Effects of antimicrobial and antioxidant activities of spice extracts on raw chicken meat quality
Huiyun Zhang,Jingjuan Wu,Xinyu Guo
Food and Bioengineering Department,Henan University of Science and Technology,Luoyang,Henan 471003,China
Abstract
Keywords:Spice extracts;Antimicrobial;Antioxidant;Raw chicken meat;TBARS values
1.Introduction
Chicken meat is favoured by consumers around the world because of its desirable nutritional qualities,such as a low fat content and a relatively high concentration of polyunsaturated fatty acids[1].Fresh meat products are usually marketed at refrigerated temperatures(2–5℃).Lipid oxidation and microbial growth may occur during refrigeration storage.Spoilage of fresh poultry meat is a financial burden to producers and requires the development of new methods to extend the shelf-life and overall safety/quality of the meat,which is the main problem faced by the poultry processing industry[2].
Lipid oxidation,which is initiated in the unsaturated fatty acids fraction in subcellular membranes,is a major cause of the deterioration and reduced shelf-life of meat products[3].Lipid oxidation may generate changes in meat quality parameters such as colour,flavour,odour,texture,and even nutritional value[4].In addition,meat and poultry products have frequently been found to be contaminated with microorganisms during the butchering and manufacturing process.These microorganisms produce undesirable quality changes in meats,especially in relation to lactic acid bacteria,a major bacterial group associated with meat spoilage[5].
Many synthetic preservatives,such as butylated hydroxylanisole(BHA),butylated hydroxyltoluene(BHT)and tertiary butylhydroquinone(THBQ),are currently being used to reduce microbial growth and thereby extend the shelf-life of meat.Because of the increasing consumer demand for“healthier”meals(free of conventional chemical preservatives),the use of natural preservatives and environmentally friendly technologies has been suggested[6].In recent years,much attention has been focused on extracts from herbs and spices,which have been used for centuries to improve the sensory characteristics and shelf-life of foods[7].Unlike synthetic compounds,natural preservatives obtained from spices are rich in phenolic compounds and they can enhance the overall quality of food by decreasing lipid oxidation and microbial growth.
Cloves and rosemary,which are commercially cultivated in China,are important aromatic spices.They are generally used as condiments to enhance the sensory quality of foods in China.In addition to their health benefits,which have been widely studied[8,9],the extracts from cloves and rosemary have been found to possess great antioxidant and antimicrobial activity[10–12].Furthermore,to the best of our knowledge,the antioxidant and antimicrobial effects of cloves or rosemary extracts,singly or combined,on fresh chicken breast meat have not been investigated.Thus,the objective of the present work was to determine the effects of cloves and rosemary,applied individually and/or in combination,on pH,microbiological analysis,colour,thiobarbituric acid reactive substances(TBARS)and sensory analysis during storage at 4℃.
2.Materials and methods
2.1.Materials
Dried cloves(Eugeniacaryophyllata)and rosemary(Rosemarinusofficinalis)were purchased from a local traditional Chinese pharmacy(Luoyang,China).
2.2.Chemicals and reagents
1,1-Diphenyl-2-picrylhydrazyl(DPPH),trolox,2,2'-azinobis(3-ethyl-benzothiazoline-6-sulonic acid)(ABTS),Folin-Ciocalteu’s reagent(FCR),sodium carbonate(Na2CO3),gallic acid,sodium nitrite(NaNO2),sodium hydroxide(NaOH),quercetin,butylated hydroxytoluene(BHT),thiobarbituric acid(TBA),and trichloroacetic acid(TCA)were purchased from Sigma Chemical Co.(St.Louis,MO,USA).Plate Count Agar,Violet Red Bile Glucose Agar,Buffered Peptone Water,de Man Rogosa&Sharpe Agar,and Glutamate Starch Phenol Red Agar were purchased from Yongxin Biological Technology Co.,Ltd.(Yixing,Jiangsu,China).Methanol and ethanol were obtained from Sinopharm Chemical Reagent Co.,Ltd.(Beijing,China).
