Calcium intake,calcium homeostasis and health
Fn Pu,Ning Chen,Shenghui Xue,∗
a La Jolla Institute of Allergy&Immunology,La Jolla,CA 92037,USA
b Hubei Exercise Training and Monitoring Key Laboratory,Hubei Provincial Collaborative Innovation Center for Exercise and Health Promotion,College of Health Science,Wuhan Sports University,Wuhan 430079,China
Abstract
Keywords:Calcium;Homeostasis;Calcium sensing receptor;Dietary calcium;Vitamin D
1.Introduction
Calcium is the 5th of the most abundant elements in the earth crust and is also the most abundant mineral in human body.The human body contains approximately 1 kg of calcium with more than 99% deposit in the bone in the form of calcium phosphate[1].Through interacting with numerous proteins distributed in different cellular compartments,calcium is involved in a large amount of aspects of life,such as muscle contraction,enzyme activation,cell differentiation,immune response,programmed cell death and neuronal activity[1–11].Such broad functions are maintained by tightly controlled calcium concentration in extracellular fluid and cellular compartments.The concentrations of calcium in blood and extracellular fluid are usually maintained at 1–2 mmol/L,while the concentration of intracellular calcium at resting state is maintained at 100 nmol/L or less by calcium ATPase,channels,and exchangers located in plasma membrane and endoplasmic reticulum(ER)membrane[2,12].During the signaling process of calcium,the concentration of intracellular calcium is increased to approximately 100μM,which triggers calcium signaling through the activation or deactivation of an array of calcium-binding proteins.In addition,pathogens,such as bacteria and viruses,can hijack calcium signaling to benefit their own life cycles including invasion,replication and proliferation[13,14].
Due to the regulation by calcium sensing receptor(CaSR)located in the parathyroid gland,the concentration of extracellular calcium is dedicatedly maintained by intestinal absorption,kidney reabsorption and bone resorption/formation.The miscommunication of these processes is responsible for calcium-related diseases,such as osteomalacia[15,16].Americans at all ages,however,do not consume enough dietary calcium compared with the recommendations by the Institute of Medicine[17].The deficiency of calcium could cause various diseases,such as osteoporosis.In this paper,we will review the key factors controlling calcium homeostasis and further discuss the diseases associated with the dysfunctional regulation of calcium and vitamin D.
2.Molecular mechanism of extracellular calcium homeostasis
Extracellular calcium homeostasis is mainly controlled by three physiological modes,including intestinal calcium absorption,renal calcium reabsorption,and bone formation/resorption[18],which is mainly regulated by CaSR through the modulation of parathyroid hormone(PTH),calcitonin and 1,25-dihydroxyvitamin D3 secretion(Fig.1)[19–21].
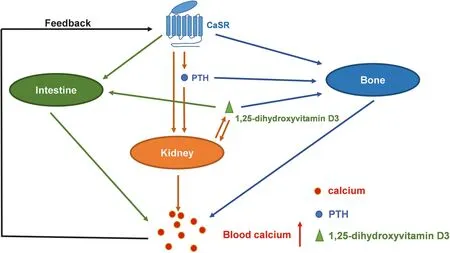
Fig.1.Regulation of calcium homeostasis by CaSR.The decrease of blood calcium level activates CaSR in parathyroid gland,which further promotes the secretion of PTH.PHT increases blood calcium level by the direct activation of calcium reabsorption in kidney and calcium release in bone.PHT also promotes the production and secretion of 1,25-dihydroxyvitamin D3 in kidney cells.1,25-Dihydroxyvitamin D3 regulates the intestinal calcium absorption,kidney calcium reabsorption and bone calcium release.CaSR expressed in bone,kidney and intestine cells are also involved in the regulation of calcium homeostasis.
