Current Considerations of Thrombectomy for Acute Myocardial Infarction
Ahmed N. Mahmoud, MD, Islam Y. Elgendy, MD and Anthony A. Bavry, MD, MPH,2
1Department of Medicine, University of Florida, Gainesville, FL, USA
2North Florida/South Georgia Veterans Health System, Gainesville, FL, USA
Introduction
ST-elevation myocardial infarction (STEMI) is due to thrombotic occlusion of an epicardial coronary artery [1, 2]. Without reperfusion there is a high incidence of death and disability [3]. Reperfusion therapy was first successfully achieved with intravenous thrombolytic therapy [4, 5]. While intravenous thrombolytic therapy is still frequently used worldwide, it is limited by incomplete reperfusion in a significant proportion of cases and it is rarely associated with intracranial hemorrhage [6–8]. Primary percutaneous coronary intervention (PCI) represents a significant achievement to safely and more predictably restore flow to the epicardial artery [9,10]. While primary PCI is effective at restoring epicardial flow, myocardial perfusion can still remain compromised at a microvascular level, presumably due to embolization of thrombotic debris [11].Impaired myocardial perfusion is evidenced by poor myocardial blush grade and incomplete ST-segment resolution, both of which are associated with worse outcomes [12–14]. In current day practice, primary PCI is usually performed with adjunctive stent placement. This is done to treat an underlying stenotic lesion or to stabilize an area of disrupted plaque. The immediate goal of thrombectomy is to improve flow in the artery and optimize stent sizing, while the longer-term goal of thrombectomy is to reduce future adverse cardiac events such as repeat revascularization and stent thrombosis. This review will discuss the evolution of thrombectomy devices during acute myocardial infarction, recent clinical trial data on the topic, and what the future of thrombectomy may hold.
Thrombus Removal Devices
A variety of devices have been developed to remove thrombus during PCI. These devices are broadly grouped into (1) embolic protection devices, which capture embolized thrombus during PCI, (2) devices which attempt to break up the thrombus to facilitate removal, and (3) aspiration or suction devices,which rely on vacuum to remove thrombus [15].
Embolic Protection Devices
Embolic protection devices (also referred to as filter devices) were studied as a means to prevent distal embolization during PCI. As such, embolic protection devices only remove thrombus that embolizes into the filter [16]. This strategy has not been found to be effective at preventing adverse events during primary PCI of a native coronary artery [17]. Possible explanations for the lack of benefit include unintended embolization as the un-deployed filter is advanced through the thrombotic lesion. Alternatively, thrombus that remains in place at the site of occlusion, is not removed from the coronary artery, and thus may contribute to sub-optimal PCI and future adverse events [18].These devices are currently only recommended during revascularization of saphenous vein graft lesions [19].
Devices to Breakup Thrombus Prior to Removal
Rheolytic thrombectomy (i.e. Angiojet) is the best-known example of this type of device. With this device, saline jets are forced inside the catheter from the distal end to proximal. Some of the saline exits and then re-enters the catheter. The anticipated effect of the saline flow is to facilitate breakup and removal of thrombus [15]. The amount of thrombus removed by rheolytic thrombectomy appears to be rather modest as evidenced by no improvement in myocardial blush grade and a small improvement in complete ST-segment resolution [17, 20]. These devices also require the need for a temporary pacemaker in a significant proportion of cases [21]. Correspondingly, this device has failed to improve clinical outcomes and may increase the risk of stroke [17, 20]. Therefore,rheolytic thrombectomy is infrequently used in clinical practice.
Aspiration or Suction Devices
Aspiration thrombectomy catheters are simple devices that are advanced into a thrombus-containing artery. The tip of the catheter has an opening, which engages the thrombus. Many brands are available with differences in tip and mouth design. Once the catheter is advanced proximal to the thrombus, vacuum is applied to the catheter which acts to suck thrombus from the artery[15]. These devices are easy to use and are more effective at improving myocardial perfusion than rheolytic thrombectomy [17, 20]. While they extract microscopic thrombotic debris approximately 70% of the time, extraction of visible thrombus (>1 mm) only occurs in 35–44% [22,23]. Although somewhat counterintuitive, angiographic evidence of thrombus has not been shown to predict successful retrieval with aspiration thrombectomy devices [22].
