transesteri fication to produce fatty acid alkylesters(FAAE)and glycerol as byproduct(Fig.2).Since methanolis the mostcommon alcoholused to produce biodiesel,another name for biodieselis fatty acid methyl ester(FAME).A wide variety of feed stocks that contain triacylglycerol can be used to produce biodiesel by transesterification.There are mainly four categories of feed stocks:algae,oilseeds,animal fats and various low-value materials such as used cooking oils[66].Biodiesels produced from different feed stocks have clear differences in carbon chain length and the degree of unsaturation,which are the two in fluential factors determining physical and chemical properties of biodiesels[65].As a fuel,there is a wealth ofliterature studying the production and properties of biodiesels including two new reviews[65,66].On the contrary,there is very little information on the use of biodiesels as a solvent in solvent extraction applications.
2.2.2.Applications ofbiodiesels in solventextraction
Soybean Oil Methyl Esters(SBMEs)are produced by the transesterification of soybean oil and methanol.SBME has high flash point(218 °C),low vapor pressure(<266 Pa)[67],high solvent power[32],low viscosity[65]and is insoluble in water[35].These properties make it attractive for solvent extraction applications.Spear etal.[67]carried out the pioneering work ofusing SBME as alternative to organic solvents.The SBME used was SoyGold®1000,which is a commercially available product.They examined the partitioning of neutral,ionizable,and metalion solutes in biphasic systems formed with SMBE and water.For the neutral solutes,partition coefficients(DSMBE)of 20 organic solutes between SBME and water were determined and compared to lg P(1-octanol–water)and significantrelationship between lg DSMBEand lg P was found.Solute distribution behavior is similar to that found for the conventionalorganic solvent–water systems,but is most similar to the olive oil–water system.Determination of free energy of transferring a methylene group for various solvent–water systems revealed that SMBE is likely an alternative to xylene.When ionizable solutes are partitioned in the SBME–water system at differing pH,the neutral species show the highest distribution and the ionized species show obvious preference for the aqueous phase.These distribution behaviors are similar to those of similar solutes in traditional extraction in the presence of conventional organic solvents,such as extraction of phenol by toluene at various pHs[7].Metal ions(e.g.,Fe3+,Co2+,and Ni2+)and actinides(UO22+,Am3+)exhibit little partitioning to the SBME phase in the absence of an extractant,indicating limited extraction power for metals,which is the same as for conventional solvents.Extractants 1-(2-pyridylazo)-2-naphthol(PAN)and(1-thiazolylazo)-2-naphthol(TAN)in SBME show selectivity of Co2+over Fe3+and Ni2+.When octyl(phenyl)-N,N-diisobutylcarbamoylmethyl phosphine oxide(CMPO)is present,partitioning of UO22+to the SBME from the aqueous solution increases significantly while the partitioning of Am3+largely depends on the concentration ofnitrate,allowing the separation ofUO22+and Am3+by this extraction system.
Following Spear's exploration,Liu et al.[35]studied extraction of rare earths by Trihexyl(tetradecyl)phosphonium bis 2,4,4-trimethylpentylphosphinate(Cyphos IL 104),which is a bifunctional ionic liquid extractant(bif-ILE),diluted in propylene carbonate,dimethylcarbonate and SBME.Acidi fied Cyphos IL 104(HNO3-Cyphos IL 104)exhibited high solubility in the three diluents and higher extraction efficiency than bis(2,4,4-trimethylpentyl)phosphinic acid(Cyanex 272)because both the quaternary phosphonium cation and the phosphonic acid anion in the organic phase extract RE(III)and that's why Cyphos IL 104 is called bif-ILE.The extraction mechanism was validated by mass spectrum analysis and this co-extraction mechanism could eliminate the loss of IL since both parts of the ionic liquid extractant are involved in the extraction.In comparison with propylene carbonate and dimethylcarbonate,SBME was concluded as the preferred diluent due to its preferable properties as mentioned above and excellent results in RE(III)extraction.In a more recent study,SBME was used to dilute sec-octylphenoxyacetic acid(CA-12)for extraction of rare earth ions(La(III),Pr(III),Nd(III),Eu(III),Gd(III),Er(III),Yb(III),Lu(III)and Y(III))[68].It is showed that Y(III)was the most difficult element to be extracted in the CA-12-SBME system,indicating that CA-12-SBME system could be applied to separate Y(III).Afractionalextraction(4 stages for extraction and 2 stages for scrubbing)was examined in a realsample and results were consistent with the data calculated by Xu model[69],con firming that the extraction system is suitable for Y(III)separation from other rare earth elements.