2.1.Calcium uptake in intestine
Intestine is the major organ responsible for calcium uptake.In general,calcium from diets is absorbed by intestine through two pathways including transcellular absorption and paracellular transport of calcium(Fig.2).In duodenum of intestine,transcellular absorption is responsible for 80% calcium uptake in low-calcium diets and less than 10% calcium uptake in high-calcium diets[22].Certain calcium channels,intracellular calcium-binding proteins and calcium pumps are responsible for transcellular absorption of calcium.This process is initiated by transient receptor potential vanilloid type 6(TRPV6)channel,a transmembrane calcium selective channel located in the brush border side membrane responsible for calcium entry[23,24].After calcium enters the cell through TRPV6 channel,calcium-buffering proteins bind to calcium and transport calcium inside the cell.At last,calcium is excluded out of the cell to blood vessels through plasma membrane ATP ase 1b(PMCA1b)located in the basolateral membrane[25].

Fig.2.Intestinal calcium absorption by transcellular absorption and paracellular transport.
TRPV6 channel belongs to the transient receptor potential(TRP)super family that contains 6 different proteins.TRPV1-4 are non-selective cation channels activated by protons,lipids,and the changes of temperature, pressure and osmolarity.TRPV5 and TRPV6 are calcium selective channels involved in renal calcium reabsorption and intestinal calcium absorption,respectively[18,23,24].TRPV6 is located in many types of cells,including the cells from intestine,prostate cancer and breast cancer[26–28].The activation of TRPV6 in the brush border membrane of intestine is the first step of calcium entry.This protein contains long intracellular N-terminal and C-terminal domains and 6 putative transmembrane domains.TRPV6 is also modified through N-linked glycosylation[29].Different from TRPV1-4,TRPV5 and TRPV6 are constitutively activated[24,30].The functional TRPV5 and TRPV6 channels are tetramers.The expression levels of TRPV6 in intestine and many types of cancer cells are regulated by vitamin D.TRPV6 is also induced by low calcium diets or at the time of weaning[31,32].Transgenic mice with TRPV6 overexpression can result in hypercalcemia and soft tissue calcification,which further supports the role of TRPV6 in calcium absorption[31,33].Additionally,TRPV6 is regulated by multiple intracellular proteins including calmodulin,S100A10/Annexin 2,Nipsnap1 and Rab11a[34–37].TRPV6 also contains a few putative phosphorylation sites,suggesting that TRPV6 is modulated by kinases[22].
The second phase of transcellular absorption for calcium is mediated by a calcium-buffering protein,calbindin D9k as an intracellular calcium-binding protein.This protein has one classical EF-hand and one pseudo EF hand.Both EF-hands cooperatively bind calcium with high affinity[38,39].Unlike parvalbumin[40,41],calbindin D9k has relatively low affinity to Mg2+.The expression level of calbindin D9k in intestine can be regulated by 1,25-dihydroxyvitamin D3,low dietary calcium conditions or at the time of weaning[31,32,42].
Although the roles of TRPV6,calbindin D9k and PMCA1 in transcellular calcium absorption are well studied,the studies with gene knockout mice suggest that TRPV6 channel and calbindin D9k are not essential for the intestinal calcium uptake and the functions of TRPV6 and calbindin D9k in transcellular calcium absorption may be compensated by other proteins[43,44].
The third step of transcellular calcium absorption is mediated by PMCA1b.As a common mechanism of PMCA,PMCA1b transports intracellular calcium to blood in an energy-dependent meaner.Animals adapted to diets with low calcium and low phosphorus or induced by vitamin D can increase the expression of PMCA1b in the basolateral membrane of intestinal cells[45,46].Besides,sodium–calcium exchangers including NCX1,are also involved in calcium extrusion in the basal lateral membrane[22].
由表7可知抽检的芯样的密度、孔隙率、稳定度和流值均符合实际设计要求,并进行了2m水头的渗透试验,发现均无渗透问题存在,说明沥青混合料的摊铺和碾压工艺是可行的。
Different from transcellular absorption of calcium,paracellular transport of calcium is a non-saturable,energy-independent pathway.Paracellular transport of calcium can be observed throughout the intestine and is the major pathway for calcium uptake,especially under the condition with high-calcium diets.Compared with transcellular calcium absorption,the molecular mechanism of paracellular calcium transport is less well studied.However,it is clear that tight junction plays a critical role in the regulation of this event.The permeability of tight junction is regulated by many proteins including claudin 2 and 12[22,31].In addition,1,25-dihydroxyvitamin D3 regulates paracellular calcium transport by suppressing the expression of claudin 3,aquaporin 8,cadherin 17,and RhoA,thus improving the permeability of tight junction[47,48].