Clinical Evidence
The Thrombus aspiration during primary percutaneous coronary intervention (TAPAS) trial was the first large size trial to compare thrombectomy compared with primary PCI alone [23]. This trial documented a marginally significant reduction in mortality. This outcome was an unexpected finding and not the primary outcome of the trial. While this possible mortality benefit should have been interpreted with caution, it ushered in enthusiasm for widespread thrombectomy use during acute myocardial infarction. Subsequent meta-analyses documented mortality benefit with thrombectomy compared with primary PCI alone [17, 20, 24]. However, the field changed dramatically after documentation of negative findings with the Thrombus Aspiration during ST-segment Elevation Myocardial Infarction(TASTE) (n=7244 patients) and Randomized Trial of Primary PCI with or without Routine Manual Thrombectomy (TOTAL) (n=10,732 patients) trials[25, 26]. An updated meta-analysis including these 2 most recent and largest trials was unable to confirm benefit from a strategy of routine thrombus removal[27]. In that analysis, aspiration thrombectomy was associated with a non-significant reduction in allcause mortality and a non-significant increase in stroke. Current practice guidelines no longer support the routine use of thrombectomy, but still consider it effective in certain circumstances (class IIb recommendation) [28]. Furthermore, the Thrombus Aspiration in Thrombus Containing Culprit Lesions in Non-ST-Elevation Myocardial Infarction(TARTOR-NSTEMI) trial had demonstrated the lack of benefit for aspiration thrombectomy in patients with NSTEMI where there may be smaller thrombus burden [29]. Also, aspiration thrombectomy has not demonstrated benefit on microvascular obstruction among patients with STEMI who present late (i.e.,beyond 12 hours) [30].
Theories Behind the Lack of Improved Cardiovascular Outcomes Following Aspiration Thrombectomy
Intuitively, the concept of thrombus removal during acute myocardial infarction still appears to be valid. During STEMI, stent implantation in a large thrombus significantly increases the risk for subsequent stent thrombosis [31]. Other studies have also confirmed an increased risk of adverse events when thrombus is present during the index PCI procedure[32]. In addition to risk of stent thrombosis, thrombus can make stent sizing difficult and increase the risk of stent malposition resulting in increased revascularization procedures [32–34].
There are multiple reasons why thrombectomy,in its present form, may be ineffective. First, it is possible that sub-optimal technique can result in embolization of thrombus into the aorta and provide a mechanism for stroke [35]. While this complication is rare, the deleterious effects of a neurological ischemic event could negate any benefit from thrombectomy. Good practice with thrombus removal should always be observed [15].
The second consideration is that not all STEMI is due to large thrombus burden [31]. A study in STEMI patients attempted to characterize the size of thrombus. An interesting finding is that approximately 70% of cases were associated with ≤ medium thrombus burden [31]. With non-large thrombus, any benefit from thrombus removal would be expected to be minimal or non-existent. Future study of thrombectomy may need to focus on the approximate 30% of cases with ≥ large thrombus.
The third reason for lack of benefit with thombectomy could be that current thrombectomy devices are not effective enough. In approximately one-third of cases, visible thrombus (>1 mm) is observed in blood that has been aspirated from a coronary artery[22]. Also, while markers of reperfusion are better with aspiration thrombectomy compared with rheolytic thrombectomy, they are still not 100% effective. This is evidenced by the myocardial blush grade≥2 of approximately 59% and complete ST-segment resolution of approximately 68% after aspiration thrombectomy [27]. Aspiration thrombectomy catheters may work best at removing soft thrombus that is small enough to be sucked into the device.In contrast, solid/firm thrombus or large thrombus may be more difficult to remove with this device.Retrievable stents were designed to remove more solid/firm thrombus from the cerebrovascular circulation. They have been studied and approved for use during acute ischemic stroke [36–38]. A meta-analysis of randomized trials documented an improvement in functional outcomes and a non-significant reduction in mortality with retrievable stent devices compared with usual care during acute ischemic stroke [39]. Since these devices have been shown to be beneficial in acute ischemic stroke, they may deserve to be studied in acute myocardial infarction.Another option might be the use of external transthoracic ultra-sound to facilitate coronary thrombolysis,which has been shown in animal models and limited human studies to be associated with improved coronary patency rates [40, 41]. However, the clinical benefit of utilizing such an approach, either alone or as an adjunct to aspiration thrombectomy, still needs to be proven in larger clinical trials.