SBME was also used as co-solvent with ethanol for microalgae lipid extraction in microwave-assisted extraction(MAE)[70].The extraction system was found to yield comparable results to those using chloroform plus ethanolas solvents or the conventional8 hour Soxhlet extraction,which uses hexane as the solvent.Employing such a solvent system avoids separation of the co-solvent because all the solvents and lipid are involved in the transesteri fication reaction.Moreover,SBME absorbs microwave more efficiently than hexane and thus is a more energy efficient solvent for MAE.This study claims that the toxic hexane can be substituted by the greener solvent biodiesel(SBME).These studies consistently highlight the promising potential of SBME for solvent extraction applications.However,SBME is unstable to oxidation because it contains unsaturated functional groups[66].This oxidation degradation alters the physical and chemical properties of SMBE which in turn may affectits suitability as a solvent.
2.3.Terpenes:D-limonene,α-pinene
2.3.1.The sources ofterpenes
Terpenes are a class ofunsaturated hydrocarbons composed ofisoprene C5 units and exist naturally in essential oils and oleoresins of plants such as conifers.The two terpenes that are most commonly used as solvents are α-pinene and D-limonene.α-Pinene is generally produced by fractional distillation of steam-distilled turpentine,which can be obtained from most conifers and from leaves,bark,and wood of various plants like rosemary,parsley,basil,yarrow,and roses.Turpentine is also a by-product of the wood and paper industry,making it an abundant and cheap raw material[36].D-Limonene is a major constituent(~90%)of essentialoil of citrus peels,which can be produced by steam distillation[11,71],high pressure-high temperature extraction[72],microwave assisted extraction[73],microwave accelerated distillation[74]or dilute acid hydrolysis of citrus peels[75].Oxidation of D-Limonene produces p-cymene[73],which is also a naturally existing compound in essential oils of tree leaves[36].Some physicaland chemical properties ofα-pinene,D-limonene and p-cymene(Table 3)are similar to that of hexane,therefore studies have been carried out to explore the possibility of replacing hexane with these renewable solvents.
2.3.2.Applications ofterpenes in solvent extraction
The first attempt to use limonene as a solvent to extract natural products was the extraction of edible oil from rice bran[10],which was carried outin comparison with extraction using the traditionalsolvent hexane and at their respective boiling points.A slightly higher amount of oilwas extracted by D-limonene and this was attributed to its slightly higher polarity than hexane and the higher operating temperature.Quality characteristics(free fatty acid content,oilcolor,phospholipid content)of crude oilextracted by these two solvents were analyzed using various analyticalmethods and were found to be comparable.Oxidation of limonene during extraction was found to be so small(<1%)that it does not affect the solvent characteristics[76].Extraction of lipids from olives using limonene provided similar results to n-hexane[60],as did the extraction of lipids from marine microalgae by pressurized limonene[77].However,in the extraction ofcarotenoid from tomatoes,extraction yields by limonene is lower than that of extraction by ethanol[78]although stillhigher than that of extraction by hexane and comparable with that of extraction by dichloromethane[11].The higher extraction yield of ethanolis attributed to its higher polarity.
The higher polarity of limonene compared to hexane enables it to extracthigher amountof lipids,nevertheless,its higher boiling pointrequires more energy for distillation separation following extraction.To tackle this problem,Virot et al.[79,80]combined microwave heating and Clevenger distillation thatremoves limonene ata much lower temperature(97.4°C)by azeotropic distillation oflimonene and water.This method could also be applied toα-Pinene and p-cymene.The azeotropic distillation was further adapted to a new procedure called Simultaneous Distillation and Extraction Process(SDEP),which extracts algallipids from wet microalgae using D-limonene,α-Pinene and p-cymene[62].Crud lipids obtained by the three solvents using SDEP were higher than conventional Soxhlet extraction(hexane as solvent)but lower than Bligh&Dyermethod(methanol/chloroform assolvent),the results can be explained by the difference of solvents'polarities.
