血清S100B、S100A6、S100P水平升高与急性冠脉综合征的相关性研究
盛乐智 陈秋静 张 奇 陆 林 沈卫峰 张瑞岩
200025 上海交通大学医学院附属瑞金医院心脏科心血管病研究所
血清S100B、S100A6、S100P水平升高与急性冠脉综合征的相关性研究
盛乐智陈秋静张奇陆 林沈卫峰张瑞岩
200025 上海交通大学医学院附属瑞金医院心脏科心血管病研究所
【摘要】目的:探讨血清终末糖基化产物受体(RAGE)的配基S100B、S100A6、S100P水平与急性冠脉综合征(ACS)相关性。方法:连续收集882例冠状动脉造影患者,检测血清S100B、S100A6、S100P、游离RAGE(sRAGE)、肿瘤坏死因子(TNF)-α水平;根据临床表现及实验室资料,将患者分为对照组(n=251)、稳定型心绞痛(SA)组(n=211)及ACS组(n=420)。结果:ACS组血清S100B、S100A6、S100P和TNF-α水平[(103.73±56.90)ng/L、(5.28±4.15) μg/L、(8.73±7.96) μg/L、(87.82±39.30)ng/L]均显著高于SA组[(81.93±27.65) ng/L、(4.36±2.45) μg/L、(3.41±3.08) μg/L、(71.88±30.70) ng/L]和对照组[(78.00±22.71) ng/L、(3.97±2.57) μg/L、(3.38±2.74) μg/L、(57.07±27.23) ng/L,P<0.01];ACS组血清sRAGE水平高于对照组[(724.01±320.37) ng/L 对(652.55±351.24) ng/L,P<0.01]。进一步将ACS组分为ST段抬高型心肌梗死(STEMI)和不稳定型心绞痛/非ST段抬高型心肌梗死(UA/NSTEMI)2个亚组进行分析,STEMI亚组的S100B、S100A6、S100P水平高于UA/NSTEMI亚组;ACS组血清S100B水平与肌钙蛋白I(cTnI)峰值水平相关(P<0.05),血清S100P水平与肌酸激酶同工酶(CK-MB)及cTnI峰值水平均有相关性(P<0.01)。多元回归分析发现,S100B、S100A6、S100P和传统危险因素均与ACS发病相关。结论:血清S100B、S100A6、S100P水平与ACS相关,与心肌缺血的损伤程度相关,它们在ACS的病理生理过程中发挥重要作用。
【关键词】急性冠脉综合征;S100B;S100A6;S100P;终末糖基化产物受体
终末糖基化产物受体(RAGE)与心血管疾病及糖尿病并发症密切相关,RAGE及其配基在动脉粥样硬化形成及心肌缺血再灌注损伤中起关键作用[1-4]。S100蛋白超家族是RAGE的配体,由超过25个成员组成,在调节炎症反应、组织退行性变、细胞增殖分化以及维持内稳中发挥重要作用[5]。一些S100家族成员如S100B、S100A6、S100P在体外实验中被证实与RAGE结合并产生作用[6],而关于S100B、S100A6和S100P是否参与了动脉粥样硬化形成或心肌缺血后病变的相关临床研究较少。
本研究检测分析急性冠脉综合征(ACS)患者和稳定型心绞痛(SA)患者血清S100B、S100A6、S100P水平,出于研究需要,同时检测游离RAGE(sRAGE)及肿瘤坏死因子(TNF)-α水平。因为sRAGE这种可溶性蛋白质被金属蛋白酶从细胞膜上切割后,可竞争性地与RAGE配体结合,发挥干预炎性配体的作用,对S100-RAGE轴产生影响;而细胞因子TNF-α在炎症反应早期有致炎症作用,后期则具有抗炎和免疫调节作用,具有双重效应[7]。
1对象与方法
1.1研究对象