The final consideration is that stent implantation into thrombus may not be as deleterious as imagined due to a variety of reasons. This could be because of increased use of intravascular ultrasound during STEMicould help to properly size stents [42]. The M-guard stent is an experimental stent which is covered by mesh material intended to capture thrombus between the stent and the wall of the vessel during implantation, thus preventing distal embolization [43]. It is possible that current generation stents may be functioning in a similar fashion and thus minimizing the impact of distal embolization. Also,potent ADP receptor antagonists are likely reducing the overall risk of stent thrombosis and future adverse cardiac events. It is important to note that the 12 month mortality remains at approximately 4–5%, therefore, opportunities remain to further reduce adverse events [44, 45].
Clinical and Angiographic Predictors of Aspiration Thrombectomy Failure
Unsuccessful aspiration thrombectomy occurs due to failure to reach or cross the culprit lesion and/or failure to collect any thrombotic material after crossing the culprit lesion [46]. This occurs in approximately 4–25% of the cases [46, 47]. Older age,marked artery tortuosity, high coronary calcification and bifurcation lesions have all been associated with a higher incidence of failed aspiration thrombectomy[46–48]. However, it is worth mentioning that the impact of aspiration thrombectomy failure on clinical outcomes remains unclear, as the incidence of mortality in those patients was similar to the patients who had successful thrombectomy [46].
Role of Glycoprotein IIb/IIIa Inhibitors Administration Prior to Aspiration Thrombectomy
Although earlier studies had suggested a potential benefit from upstream glycoprotein IIb/IIIa inhibitors during primary PCI, recent studies have demonstrated the lack of clinical benefit with glycoprotein IIb/IIIa inhibitors in the era of potent ADP antagonists [49, 50]. In the intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction(INFUSE-AMI) trial evaluated the potential benefit of local intra-coronary delivery of abciximab with a local infusion catheter, there was no clinical outcome benefit in the arm who underwent aspiration thrombectomy compared with no aspiration thrombectomy [51]. In addition, a meta-regression analysis of randomized trials did not illustrate any added clinical benefit with the concomitant use of glycoprotein IIb/IIIa inhibitors with aspiration thrombectomy [27].
Case Examples
Case 1
An 82-year-old male with prior PCI to the right coronary artery (RCA) developed an inferior STEMI when his dual anti-platelet therapy was held before an invasive procedure. Emergent coronary angiography revealed an occluded distal RCA (Figure 1).The area of occlusion was dilated multiple times with 2.5 mm and 3.0 mm balloons; however, this was unsuccessful in restoring coronary flow. At this point, aspiration thrombectomy was performed.The thrombus was too large to be sucked into the device; therefore, constant suction was applied as the device was removed from the body. The thrombus was noted to become dislodged from the catheter tip within the hemostatic valve. It was removed from the guide catheter by aspirating from the side port of the hemostatic valve (Figure 2). Final angiogram revealed a good result after drug-eluting stent implantation (Figure 3). This case illustrates the application of selective thrombectomy after failure to restore flow with initial balloon angioplasty. It also illustrates a possible mechanism for stroke if the thrombus was not recognized to be retained in the guide catheter and/or hemostatic valve.
Case 2
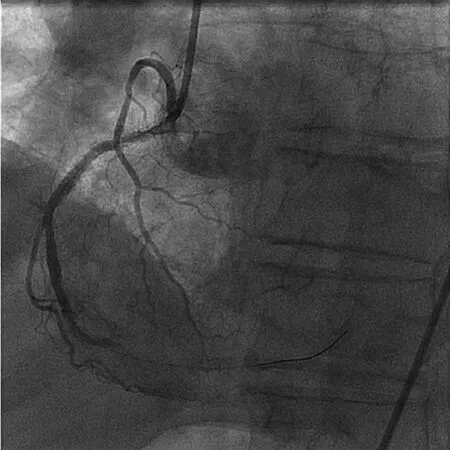
Figure 1 Distal Right Coronary Artery Occlusion.