In addition to these experimental explorations, a theoretical evaluation of nine solvents,includingα-Pinene,was done using Hansen solubility parameters for the dissolving mechanism of aroma compounds in blackcurrant buds.Theoreticallyα-Pinene is a good alternative to replace hexane ifthe Hansen parameters for hexane obtained by Chemat et al.[61]are used,but experimentally the pro file of lipid extracted by α-pinene is different from that of hexane[61].
2.4.Discussion
Biodiesel(SBME)has been examined for suitability as a solvent in extraction of neutral,ionizable and metalsolutes from water and its overall performance was similar to traditional VOCs,making it a promising alternative solvent in solvent extraction processes.D-Limonene,α-pinene and p-cymene have been applied in extraction of natural products,including lipids and carotenoids,from a variety of feed stocks and were comparable(in terms oflipid pro files)orprovided even better results(in terms of yield)compared with traditionalsolvents,despite their high boiling points that require higher energy consumption for distillation following extraction,which could be partly overcome by azeotropic distillation.Extraction of lipids from plants or algae is not rigorous liquid–liquid extraction as only one liquid phase is involved in the process,except for the case which involves aqueous phase and limonene phase in the extraction ofcarotenoid[11].Nevertheless,all of these processes share the same characteristics that they are separation processes making use of solubilities.More experimental work covering extraction of different types of species using these three solvents are warranted for a comprehensive examination of their performance in solvent extraction.
Another solvent that has been identified as a potential alternative green solvent in Table 1,namely DMF,has not received any investigation for solvent extraction,to the best of the authors'knowledge.An examination of DMF would be worthwhile.Thus far,these studies are limited to laboratory scale thermodynamics of extraction systems involving these alternative solvents,kinetic studies and pilot plant trials are rarely found,although both of which are important aspects of solvent extraction.Moreover,searching for more potentialbio-derived solvents with diversity in physicochemical properties that are required by selectivity of solventextraction needsto continue while also examining existing solvents.Currently the prices of these bio-derived solvents are comparatively high[66,71]and the supply of feedstock for their production in large scale is insufficient[66],in addition to the technical problems of these bio-derived solvents as discussed above.Overcoming these problems willpromote industrialization ofbio-derived solvents in solvent extraction.
3.Summary and Outlook
Bio-derived solvents,produced from biomass through a biore finery,are a group ofenvironmentally benign,renewable and bio-degradable solvents that can contribute to an environmentally sustainable solvent extraction industry.Biodiesel(SBME)has presented comparable performance to traditional solvents for extraction of neutral,ionisable and metal species and thus is a good alternative solvent.Terpenes(α-pinene,D-limonene and its derivative p-cymene)are promising alternatives as shown by their capability in extraction of natural products and their physicochemical properties.DMF has not been investigated but also shows potentialfor use as a green solvent.Further work is required to examine this new category of solvent,including investigating extraction performance,kinetics studies and pilot plant trials as well as searching for new bio-derived solvents.
Acknowledgements
Support from the Australian Research Council(project ID:LP140100650)and the Particulate Fluids Processing Centre is acknowledged.Zheng Liis gratefulfor financialsupport from the China Scholarship Council.
[1]J.Rydberg,M.Cox,C.Musikas,G.R.Choppin,Solvent Extraction Principles and Practice,2nd ed.Marcel Dekker,Inc.,New York,2004.
[2]J.D.Kinrade,J.C.Van Loon,Solvent extraction for use with flame atomic absorption spectrometry,Anal.Chem.46(13)(1974)1894–1898.
[3]G.W.Stevens,J.M.Perera,F.Grieser,Inter facialas pects of metal ion extraction in liquid–liquid systems,Rev.Chem.Eng.17(2)(2001)87–110.
[4]J.M.Perera,G.W.Stevens,The role of additives in metal extraction in oil/water systems,Solvent Extr.Ion Exch.29(3)(2011)363–383.
[5]D.Ciceri,L.R.Mason,D.J.E.Harvie,J.M.Perera,G.W.Stevens,Extraction kinetics of Fe(III)by di-(2-ethylhexyl)phosphoric acid using a Y–Y shaped micro fluidic device,Chem.Eng.Res.Des.92(3)(2014)571–580.