2.2.Calcium reabsorption in kidney
Kidney is another essential organ for calcium sensing and reabsorption.Calcium is absorbed in nephron with the highest absorption in proximal tubules[18].The renal calcium reabsorption comprises of two pathways including paracellular pathway and transepithelial pathway.Similar as transcellular calcium absorption in intestine,transepithelial calcium reabsorption in kidney also contains three major steps.First,calcium is transported to the intracellular space through calcium channels located in the epical membrane.TRPV5 is the major calcium channel located in the epical plasma membrane responsible for calcium transportation.TRPV5 is a calcium-selective channel with 75% amino acid identity to TRPV6[18,49].The N-terminal of TRPV5 contains six ankyrin repeats involved in the tetramer formation and protein–protein interaction.The C-terminal of TRPV5 contains a phosphorylation site of protein kinase C.The kidney calcium reabsorption is impaired in TRPV5 knockout mice,suggesting the critical role of TRPV5 in this process[18,50].TRPV5 is regulated by many biomolecules,and can be activated by kallikrein or bradykinin receptor through PLC/DAG/PKC pathway[51].Similar to TRPV6,TRPV5 is regulated by annexin-2,Rab11a,calmodulin and other proteins[18,24].Second,the intracellular calcium binds to calciumbuffering proteins,such as calbindin D28k and calbindin D9k,and passively diffused to the basolateral membrane through calcium gradient.Calbindin D28k contains three pairs of EF-hand motifs with 6 calcium-binding sites.This protein dynamically controls calcium reabsorption through the interaction with TRPV5 at low intracellular calcium concentration[52].Renal calcium reabsorption is disrupted in calbindin D28k knockout mice with high calcium diets[18,53],while other group shows that the effect of calbindin D28k on calcium transport is compensated by other proteins including calbindin D9k[18,54].At last,calcium is transported to blood by PMCA1b and/or NCX1 located in the basolateral membrane.
Calcium reabsorption in kidney is modulated by PTH,1,25-dihydroxyvitamin D3 and estrogen.PTH reduces the expression levels of TRPV5,calbindin D28k,PMCA1b and NCX1 in kidney cells through PTH receptor(PTHR)-mediated signaling pathway.PTH also stimulates the production of 1,25-dihydroxyvitamin D3 in proximal tubules.1,25-Dihydroxyvitamin D3,produced from kidney and other organs,down-regulates the expression of TRPV5,NCX1 and calbindin D28k in kidney cells. The expression level of PMCA 1b in these cells,however,is not significantly affected by 1,25-dihydroxyvitamin D3.Additionally,animal experiments also suggest that certain estrogen can increase the expression of renal reabsorption-related proteins,such as TRPV5,PMCA1b,calbindin D28k and NCX1[18,55].
2.3.Bone calcium regulation
Besides intestinal absorption and renal reabsorption of calcium,bone resorption is an important mechanism to modulate calcium level in blood.Bone is constantly remodeled by osteoblasts and osteoclast.Osteoblasts facilitate bone formation,while osteoclasts break bone tissue and release calcium.The development and activation of osteoclasts are mediated by receptor activator of NF-κB(RANK)ligand(RANKL).The expression of RANKL is promoted by vitamin D3,PTH,PTHrP,TNF- α,IL-1,IL-6,IL-11 and IL-17 and inhibited by TGFβ[57–62].On the other hand,the activity of RANKL is also inhibited by osteoprotegerin(OPG),a secreted protein functioning as a decoy receptor for RANKL.OPG disrupts the interaction between RANK and RANKL by competitively binding to RANKL,and functions as an inhibitor for the development and activation of osteoclasts.The expression level of OPG in human bone marrow cells is inhibited by 1,25-dihydroxyvitamin D3andPTH[63–65].Thus,1,25-dihydroxyvitamin D3 and PTH modulate calcium resorption in bone by increasing RNAKL expression and decreasing OPG expression.