A 63-year-old male presented with anterior STEMI.Emergent coronary angiography revealed an occluded mid left anterior descending artery (LAD) (Figure 4).After balloon angioplasty, normal coronary flow was restored with no/minimal thrombus burden at a stenotic lesion (Figure 5). The lesion was attempted to be direct stented; however, a stent would not pass due to significant calcium burden. Rotational atherectomy was performed with a 1.25 mm and a 1.5 mm burr.This allowed for optimal implantation of 2 drug-eluting stents (Figure 6). In this case, balloon angioplasty restored normal flow and uncovered no/minimal thrombus burden; therefore, selective thrombectomy was not felt to be indicated.
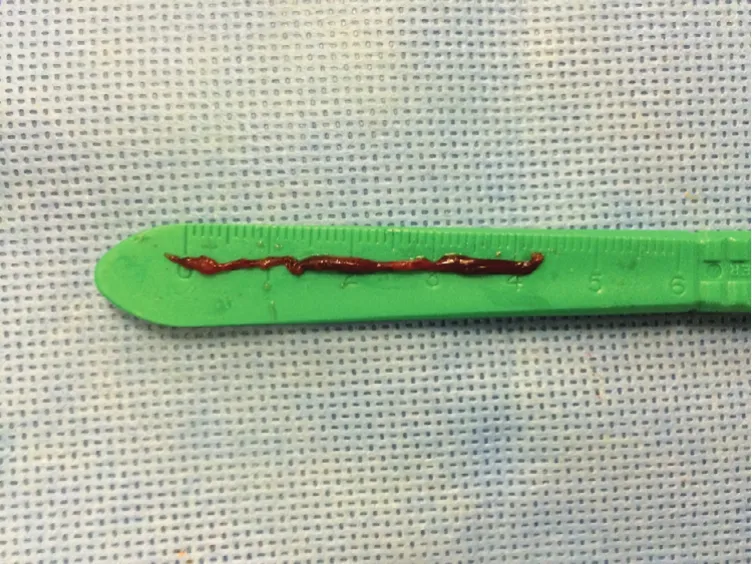
Figure 2 4.5-centimeter Thrombus Retrieved from the Distal Right Coronary Artery Following Aspiration Thrombectomy.
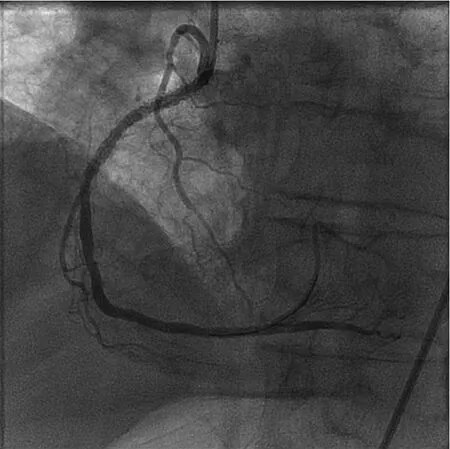
Figure 3 Good Angiographic Result in the Right Coronary Artery Following Aspiration Thrombectomy and Drug-Eluting Stent Implantation.This case illustrates an application of selective thrombectomy.
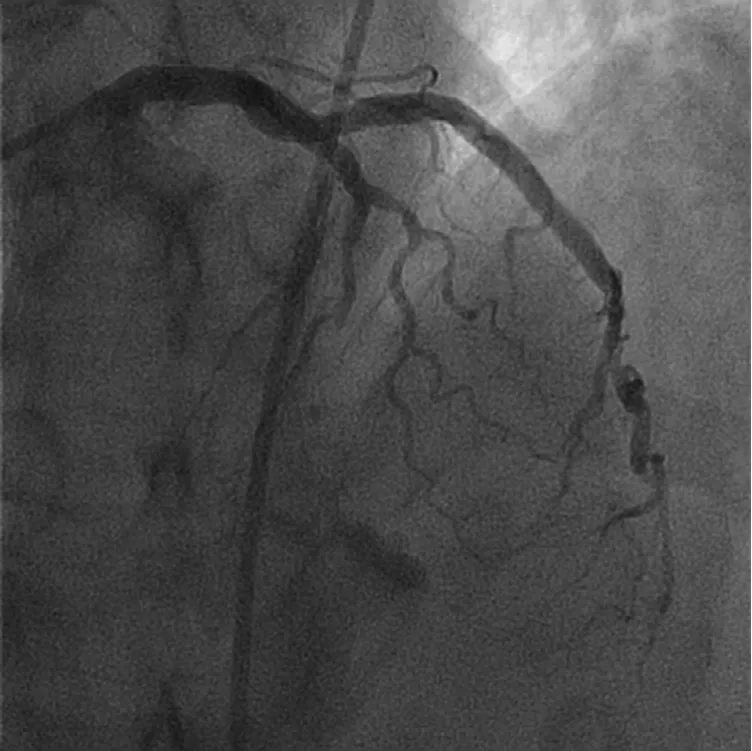
Figure 4 Mid Left Anterior Descending Artery Occlusion.