选择2014年3月至2015年2月在我院心脏科接受冠状动脉(冠脉)造影术(CAG)的882例患者。这些患者因严重或顽固胸闷、胸痛症状而入院,经危险分层评估后,选择侵入性或缺血驱动性策略,立即或择期接受血管造影检查[8]。SA、不稳定型心绞痛(UA)、非ST段抬高型心肌梗死(NSTEMI)的定义依据美国心脏病学会(ACC)/美国心脏协会(AHA)2010指南,ST段抬高型心肌梗死(STEMI)的诊断依据其2013指南,2型糖尿病的诊断依据美国糖尿病协会标准[9]。由于心力衰竭可能对S100蛋白水平有潜在影响,故排除陈旧性心肌梗死和(或)严重心力衰竭患者。外伤史、恶性肿瘤、结缔组织病、心脏瓣膜病、心肌病、感染及严重肾功能衰竭亦纳入排除标准。
根据临床表现和各项检查结果,将患者分为对照组(n=251,管腔狭窄≤30%)、SA组(n=211)和ACS组(n=420),其中ACS组又分为STEMI组(n=260)和UA/NSTEMI组(n=160)。
本研究经医院伦理委员会批准,并征得所有患者知情同意。
1.2方法
1.2.1CAG
通过桡动脉或股动脉路径行CAG,心外膜主要冠脉包括左主干、前降支、回旋支和右冠状动脉,左主干狭窄≥50%者可认为已达到双支病变。
1.2.2生化检测
所有血样本均取空腹血,ACS患者在入院24 h内采血,所有血样本经离心后取上层血清储存于-80 ℃环境以备用。按照标准实验室技术检测血糖、糖化血红蛋白、尿素氮、肌酐、尿酸、血脂项目,心肌酶谱包括肌酸激酶(CK)、肌酸激酶同工酶(CK-MB)、肌钙蛋白I(cTnI)。使用酶联免疫试剂盒检测血清S100B、S100A6、S100P、sRAGE、TNF-α水平。
1.2.3统计分析
所有数据采用SPSS15.0软件进行分析,计量资料以均数±标准差表示,分类资料描述为频率或百分比,组间差异进行卡方检验;连续变量正态分布采用Kolmolgorov-Smirnov检验,非正态分布的连续变量进行对数或平方根转换,组间比较采用单因素方差分析,变量间的相关分析采用Pearson和Spearman相关,构建Logistic多元回归模型评估发生ACS的独立因素。以P<0.05为差异具有统计学意义。
2结果
2.1临床资料和生化检测
SA和ACS组中老年、男性和吸烟者均多于对照组(P<0.01),高血压和高脂血症的比例也高于对照组(P<0.01),2型糖尿病的发病率在3组间无统计学差异。生化检测显示SA和ACS组的低密度脂蛋白胆固醇(LDL-C)、载脂蛋白B、血尿素氮和肌酐水平高于对照组,但高密度脂蛋白胆固醇(HDL-C)和载脂蛋白A水平则低于对照组(P<0.01);ACS组患者较SA组患者多支病变更为常见(P<0.01)。
2.2血清S100B、S100A6、S100P和炎症因子水平
ACS组血清S100B、S100A6、S100P水平较其他两组显著升高,SA和对照组之间无明显差异;对照组、SA组、ACS组的TNF-α水平依次递升;ACS组的sRAGE水平高于对照组,与SA组并无显著差异,见表1。

表1 各组血清S100蛋白、sRAGE及炎症因子水平比较
注:与对照组比较,(1)P<0.01;与SA组比较,(2)P<0.01;与UA/NSTEMI组比较,(3)P<0.05
进一步比较ACS组亚组间的差异,STEMI组患者的S100B、S100A6、S100P、TNF-α水平均高于UA/NSTEMI组,而sRAGE水平无差异。
另外,S100B水平在SA组中与S100A6水平显著相关(r=0.167,P<0.05),在ACS组中与S100P显著相关(r=0.313,P<0.001)。在所有分组中,S100A6水平均与TNF-α水平相关(P<0.05)。2.3血清S100B、S100A6、S100P与心肌缺血损伤程度的相关性