2.4.Calcium homeostasis by CaSR regulation
Calcium level in blood is mainly maintained by CaSR located in the parathyroid gland.CaSR belongs to family C of Gprotein coupled receptor.Other members in this family include metabotropic glutamate receptor,GABABreceptor and taste receptor[66–69].CaSR has seven transmembrane domains.The length of the extracellular and intracellular domain of CaSR varies in different cell types due to alternative splicing.The Nterminal of extracellular domain of CaSR contains more than500 residues.It interacts with multiple ligands,indicating that this protein has multiple functions in sensing micro-environmental changes.CaSR is usually expressed as homodimer or heterodimers in the plasma membrane.A cysteine rich region located in the extracellular domain of CaSR is critical for the dimerization of CaSR[70,71].
The C-terminal of intracellular domain of CaSR interacts with many cell signaling proteins[72].Protein kinase C regulates CaSR functions by the phosphorylation of several serine residues in the intracellular domain.An ubiquitin ligase,dorfin,dynamically interacts with intracellular domain of CaSR,and regulates trafficking and degradation of CaSR[73].Like other G-protein coupled receptors,CaSR is phosphorylated by G protein receptor kinases(GRKs)in the second or third loop[72].The β-arrestin binds to the phosphorylated loop and blocks the interaction between CaSR and G-protein.The β-arrestin binding also facilitates the receptor internalization and activates ERKs in a G protein-independent manner[74].Filamin is a scaffold protein that interacts with CaSR and modulates CaSR signaling[75].CaSR also interacts with caveolin-1,a 22 kDa transmembrane protein extensively expressed in caveolea[76],and regulates the expression and activation of inducible nitric oxide synthase(iNOS)[77].Caveolin-1 also can be involved in trafficking of cholesterol and sphingolipids,and serve as a scaffold protein to modulate signaling transduction.However,the functions of intestinal chloride ion channel and exchangers regulated by CaSR are not clear yet,although CaSR is able to modulate Ca2+and IP3 dependent Cl−current in CaSR-overexpressed oocytes[78].
One of the major functions of CaSR is regulating systemic Ca2+homeostasis[79,80].Ca2+is the primary ligand of CaSR.At least four calcium-binding sites are reported in the extracellular domain of CaSR.CaSR cooperatively binds to Ca2+when extracellular concentration of Ca2+increases[81,82].CaSR is also able to sense Mg2+,amino acids,pH,antibiotics and peptides,and subsequently activates downstream Gi(G inhibitory)and Gqas well as G12/13pathways[80].The activation of CaSR can inhibit cAMP production,activate ERK pathway through Gipathway,activate PLC-IP3 cascades,and release Ca2+from ER via Gqpathway.It also can activate Rho and phospholipids D by G12/13pathway[72].In healthy individuals,a slight change of calcium concentration in the extracellular fluid can trigger the downstream signaling of CaSR in the parathyroid gland,which can further induce the change of PTH secretion and subsequently influence the cells in intestine,kidney and bone to adjust the concentration of extracellular calcium[70,83].
CaSR regulates extracellular calcium levels in several aspects(Fig.1).First,CaSR regulates the secretion of PTH that has direct effects on calcium reabsorption in kidney and bone remodeling.Second,the increase of PTH stimulates the production and secretion of 1,25-dihydroxyvitamin D3 in proximal tubules as the key molecule for the regulation of the intestinal calcium absorption,kidney calcium reabsorption and bone calcium release[83].Third,the activation of CaSR,in some cases,inhibits the activity of osteoclasts,which further suppresses bone calcium release[84].Forth,CaSR further modulates extracellular calcium level by regulating calcitonin selection in thyroidal C-cells[85,86].Moreover,CaSR is highly expressed in the digestive system including pancreas,stomach,small intestine and large intestine.In small intestine,CaSR modulates motility and development of intestine,NaCl and H2O transport,Ca2+/Mg2+absorption and nutrient absorption[87].Furthermore,CaSR expressed in renal tubules also can directly regulate the filtering and reabsorption of metal ions,including calcium[70].