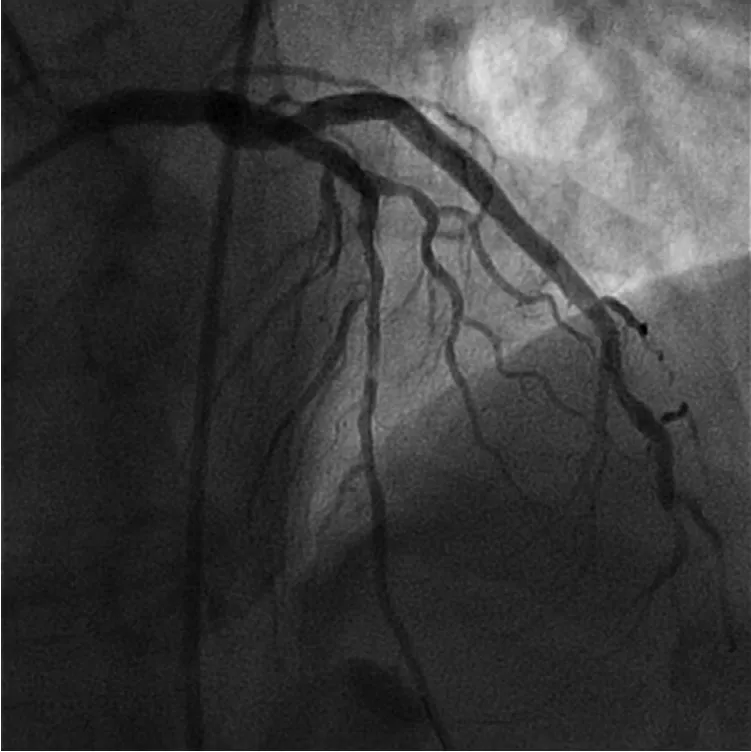
Figure 5 Normal Coronary Flow in the Left Anterior Descending Artery Following Balloon Angioplasty.There is no/minimal thrombus burden associated with the stenosis.
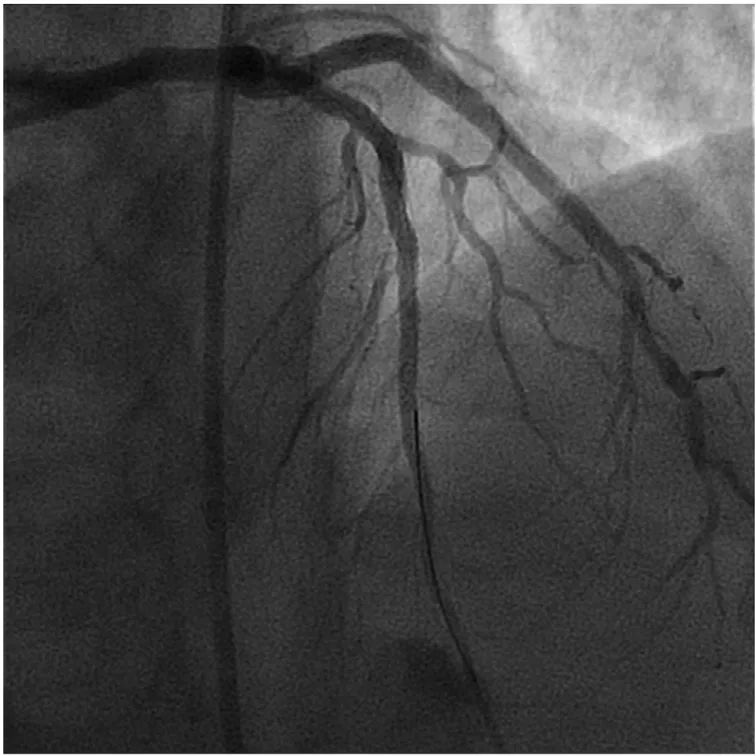
Figure 6 Good Angiographic Result in the Left Anterior Descending Artery Following Rotational Atherectomy and Drug-Eluting Stent Implantation.This case illustrates an example where thrombectomy was not necessary.