[6]Z.Zhou,W.Qin,S.Liang,Y.Tan,W.Fei,Recovery of lithium using tributyl phosphatein methyl isobutyl ketone and FeCl3,Ind.Eng.Chem.Res.51(39)(2012)12926–12932.
[7]Z.Li,K.A.Mumford,K.H.Smith,Y.Wang,G.W.Stevens,Extraction ofphenolby toluene in the presence ofsodium hydroxide,Sep.Sci.Technol.49(18)(2014)2913–2920.
[8]V.S.Kislik,Solvent Extraction Classicaland NovelApproaches,Elsevier,Oxford,2012.
[9]Industrial Scale Natural Products Extraction,Wiley-VCH Verlag GmbH&Co.KGaA,Weinheim,2011.
[10]P.K.Mamidipally,S.X.Liu,First approach on rice bran oilextraction using limonene,Eur.J.Lipid Sci.Technol.106(2)(2004)122–125.
[11]Z.Chemat-Djenni,M.A.Ferhat,V.Tomao,F.Chemat,Carotenoid extraction from tomato using a green solvent resulting from orange processing waste,J.Essent.Oil Bear.Plants 13(2)(2010)139–147.
[12]S.F.Shen,K.H.Smith,S.Cook,S.E.Kentish,J.M.Perera,T.Bowser,G.W.Stevens,Phenol recovery with tributyl phosphate in a hollow fiber membrane contactor:experimentaland modelanalysis,Sep.Purif.Technol.69(1)(2009)48–56.
[13]F.Wang,F.He,J.Zhao,N.Sui,L.Xu,H.Liu,Extraction and separation of cobalt(II),copper(II)and manganese(II)by Cyanex272,PC-88A and their mixtures,Sep.Purif.Technol.93(2012)8–14.
[14]World Health Organization,Indoor Air Quality:Organic Pollutants.Reports on a WHO Meeting,Euro Reports and Studies 111.;Copenhagen,1989.
[15]The European Chemicals Agency,http://echa.europa.eu/regulations/reach(Accessed on 31 October 2014).
[16]J.L.Williams,Oil Price History and Analysis,http://www.wtrg.com/prices.htm(Accessed on 14 November 2014).
[17]C.S.M.Pereira,V.M.T.M.Silva,A.E.Rodrigues,Ethyl lactate as a solvent:properties,applications and production processes—a review,Green Chem.13(10)(2011)2658–2671.
[18]Historical Crude Oil Price,http://www.infomine.com/investment/metal-prices/crude-oil/all/(Accessed on 31 October 2014).
[19]J.H.Clark,Green chemistry:today(and tomorrow),Green Chem.8(1)(2006)17–21.
[20]F.M.Kerton,R.Marriott,Alternative Solvents for Green Chemistry,2nd ed.Royal Society of Chemistry,Cambridge,2013.
[21]P.G.Jessop,Searching for green solvents,Green Chem.13(6)(2011)1391–1398.
[22]Y.Zhang,B.R.Bakshi,E.S.Demessie,Life cycle assessment of an ionic liquid versus molecular solvents and their applications,Environ.Sci.Technol.42(5)(2008)1724–1730.
[23]R.K.Henderson,C.Jimenez-Gonzalez,D.J.C.Constable,S.R.Alston,G.G.A.Inglis,G.Fisher,J.Sherwood,S.P.Binks,A.D.Curzons,Expanding GSK's solvent selection guide—Embedding sustainability into solventselection starting atmedicinalchemistry,Green Chem.13(4)(2011)854–862.
[24]P.J.Dunn,A.Wells,M.T.Williams,Green Chemistry in the Pharmaceutical Industry,Wiley,2010.
[25]P.T.Anastas,J.C.Warner,Green chemistry:Theory and practice,Oxford University Press,New York,1998.
[26]F.Chemat,M.A.Vian,G.Cravotto,Green extraction ofnatural products:conceptand principles,Int.J.Mol.Sci.13(7)(2012)8615–8627.
[27]F.M.Kerton,R.Marriott,Renewable solvents,Alternative Solvents for Green Chemistry,2nd ed.Royal Society of Chemistry,Cambridge 2013,pp.97–117.
[28]K.Srinivas,T.M.Potts,J.W.King,Characterization of solvent properties of methyl soyate by inverse gas chromatography and solubility parameters,Green Chem.11(10)(2009)1581–1588.