3.Calcium diets,supplements,vitamin D and diseases
Food is an important source for calcium uptake.The common calcium-rich foods include milk,yogurt,cheese,shrimp,soybean,soy milk,tofu,broccoli,orange,kale and others.The major forms of calcium supplements are calcium carbonate and calcium citrate.Americans at all ages,however,do not take enough dietary calcium compared with the recommendations by the Institute of Medicine[17].Sufficient calcium intake is important for human health and calcium deficiency could lead to diseases,such as osteoporosis and rickets[88,89].
Bone is a living and constantly remodeling tissue[90].The old or damaged bone is resorbed by osteoclasts and the new bone is constructed by osteoblasts[56].Bone is primarily composed of organic components and inorganic components.The organic components are consisted mainly of type I collagen responsible for bone flexibility.The inorganic components comprised of hydroxyapatite,insoluble salts containing calcium and phosphorus provide bone strength against compression[90].The concentration of calcium in serum is usually kept in a very limited range so as to prevent the disorder of some physiological functions,such as muscle contraction[91].Bone is a mineral reservoir for calcium and phosphorus.Over 99% of total calcium in human body is stored in bone and teeth.Calcium plays important roles in the formation process of new bone and maintenance of existing bone by collaborating with other factors such as phosphorus,vitamin D and calcium-binding proteins[92].
Dietary calcium intake is critical for the calcium homeostasis of bone.Supplementary diets containing calcium with average recommended dietary allowance for children increase bone mineral density(BMD)and reduce the risk of fracture[93].As reviewed in the previous section,calcium homeostasis is well maintained in the cells from intestine,kidney and bone.When serum calcium level is low,CaSR promotes the secretion of PTH and indirectly increases 1,25-dihydroxyvitamin D3 level.When dietary calcium intake from intestine is low,blood calcium level is maintained by kidney reabsorption and bone calcium release at the expense of bone strength[94].On the other hand,lack of vitamin D could cause serious health problems.Low vitamin D level limits the synthesis of 1,25-dihydroxyvitamin D3,which further reduces blood calcium level through the inhibition of intestinal calcium absorption and renal calcium absorption.The PTH level,however,increases in response to the reduced blood calcium level.The increase of PTH promotes bone remodeling,while low calcium inhibits bone mineralization,correspondingly leading to the increased osteoid[94].
Some studies have demonstrated that patients with specific diseases or disease treatments may have skeletal abnormalities.For example,valproate,a chronic antiepileptic therapy,may cause low bone mass in pediatric patients and sufficient intake of calcium can offset this harmful effect[95].BMD is significantly lower in patients with Parkinson’s disease than the healthy people and the lower BMD is correlated with severe progression of the disease[96].The loss of BMD is the early sign of osteopenia that can turn into osteoporosis.Osteoporosis can lead to fracture and other severe bone diseases.Current studies reveal that adequate intake of calcium can decrease the risk of osteoporosis,fracture and diabetes in some cases[92].