分析ACS组患者血清S100B、S100A6、S100P水平与心肌酶谱的关系,以探讨此3种蛋白水平能否反映心肌缺血损伤严重度。结果发现血清S100B水平与cTnI的峰值水平相关(r=0.144,P<0.05),S100P水平与CK-MB(r=0.229,P<0.001)及cTnI(r=0.190,P<0.01)峰值水平均相关。
2.4Logistic多元回归分析
采用多元回归分析法,将传统心血管危险因素和炎症因子进行修正,发现年龄、吸烟史、低水平HDL-C、TNF-α、S100B、S100A6、S100P均与ACS患者独立相关,而sRAGE则未能进入回归方程,见表2。
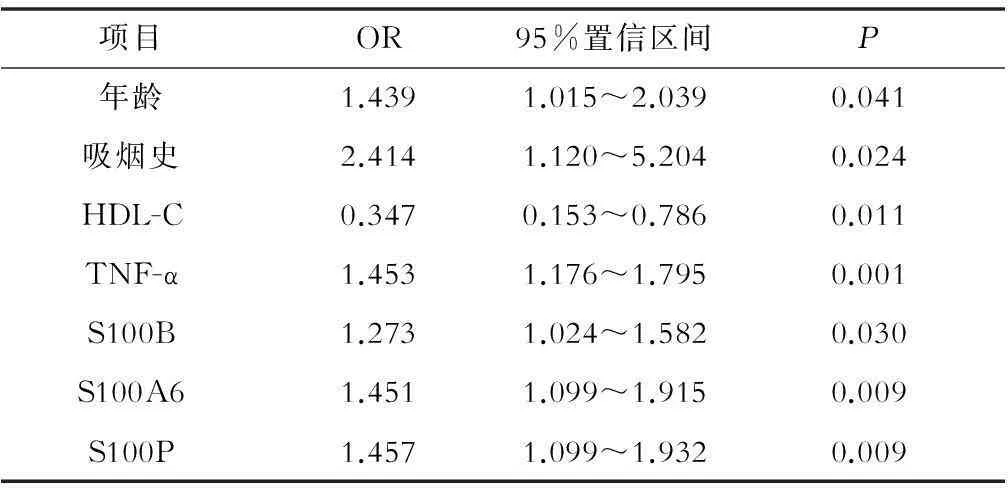
表2 ACS多因素分析
3讨论
本研究发现,血清S100B、S100A6、S100P水平与ACS相关,与心肌缺血的损伤程度相关,说明这些S100家族成员在ACS的病理生理过程中发挥了重要作用。既往研究表明,一些S100蛋白家族成员参与心血管疾病,如S100B、S100A1、S100A4和S100A8等[5]。本研究发现,ACS患者血清S100B、S100A6、S100P水平增高,并且与心肌缺血的损伤程度相关。S100B可由胶质细胞、少突细胞等多种神经组织细胞表达分泌至胞外,并介导生物学功能。以往有细胞生物实验研究发现,S100B能活化RAGE调节性炎症通道,提高黏附分子和炎症因子的表达,导致动脉粥样硬化形成,且缺血的心肌细胞能表达并分泌S100B[6,10-11]。目前关于S100A6的研究主要集中在肿瘤和纤维化疾病,但相关研究表明,心肌细胞、血管平滑肌细胞和内皮细胞均可表达S100A6[11-14]。有研究发现,心肌梗死后心肌细胞的S100B和S100A6蛋白及信使核糖核酸水平上调[15-19]。我们的研究则证实, ACS患者血清S100A6水平升高,并与TNF-α相关,提示S100A6与炎症密切相关。许多肿瘤组织和肿瘤细胞系中发现S100P升高[20-22]。体内和体外实验表明,S100P可以激活丝裂原活化蛋白激酶(MAPK)和核转录因子-κB(NF-κB)通路,通过上调细胞周期因子周期蛋白D1和周期蛋白依赖性激酶,促进肿瘤细胞增殖;而抑制RAGE,能拮抗并消除这些效应[23-25]。本研究首次证实S100P参与了心肌梗死。
既往有研究认为,冠状动脉疾病或ACS患者的sRAGE水平降低[26-28],然而本研究发现,ACS患者sRAGE水平高于对照组。结果不一致的原因首先可能在于样本量的差异;其次糖尿病患者在本研究中所占比例很高,而糖尿病会影响sRAGE水平[27]。
S100蛋白、高迁移率族蛋白(HMGB1、HMGB2)和b-缩氨酸淀粉体等配基与RAGE结合能诱发炎症反应[1-3];而抑制RAGE与相关配基结合,能显著减少心肌损伤和细胞凋亡[4、29-30],说明RAGE-配体调节机制密切参与心肌缺血损伤。心肌缺血或坏死促进了梗死区及梗死周边区域S100B、S100A6、S100P、RAGE等炎症因子的产生,从而激活了S100-RAGE轴,诱发炎症因子的表达进一步增加,大量的S100族蛋白和其他炎症因子的释放加剧心肌损伤、细胞凋亡和心肌重构,形成恶性循环,导致心功能不全。
参考文献
[1]Yan SF, Ramasamy R, Schmidt AM. Receptor for AGE (RAGE) and its ligands-cast into leading roles in diabetes and the inflammatory response[J].J Mol Med,2009,87(3):235-247.