Conclusions
In conclusion, thrombus removal still remains a valid concept during acute myocardial infarction. Currently available aspiration thrombectomy devices are recommended for selective, rather than routine use during STEMI. Future studies will need to focus on safe removal of thrombus (i.e. without increasing the risk of stroke), appropriate lesions from which to remove thrombus (i.e. only thrombi of a certain size/characteristic), and more effective thrombus removal devices. Regarding the latter,devices that are more capable of removing solid/firm thrombi (i.e. retrievable stents) deserve study during STEMI. Opportunities still exist with thrombus management to improve future adverse cardiac events in acute myocardial infarction patients.
Conflict of Interest
The authors declare no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial or notfor-profit sectors.
REFERENCES
1. DeWood MA, Spores J, Notske R, Mouser LT, Burroughs R,Golden MS, et al. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med 1980;303:897–902.
2. Worthley SG, Osende JI, Helft G,Badimon JJ, Fuster V. Coronary artery disease: pathogenesis and acute coronary syndromes. Mt Sinai J Med 2001;68:167–81.
3. Wood FO, Leonowicz NA, Vanhecke TE, Dixon SR, Grines CL. Mortality in patients with ST-segment elevation myocardial infarction who do not undergo reperfusion. Am J Cardiol 2012;110:509–14.
4. Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico (GISSI). Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Lancet 1986;1:397–402.
5. The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993;329:673–82.
6. Simes RJ, Topol EJ, Holmes DR, Jr.,White HD, Rutsch WR, Vahanian A,et al. Link between the angiographic substudy and mortality outcomes in a large randomized trial of myocardial reperfusion. Importance of early and complete infarct artery reperfusion.GUSTO-I Investigators. Circulation 1995;91:1923–8.
7. Brass LM, Lichtman JH, Wang Y, Gurwitz JH, Radford MJ,Krumholz HM. Intracranial hemorrhage associated with thrombolytic therapy for elderly patients with acute myocardial infarction: results from the Cooperative Cardiovascular Project. Stroke 2000;31:1802–11.
8. Kucia AM, Zeitz CJ. Failed reperfusion after thrombolytic therapy:recognition and management.Heart Lung 2002;31:113–21.
9. Hartzler GO, Rutherford BD,McConahay DR. Percutaneous transluminal coronary angioplasty: application for acute myocardial infarction. Am J Cardiol 1984;53:117c–21c.
10. Prida XE, Holland JP, Feldman RL,Hill JA, MacDonald RG, ConticR,et al. Percutaneous transluminal coronary angioplasty in evolving acute myocardial infarction. Am J Cardiol 1986;57:1069–74.
11. Henriques JP, Zijlstra F, Ottervanger JP, de Boer MJ, van’t Hof AW,Hoorntje JC, et al. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction.Eur Heart J 2002;23:1112–7.
12. van’t Hof AW, Liem A, de Boer MJ,Zijlstra F. Clinical value of 12-lead electrocardiogram after successful reperfusion therapy for acute myocardial infarction. Zwolle Myocardial Infarction Study Group. Lancet 1997;350:615–9.
13. van’t Hof AW, Liem A, Suryapranata H, Hoorntje JC, de Boer MJ, Zijlstra F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction:myocardial blush grade. Zwolle Myocardial Infarction Study Group.Circulation 1998;97:2302–6.
14. Stone GW, Peterson MA, Lansky AJ, Dangas G, Mehran R, Leon MB.Impact of normalized myocardial perfusion after successful angioplasty in acute myocardial infarction.J Am Coll Cardiol 2002;39:591–7.
15. Bavry A. Management of thrombotic lesions. In Cardiovascular intervention: a companion to Braunwald’s heart disease E-book.Amsterdam: Elsevier; 2015:223–8.
16. Bangalore S, Bhatt DL. Embolic protection devices. Circulation 2014;129:e470–6.
17. Bavry A, Kumbhani D, Bhatt D.Role of adjunctive thrombectomy and embolic protection devices in acute myocardial infarction:a comprehensive meta-analysis of randomized trials. Eur Heart J 2008;29:2989–3001.