[29]L.Lomba,B.Giner,I.Bandres,C.Lafuente,M.a.R.Pino,Physicochemical properties of green solvents derived from biomass,Green Chem.13(8)(2011)2062–2070.
[30]S.Aparicio,R.Alcalde,The green solventethyllactate:An experimental and the oreticalcharacterization,Green Chem.11(1)(2009)65–78.
[31]J.I.Garcia,H.Garcia-Marin,J.A.Mayoral,P.Perez,Green solvents from glycerol.Synthesis and physico-chemical properties of alkylglycerolethers,Green Chem.12(3)(2010)426–434.
[32]G.Knothe,K.R.Steidley,Fatty acid alkylesters as solvents:evaluation of the kauributanol value.comparison to hydrocarbons,dimethyldiesters,and other oxygenates,Ind.Eng.Chem.Res.50(7)(2011)4177–4182.
[33]J.Hu,Z.Du,Z.Tang,E.Min,Study on the solvent power of a new green solvent:Biodiesel,Ind.Eng.Chem.Res.43(24)(2004)7928–7931.
[34]Y.Gu,F.Jerome,Glycerolas a sustainable solvent for green chemistry,Green Chem.12(7)(2010)1127–1138.
[35]Y.Liu,L.Zhu,X.Sun,J.Chen,Toward greener separations ofrare earths:Bifunctional ionic liquid extractants in biodiesel,AICHE J.56(9)(2010)2338–2346.
[36]F.Chemat,Terpenes as green solvents for natural products extraction,Alternative Solvents for Natural Products Extraction,Springer 2014,pp.205–219.
[37]S.Ramachandran,P.Fontanille,A.Pandey,C.Larroche,Gluconic acid:Properties,applications and microbial production,Food Technol.Biotechnol.44(2)(2006)185–195.
[38]A.N.Patel,Ternary phase equilibrium studies of furfural-water–solvent systems,J.Chem.Technol.Biotechnol.Chem.Technol.34(4)(1984)161–164.
[39]Wikipedia FurfurylAlcohol,http://en.wikipedia.org/wiki/Furfuryl_alcohol(Accessed on 17 November 2014(17 November)).
[40]A.Apelblat,E.Manzurola,Solubility of suberic,azelaic,levulinic,glycolic,and diglycolic acids in water from 278.25 K to 361.35 K,J.Chem.Thermodyn.22(3)(1990)289–292.
[41]E.Christensen,A.Williams,S.Paul,S.Burton,R.L.McCormick,Properties and performance of levulinate esters as dieselblend components,Energy Fuel25(11)(2011)5422–5428.
[42]I.T.Horvath,H.Mehdi,V.Fabos,L.Boda,L.T.Mika,[Gamma]-valerolactone-a sustainable liquid for energy and carbon-based chemicals,Green Chem.10(2)(2008)238–242.
[43]Y.Román-Leshkov,C.J.Barrett,Z.Y.Liu,J.A.Dumesic,Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates,Nature 447(7147)(2007)982–985.
[44]J.Sherwood,M.De bruyn,A.Constantinou,L.Moity,C.R.McElroy,T.J.Farmer,T.Duncan,W.Raverty,A.J.Hunt,J.H.Clark,Dihydrolevoglucosenone(Cyrene)as a bio-based alternative for dipolar aprotic solvents,Chem.Commun.50(68)(2014)9650–9652.
[45]D.S.Flett,J.Melling,M.Cox,Commercialsolvent systems for inorganic processes,Handbook of Solvent Extraction,John Wiley&Sons,New York;Chichester;Brisbane;Toronto;Singapore 1983,pp.629–647.
[46]R.A.Clará,A.C.G.Marigliano,H.N.Sólimo,Density,viscosity,and refractive index in the range(283.15 to 353.15)K and vapor pressure ofα-pinene,D-limonene,(±)-linalool,and citral over the pressure range 1.0 kPa atmospheric pressure,J.Chem.Eng.Data 54(3)(2009)1087–1090.
[47]R.Francesconi,C.Castellari,F.Comelli,Densities,viscosities,refractive indices,and excess molar enthalpies of methyl tert-butyl ether+components of pine resins and essentialoils at 298.15 K,J.Chem.Eng.Data 46(6)(2001)1520–1525.