[2]Basta G.Receptor for advanced glycation endproducts and atherosclerosis: From basic mechanisms to clinical implications[J].Atherosclerosis,2008, 196(1):9-21.
[3]Farmer DG, Kennedy S. RAGE,vascular tone and vascular disease[J].Pharmacol Ther,2009,124(2):185-194.
[4]Yan SF, Ramasamy R, Schmidt AM. The receptor for advanced glycation endproducts (RAGE) and cardiovascular disease[J]. Expert Rev Mol Med,2009, 12(11):e9.
[5]Donato R. RAGE: a single receptor for several ligands and different cellular responses: the case of certain S100 proteins[J].Curr Mol Med,2007,7 (8):711-724.
[6]Leclerc E, Fritz G, Vetter SW, et al.Binding of S100 proteins to RAGE: an update[J]. Biochim Biophys Acta,2009,1793(6):993-1007.
[8]张奇,沈卫峰.非ST段抬高型急性冠脉综合征治疗策略流程——2014AHA/ACC指南解读[J].国际心血管病杂志,2015,42(2):65-67.
[9]American Diabetes Association. Standards of medical care in diabetes-2009[M]. Diabetes Care,2009,32 Suppl 1:S13-S61.
[10]Donato R, Sorci G, Riuzzi F, et al. S100B's double life: intracellular regulator and extracellular signal[J].Biochim Biophys Acta,2009,1793 (6):1008-1022.
[12]Engelkamp D, Schäfer BW, Erne P,et al.S100 alpha, CAPL and CACY: molecular cloning and expression analysis of three calcium-binding proteins from human heart[J].Biochemistry,1992,31(42):10258-10264.
[13]Mandinova A, Atar D, Schäfer BW, et al. Distinct subcellular localization of calcium binding S100 proteins in human smooth muscle cells and their relocation in response to rises in intracellular calcium[J].J Cell Sci,1998,111(Pt14):2043-2054.
[14]Hsieh HL, Schäfer BW, Cox JA,et al. S100A13 and S100A6 exhibit distinct translocation pathways in endothelial cells[J]. J Cell Sci,2002,115 (Pt15):3149-3158.
[15]Tsoporis JN, Marks A, Kahn HJ, et al. Inhibition of norepinephrine-induced cardiac hypertrophy in s100beta transgenic mice[J]. J Clin Invest,1998,102 (8):1609-1616.
[16]Tsoporis JN, Marks A, Haddad A,et al.S100B expression modulates left ventricular remodeling after myocardial infarction in mice[J].Circulation, 2005,111(5):598-606.
[17]Tsoporis JN, Izhar S, Leong-Poi H, et al. S100B interaction with the receptor for advanced glycation end products (RAGE): a novel receptor-mediated mechanism for myocyte apoptosis postinfarction[J]. Circ Res,2010,106 (1):93-101.
[18]Stanton LW, Garrard LJ, Damm D, et al. Altered patterns of gene expression in response to myocardial infarction[J]. Circ Res,2000,86(9):939-945.
[19]Tsoporis JN, Marks A, Haddad A, et al. S100A6 is a negative regulator of the induction of cardiac genes by trophic stimuli in cultured rat myocytes[J]. Exp Cell Res,2005,303(2):471-481.
[20]Arumugam T, Simeone DM, Van Golen K,et al. S100P promotes pancreatic cancer growth, survival and invasion[J]. Clin Cancer Res,2005,11(15):5356-5364.
[21]Kim B, Lee HJ, Choi HY, et al. Clinical validity of the lung cancer biomarkers identified by bioinformatics analysis of public expression data[J]. Cancer Res,2007,67(15):7431-7438.
[22]Austermann J, Nazmi AR, Muller-Tidow C,et al. Characterization of the Ca2+-regulated ezrin-S100P interaction and its role in tumor cell migration[J]. J Biol Chem,2008,283(43):29331-29340.
[23]Arumugam T, Simeone DM, Schmidt AM,et al. S100P stimulates cell proliferation and survival via receptor for activated glycation end products (RAGE)[J].J Biol Chem,2004,279(7):5059-5065.