18. Srinivasan M, Rihal C, Holmes DR,Prasad A. Adjunctive thrombectomy and distal protection in primary percutaneous coronary intervention: impact on microvascular perfusion and outcomes.Circulation 2009;119:1311–9.
19. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011;58:e44–122.
20. Kumbhani DJ, Bavry AA, Desai MY, Bangalore S, Bhatt DL.Role of aspiration and mechanical thrombectomy in patients with acute myocardial infarction undergoing primary angioplasty:an updated meta-analysis of randomized trials. J Am Coll Cardiol 2013;62:1409–18.
21. Ali A, Cox D, Dib N, Brodie B, Berman D, Gupta N, et al.Rheolytic thrombectomy with percutaneous coronary intervention for infarct size reduction in acute myocardial infarction: 30-day results from a multicenter randomized study. J Am Coll Cardiol 2006;48:244–52.
22. Vlaar PJ, Svilaas T, Vogelzang M,Diercks GF, de Smet BJ, van den Heuvel AF, et al. A comparison of 2 thrombus aspiration devices with histopathological analysis of retrieved material in patients presenting with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2008;1:258–64.
23. Svilaas T, Vlaar PJ, van der Horst IC, Diercks GF, de Smet BJ, van den Heuvel AF, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med 2008;358:557–67.
24. Kumbhani DJ, Bavry AA, Desai MY, Bangalore S, Byrne RA, Jneid H, et al. Aspiration thrombectomy in patients undergoing primary angioplasty: totality of data to 2013. Catheter Cardiovasc Interv 2014;84:973–7.
25. Frobert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T,Maeng M, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med 2013;369:1587–97.
26. Jolly SS, Cairns JA, Yusuf S,Meeks B, Pogue J, Rokoss MJ, et al. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med 2015;372:1389–98.
27. Elgendy IY, Huo T, Bhatt DL,Bavry AA. Is aspiration thrombectomy beneficial in patients undergoing primary percutaneous coronary intervention? meta-analysis of randomized trials. Circ Cardiovasc Interv 2015;8:e002258.
28. Levine GN, O’Gara PT, Bates ER, Blankenship JC, Kushner FG,Bailey SR, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions.Circulation 2016;133:1135–47.
29. Thiele H, de Waha S, Zeymer U,Desch S, Scheller B, Lauer B, et al.Effect of aspiration thrombectomy on microvascular obstruction in NSTEMI patients: the TATORTNSTEMI trial. J Am Coll Cardiol 2014;64:1117–24.
30. Desch S, Stiermaier T, de Waha S,Lurz P, Gutberlet M, Sandri M, et al.Thrombus aspiration in patients with ST-segment elevation myocardial infarction presenting late after symptom onset. JACC Cardiovasc Interv 2016;9:113–22.
31. Sianos G, Papafaklis MI, Daemen J, Vaina S, van Mieghem CA, van Domburg RT, et al. Angiographic stent thrombosis after routine use of drug-eluting stents in ST-segment elevation myocardial infarction: the importance of thrombus burden. J Am Coll Cardiol 2007;50:573–83.
32. Singh M, Berger PB, Ting HH,Rihal CS, Wilson SH, Lennon RJ,et al. Influence of coronary thrombus on outcome of percutaneous coronary angioplasty in the current era (the Mayo Clinic experience).Am J Cardiol 2001;88:1091–6.
33. Kim YS, Koo BK, Seo JB, Park KW,Suh JW, Lee HY, et al. The incidence and predictors of postprocedural incomplete stent apposition after angiographically successful drugeluting stent implantation. Catheter Cardiovasc Interv 2009;74:58–63.
34. Guo N, Maehara A, Mintz GS, He Y, Xu K, Wu X, et al. Incidence,mechanisms, predictors, and clinical impact of acute and late stent malapposition after primary intervention in patients with acute myocardial infarction: an intravascular ultrasound substudy of the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial.Circulation 2010;122:1077–84.
35. Vaduganathan M, Bhatt DL.Manual thrombectomy in myocardial infarction: aspiring for better. J Am Heart Assoc 2015;4:002201.
36. Berkhemer OA, Fransen PS,Beumer D, van den Berg LA,Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20.
37. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–30.