[48]R.Daniel,H.Xu,C.Wang,D.Richardson,S.Shuai,Combustion performance of2,5-dimethylfuran blends using dual-injection compared to direct-injection in a SI engine,Appl.Energy 98(2012)59–68.
[49]I.Fichan,C.Larroche,J.B.Gros,Water solubility,vapor pressure,and activity coefficients of terpenes and terpenoids,J.Chem.Eng.Data 44(1)(1998)56–62.
[50]K.A.Kobe,T.S.Okabe,M.T.Ramstad,P.M.Huemmer,p-Cymene studies.VI.Vapor pressure of p-cymene,some of its derivatives and related compounds,J.Am.Chem.Soc.63(12)(1941)3251–3252.
[51]R.L.Schmidt,J.C.Randall,H.L.Clever,The surface tension and density ofbinary hydrocarbon mixtures:benzene-n-hexane and benzene-n-dodecane,J.Phys.Chem.70(12)(1966)3912–3916.
[52]M.Goral,B.Wisniewska-Goclowska,A.Skrzecz,I.Owczarek,K.Blazej,M.C.Haulait-Pirson,G.T.Hefter,F.Kapuku,Z.Maczynska,C.L.Young,IUPAC-NIST solubility data series.81.Hydrocarbons with water and seawater—revised and updated.Part 4.C6H14 hydrocarbons with water,J.Phys.Chem.Ref.Data 34(2)(2005)709–713.
[53]R.Lun,D.Varhanickova,W.-Y.Shiu,D.Mackay,Aqueous solubilities and octanol–water partition coefficients of cymenes and chlorocymenes,J.Chem.Eng.Data 42(5)(1997)951–953.
[54]C.Dejoye Tanzi,M.Abert Vian,C.Ginies,M.Elmaataoui,F.Chemat,Terpenes as green solvents for extraction of oil from microalgae,Molecules 17(7)(2012)8196–8205.
[55]Ag Processing,Inc.http://www.agp.com/(The of ficialwebsite of Ag Processing Inc(AGP®),Accessed on 12 January 2015).
[56]S.V.D.Freitas,M.B.Oliveira,A.J.Queimada,M.J.Pratas,Á.S.Lima,J.A.P.Coutinho,Measurement and prediction of biodiesel surface tensions,Energy Fuel 25(10)(2011)4811–4817.
[57]G.Tian,R.Daniel,H.Xu,DMF—a new biofuelcandidate,in:M.A.D.S.Bernardes(Ed.),Biofuel Production-Recent Developments and Prospects,InTech 2011,pp.487–520.
[58]C.M.Hansen,Hansen Solubility Parameters:A User's Handbook,2nd ed.CRC Press,Boca Raton,2007.
[59]D.L.Williams,K.D.Kuklenz,A determination of the Hansen solubility parameters of hexanitrostilbene(HNS),Propellants,Explos.,Pyrotech.34(5)(2009)452–457.
[60]M.Virot,V.Tomao,C.Ginies,F.Chemat,Total lipid extraction of food using D-limonene as an alternative to n-hexane,Chromatographia 68(3–4)(2008)311–313.
[61]A.Filly,A.S.Fabiano-Tixier,Y.Lemasson,C.Roy,X.Fernandez,F.Chemat,Extraction of aroma compounds in blackcurrant buds by alternative solvents:theoretical and experimental solubility study,C.R.Chim.17(12)(2014)1268–1275.
[62]C.Dejoye Tanzi,M.Abert Vian,F.Chemat,New procedure for extraction of algal lipids from wet biomass:a green clean and scalable process,Bioresour.Technol.134(2013)271–275.
[63]A.F.M.Barton,CRC Handbook of Solubility Parameters and Other Cohesion Parameters,CRC Press,1991.
[64]ASTM,Standard Specification for Biodiesel Fuel Blend Stock(B100)for Middle Distillate Fuels;D6751-08,2008.
[65]S.K.Hoekman,A.Broch,C.Robbins,E.Ceniceros,M.Natarajan,Review of biodiesel composition,properties,and specifications,Renew.Sust.Energ.Rev.16(1)(2012)143–169.
[66]B.Moser,Biodiesel production,properties,and feed stocks,in:D.Tomes,P.Lakshmanan,D.Songstad(Eds.),Biofuels,Springer,New York 2011,pp.285–347.