The role of calcium in cancer has been explored for almost 100 years[97].The relationship between calcium and cancer,however,is still controversial.A previous randomized trial has demonstrated that the supplementation of calcium and vitamin D for seven years has no effects on colorectal cancer[98].High calcium intake,however,seems to reduce the risk of breast cancer and the increase the risk of prostate cancer[99].Breast cancer is the most common cancer in women in the United States and China[100].Early diagnosis,prognosis and treatment of cancers through biomarkers,such as HER-2 and gastrin-releasing peptide receptor(GRPR),are actively studied in animal models[101–106].On the other hand,cancer prevention through diet interventions is a hot topic with the aim of reducing and preventing cancer occurrence.High calcium intake seems to reduce the risk of some cancers,such as breast cancer[99].Vitamin D and calcium shows anti-breast cancer effects through regulating signaling pathways associated with cell proliferation,invasion and apoptosis.Epidemiological studies show that the intake of dietary and supplementary calcium and vitamin D reduces the risk of breast cancer.Additionally,the levels of serum calcium and vitamin D metabolites are inversely correlated with breast cancer[107].The mutations in vitamin D receptor and calcium sensing receptor are also identified in breast cancer tissue,suggesting the involvement of calcium and vitamin D signaling in breast cancer[107–109].TRPV6 can exhibit the up-regulation by 2–15 folds in breast cancer tissue when compared with that in normal breast tissue.The expression level of TRPV6 is reduced in breast cancer cell lines in the presence of tamoxifen,an antagonist of estrogen receptor[110],and can be up-regulated by 1,25-vitamin D.The overexpression of TRPV6 is also observed in the highly invasive area of breast cancer[111].Therefore,further studies on the relationship between dietary vitamin D/calcium and TRPV6 in breast cancer are highly desired.
Additionally,dietary calcium involves in cardiovascular diseases.High calcium intake could induce fatty acids and bile to bind to calcium,which further inhibits intestinal calcium absorption and therefore reduces cholesterol level in blood[112–114].Blood calcium also regulates blood pressure by modulating renin-angiotensin system[113–115].
Whether calcium supplements can be beneficial or harmful to the health of people is still controversial.Although the effects of calcium supplements or casual calcium uptake on health outcomes have been systematically reviewed[99],the risks of calcium supplements for cardiovascular diseases have not been completely understood[116].High calcium intake can slightly improve BMD in children and pregnant woman.There is no consistent conclusion between calcium intake and cardiovascular diseases,except blood pressure.Similarly,although some studies show that people with high calcium intake has lower chance of overweight and obesity[117],the relationship between calcium and obesity is still controversial.Calcium supplementation in diets can contribute to the reduced rate of bone loss and fracture incidence in elders;however,it can also increase the risks of acute gastrointestinal events,kidney stone,and cardiovascular diseases such as myocardial infarction and stroke[118,119].Based on the meta-analysis,only 10% fracture incidence is reduced due to the calcium supplementation,but the incidences of myocardial infarction and stroke are increased up to 27%–31% and 12%–20%,respectively[119].Moreover,high calcium intake for men also has the potential for the risk of advanced and fatal prostate cancer[17,120,121].
4.Conclusion
Calcium is the most abundant mineral in human body with 99% deposit in bone.The intestinal absorption,kidney reabsorption and bone resorption are three major events for calcium homeostasis regulated by CaSR through a series of complicated mechanisms.Any miscommunication of these processes can lead to diseases associated with dysfunctional regulation of calcium.In addition,calcium in diets and supplements along with vitamin D play critical roles in calcium homeostasis.Low calcium intake or low vitamin D level can also result in bone diseases.High calcium intake can reduce the risk of breast cancer and contribute to the reduced rate of bone loss and fracture incidence in elders.On the other hand,although high calcium intake can reduce the risk of many diseases,it also can increase the risks of acute gastrointestinal events,kidney stone,and cardiovascular diseases such as myocardial infarction and stroke.Therefore,the consumption and supplementation of calcium should abide by the health status of individuals.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China(No.31571228),Chutian Scholar Program from Education Department of Hubei Province and Innovative Start-up Foundation from Wuhan Sports University to NC.
- 食品科学与人类健康(英文)的其它文章
- Bioactive peptides on endothelial function
- Phenolics extract of Tetrapleura tetraptera fruit inhibits xanthine oxidase and Fe2+-induced lipid peroxidation in the kidney,liver,and lungs tissues of rats in vitro
- Characterization of volatiles Tribolium castaneum(H.)in flour using solid phase microextraction–gas chromatography mass spectrometry(SPME–GCMS)
- Nutritional composition,in vitro antioxidant and anti-diabetic potentials of Breynia retusa(Dennst.)Alston
- Effects of antimicrobial and antioxidant activities of spice extracts on raw chicken meat quality
- GUIDE FOR AUTHORS