38. Saver JL, Goyal M, Bonafe A,Diener HC, Levy EI, Pereira VM,et al. Stent-retriever thrombectomy after intravenous t-PA vs.t-PA alone in stroke. N Engl J Med 2015;372:2285–95.
39. Elgendy IY, Kumbhani DJ,Mahmoud A, Bhatt DL, Bavry AA. Mechanical thrombectomy for acute ischemic stroke: a metaanalysis of randomized trials. J Am Coll Cardiol 2015;66:2498–505.
40. Cohen MG, Tuero E, Bluguermann J, Kevorkian R, Berrocal DH,Carlevaro O, et al. Transcutaneous ultrasound-facilitated coronary thrombolysis during acute myocardial infarction. Am J Cardiol 2003;92:454–7.
41. Siegel RJ, Atar S, Fishbein MC,Brasch AV, Peterson TM, Nagai T,et al. Noninvasive, transthoracic,low-frequency ultrasound augments thrombolysis in a canine model of acute myocardial infarction. Circulation 2000;101:2026–9.
42. Witzenbichler B, Maehara A,Weisz G, Neumann FJ, Rinaldi MJ,Metzger DC, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents(ADAPT-DES) study. Circulation 2014;129:463–70.
43. Stone GW, Abizaid A, Silber S,Dizon JM, Merkely B, Costa RA, et al. Prospective, randomized, multicenter evaluation of a polyethylene terephthalate micronet mesh-covered stent (MGuard) in ST-segment elevation myocardial infarction: the MASTER trial. J Am Coll Cardiol 2012;60:1975–84.
44. Lagerqvist B, Frobert O, Olivecrona GK, Gudnason T, Maeng M,Alstrom P, et al. Outcomes 1 year after thrombus aspiration for myocardial infarction. N Engl J Med 2014;371:1111–20.
45. Jolly SS, Cairns JA, Yusuf S,Rokoss MJ, Gao P, Meeks B, et al.Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet 2016;387:127–35.
46. Vink MA, Kramer MC, Li X,Damman P, Rittersma SZ, Koch KT, et al. Clinical and angiographic predictors and prognostic value of failed thrombus aspiration in primary percutaneous coronary intervention. JACC Cardiovasc Interv 2011;4:634–42.
47. Claessen BE, Dangas GD.Thrombus aspiration in primary percutaneous coronary intervention how to manage failure. JACC Cardiovasc Interv 2011;4:643–4.
48. Lorgis L, Fayard M, Dentan G,Richard C, Buffet P, L’Huillier I, et al. Clinical predictors of successful thrombectomy with the Export®aspiration catheter in the acute phase of myocardial infarction.Data from the RICO survey working group. Arch Cardiovasc Dis 2010;103:522–9.
49. Le May MR, Wells GA, Glover CA, So DY, Froeschl M, Marquis JF, et al. Primary percutaneous coronary angioplasty with and without eptifibatide in ST-segment elevation myocardial infarction: a safety and efficacy study of integrilin-facilitated versus primary percutaneous coronary intervention in ST-segment elevation myocardial infarction (ASSIST). Circ Cardiovasc Interv 2009;2:330–8.
50. Mehilli J, Kastrati A, Schulz S,Früngel S, Nekolla SG, Moshage W, et al. Abciximab in patients with acute ST-segment–elevation myocardial infarction undergoing primary percutaneous coronary intervention after clopidogrel loading: a randomized double-blind trial. Circulation 2009;119:1933–40.
51. Stone GW, Maehara A,Witzenbichler B, Godlewski J,Parise H, Dambrink JH, et al.Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: the INFUSE-AMI randomized trial. J Am Med Assoc 2012;307:1817–26.
 Cardiovascular Innovations and Applications2016年2期
Cardiovascular Innovations and Applications2016年2期
- Cardiovascular Innovations and Applications的其它文章
- Transient Pulmonary Atelectasis after Ketamine Sedation during Cardiac Catheterization in Spontaneously Breathing Children with Congenital Heart Disease
- Identification and Management of Iatrogenic Aortocoronary Dissection
- Cardiovascular Abnormalities Among Patients with Spontaneous Subarachnoid Hemorrhage.A Single Center Experience
- Coronary Artery Chronic Total Occlusion
- Carotid Artery Stenting: 2016 and Beyond
- The Transradial Approach for Cardiac Catheterization and Percutaneous Coronary Intervention: A Review