[24]Kim JK, Jung KH, Noh JH, et al. Targeted disruption of S100P suppresses tumor cell growth by down-regulation of cyclin D1 and CDK2 in human hepatocellular carcinoma[J]. Int J Oncol,2009,35(6):1257-1264.
[25]Fuentes MK, Nigavekar SS, Arumugam T, et al. RAGE activation by S100P in colon cancer stimulates growth migration, and cell signaling pathways[J]. Dis Colon Rectum,2007,50(8):1230-1240.
[26]Geroldi D, Falcone C, Emanuele E. Soluble receptor for advanced glycation end products: from disease marker to potential therapeutic target[J]. Curr Med Chem,2006,13(17):1971-1978.
[27]Yan XX, Lu L, Peng WH, et al. Increased serum HMGB1 level is associated with coronary artery disease in nondiabetic and type 2 diabetic patients[J]. Atherosclerosis,2009,205(2):544-548.
[28]McNair ED, Wells CR, Mabood Qureshi A, et al. Modulation of high sensitivity C-reactive protein by soluble receptor for advanced glycation end products[J].Mol Cell Biochem,2010,341(1-2):135-138.
[29]Bucciarelli LG, Kaneko M, Ananthakrishnan R, et al. Receptor for advanced glycation endproducts: key modulator of myocardial ischemic injury[J]. Circulation,2006,113(9):1226-1234.
[30]Bucciarelli LG, Ananthakrishnan R, Hwang YC, et al. RAGE and modulation of ischemic injury in the diabetic myocardium[J]. Diabetes,2008,57 (7):1941-1951.
(收稿:2015-06-17 修回:2015-11-21)
(本文编辑:丁媛媛)
Association of increased S100B, S100A6 and S100P in serum levels with acute coronary syndrome.
SHENGLezhi,CHENQiujing,ZHANGQi,LULin,SHENWeifeng,ZHANGRuiyan.
DepartmentofCardiology,RuijinHospital,InstituteofCardiovascularDiseases,SchoolofMedicine,ShanghaiJiaotongUniversity,Shanghai200025,China
【Abstract】Objective: To investigate whether the serum levels of receptor for advanced glycation endproduct (RAGE) and ligands S100B, S100A6 and S100P were related to acute coronary syndrome (ACS). Methods: Serum levels of S100B, S100A6, S100P, soluble RAGE (sRAGE), and TNF- were analyzed in 882 consecutive patients. Based upon clinical and laboratory findings, the patients were categorized as control group (n=251), stable angina (SA) group (n=211), and ACS group (n=420). Results: Serum S100B, S100A6, S100P and TNF-α levels[(103.73±56.90)ng/L,(5.28±4.15) μg/L,(8.73±7.96) μg/L,(87.82±39.30)ng/L] were higher in ACS group than in SA group [(81.93±27.65) ng/L,(4.36±2.45) μg/L,(3.41±3.08) μg/L,(71.88±30.70) ng/L]as well as in control group[(78.00±22.71) ng/L,(3.97±2.57) μg/L,(3.38±2.74) μg/L,(57.07±27.23) ng/L(each P<0.01)], and sRAGE levels were higher in ACS patients versus controls[(724.01±320.37) ng/L vs (652.55±351.24) ng/L , with P<0.01]. These bio-measurements were further made by classifying ACS patients into ST-segment elevation myocardial infarction (STEMI) and unstable angina/non-ST-segment elevation myocardial infarction (UA/NSTEMI) subgroups, and STEMI patients had the higher S100B, S100A6 and S100P levels than UA/NSTEMI patients.In ACS group,S100B level was correlated with troponin I(cTnI)level(P<0.05),while S100P level was correlated with CK-MB and cTnI levels(P<0.01). In multivariable regression analysis,S100B,S100A6,S100P and conventional risk factors were independently associated with ACS in patients. Conclusion: It indicates that serum levels of S100B, S100A6 and S100P are associated with ACS, and that serum levels of these proteins are related to the severity of myocardial infarction, implying that they are involved in the pathophysiology of ACS.
【Key words】Acute coronary syndrome; S100B; S100A6; S100P; Receptor for advanced glycation end products
doi:10.3969/j.issn.1673-6583.2016.02.015
通信作者:张瑞岩,Email:zhangruiyan@263.net