[67]S.K.Spear,S.T.Grif fin,K.S.Granger,J.G.Huddleston,R.D.Rogers,Renewable plantbased soybean oil methyl esters as alternatives to organic solvents,Green Chem.9(2007)1008–1015.
[68]W.Wang,H.Yang,Y.Liu,H.Cui,J.Chen,The application of biodiesel and secoctylphenoxyacetic acid(CA-12)for the yttrium separation,Hydrometallurgy 109(1–2)(2011)47–53.
[69]G.Xu,Theory of counter current extraction I.Equations of optimization and their applications,Acta Sci.Nat.Univ.Pekin.1(1978)51–66.
[70]J.Iqbal,C.Theegala,Microwave assisted lipid extraction from microalgae using biodieselas co-solvent,AlgalRes.2(1)(2013)34–42.
[71]R.Ciriminna,M.Lomeli-Rodriguez,P.Demma Cara,J.A.Lopez-Sanchez,M.Pagliaro,Limonene:a versatile chemicalof the bioeconomy,Chem.Commun.50(97)(2014)15288–15296.
[72]C.G.Lopresto,F.Petrillo,A.A.Casazza,B.Aliakbarian,P.Perego,V.Calabrò,A nonconventionalmethod to extract D-limonene from waste lemon peels and comparison with traditional Soxhlet extraction,Sep.Purif.Technol.137(2014)13–20.
[73]J.H.Clark,E.M.Fitzpatrick,D.J.Macquarrie,L.A.Pfaltzgraff,J.Sherwood,p-Cymenesulphonic acid:An organic acid synthesised from citrus waste,Catal.Today 190(1)(2012)144–149.
[74]M.A.Ferhat,B.Y.Meklati,J.Smadja,F.Chemat,An improved microwave Clevenger apparatus for distillation of essential oils from orange peel,J.Chromatogr.A 1112(1–2)(2006)121–126.
[75]M.Pourbafrani,G.Forgács,I.S.Horváth,C.Niklasson,M.J.Taherzadeh,Production of biofuels,limonene and pectin from citrus wastes,Bioresour.Technol.101(11)(2010)4246–4250.
[76]S.X.Liu,P.K.Mamidipally,Quality comparison of rice bran oil extracted with D-limonene and hexane,Cereal Chem.82(2)(2005)209–215.
[77]M.T.Golmakani,J.A.Mendiola,K.Rezaei,E.Ibáñez,Pressurized limonene as an alternative bio-solvent for the extraction of lipids from marine microorganisms,J.Supercrit.Fluids 92(2014)1–7.
[78]M.Castro-Puyana,M.Herrero,I.Urreta,J.Mendiola,A.Cifuentes,E.Ibáñez,S.Suárez-Alvarez,Optimization of clean extraction methods to isolate carotenoids from the microalga Neochloris oleoabundans and subsequent chemicalcharacterization using liquid chromatography tandem mass spectrometry,Anal.Bioanal.Chem.405(13)(2013)4607–4616.
[79]M.Virot,V.Tomao,C.Ginies,F.Visinoni,F.Chemat,Green procedure with a green solvent for fats and oils'determination:microwave-integrated Soxhlet using limonene followed by microwave Clevenger distillation,J.Chromatogr.A 1196–1197(0)(2008)147–152.
[80]S.Chemat,V.Tomao,F.Chemat,Limonene as green solvent for extraction ofnatural products,Green Solvents I,Springer 2012,pp.175–186.
 Chinese Journal of Chemical Engineering2016年2期
Chinese Journal of Chemical Engineering2016年2期
- Chinese Journal of Chemical Engineering的其它文章
- Relationship between breakthrough curve and adsorption isotherm of Ca(II)imprinted chitosan microspheres for metaladsorption☆
- Experimental study on the effects of big particles physical characteristics on the hydraulic transport inside a horizontal pipe
- Investigation of extraction fraction in con fined impinging jet reactors for tri-butyl-phosphate extracting butyric acid process☆
- Experimental evaluation and modeling of liquid jet penetration to estimate droplet size in a three-phase riser reactor
- Photorheologically reversible micelle composed ofpolymerizable cationic surfactant and 4-phenylazo benzoic acid☆
- Review on current advances,future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents☆