2.3.Preparation of spice extracts
Aliquots(50 geach)of powdered and dried spices were mixed into 400 mL of 95%(v/v)ethanol for 12 h in enclosed flasks with constant shaking(100 rpm).After filtration with Whatman No.2 filter paper,the residue was re-extracted with an additional 200 mL of 95% ethanol for an additional 12 h and then filtered.The combined filtrates were concentrated in a rotary evaporator(RE 52AA,Yarong Biochemical Analysis Co.,Ltd.,Shanghai,China)(50℃)with a vacuum pump,and the extracts were freeze dried.Dried extracts were placed in sealed bottles and stored at 4℃ before use.The extracts were dissolved in 95% ethanol for analysis of antioxidant and antimicrobial properties and were dissolved in distilled water(1%,w/v)for application on chicken meat products.The extraction yields of the spice ethanolic extracts were calculated using the following equation:
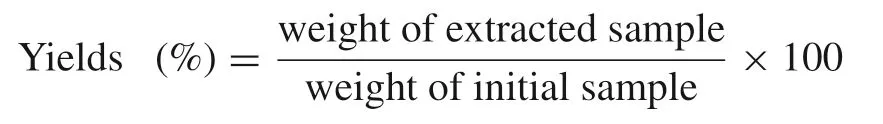
2.4.Analysis of spice extracts
2.4.1.Antioxidantactivity
2.4.1.1.DPPHradicalscavengingactivity.For the DPPH radical scavenging activity(RSA)assay,the procedure of Hatano et al.[13]was followed.Briefly,aliquots of 0.5 mL of the DPPH•solution(50 mg/mL)was mixed with 4.5 mL of methanol,and 0.1 mL of spice extracts at various concentrations(0.1–10.0 mg/mL)was added.The mixture was vortexed for 1 min and then left to stand at room temperature for 30 min in the dark,and the absorbance was read at 517 nm using a UV–vis spectrophotometer(Carry 100 UV-VIS,Agilent Technologies,Santa Clara,CA,USA)against a blank.The inhibitory percentage of DPPH was calculated according to the following equation:

The EC50value(mg/mL)was calculated as the concentration at which the DPPH radical scavenging activity was 50%.
2.4.1.2.Metalion-chelatingassay.The assay for metal chelation(Fe2+)was carried out according to the method of Wang and Xiong[14].Briefly,1 mL of 20μmol/L FeCl2was mixed with 2 mL of 50μmol/L ferrozine,which produces a chromophore that absorbs strongly at 562 nm.After the addition of 0.5 mL of spice extracts(0.1–10.0 mg/mL),the colour change was measured spectrophotometrically at 562 nm.The ability of extracts to chelate ferrous ion was calculated as follows:
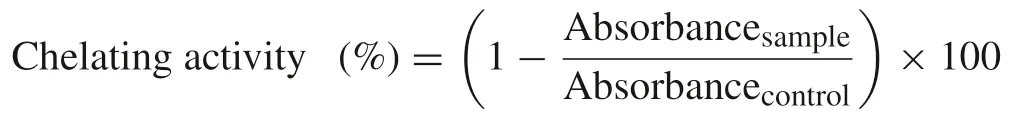
The EC50value(mg/mL)was calculated as the concentration at which the chelating activity was 50%.
2.4.2.Totalphenoliccontent
The Folin-Ciocalteu reagent assay was used to determine the total phenolic content of extracts[15].A 0.1-mL aliquot of the extract was mixed with 0.1 mL of Folin-Ciocalteu reagent(previously diluted three-fold with distilled water)and allowed to react for 3 min,and then 0.3 mL of 2% sodium carbonate(Na2CO3)solution was added.The mixture was allowed to stand for another 2 h before the absorbance was measured at 760 nm.Gallic acid was used as the standard for the calibration curve.The total phenolic content was expressed in mg gallic acid equivalents(GAE)per gram of sample(mg/g).All determinations were performed in triplicate.
2.4.3.Totalflavonoidcontent
The total flavonoid contents of the spice extracts were determined by a modified colourimetric method described by Sakanaka et al.[16],using quercetin as a standard.Extracts or standard solutions(250μL)were mixed with distilled water(1.25 mL)and 75μL of 5% sodium nitrite(NaNO2)solution,followed by the addition of 150μL of 10% aluminium chloride(AlCl3)solution 5 min later.After 6 min,0.5 mL of 1 mol/L sodium hydroxide(NaOH)and 0.6 mL distilled water were added.The solutions were then mixed,and the absorbance was measured at 510 nm.The results were expressed as mg quercetin/g of sample.All determinations were performed in triplicate.
2.4.4.Antibacterialactivityofextracts
2.4.4.1.Microbialstrains.Listeriamonocytogenes(NICPBP 54002),E.coli(strain ATCC 25922),Pseudomonasfluorescens(strain AS1.1802)andL.sake(strain AS1.80)stocks were obtained from the Microbial Research Institute of Chinese Academy of Science(Beijing,China).L.monocytogeneswas transferred into a sterile TSB-YE(tryptic soy broth-yeast extract)medium and incubated at 37℃ for 24 h.E.coliandP.fluorescenswere suspended in a sterile broth medium(1% peptone,0.3% beef broth and 0.5% sodium chloride)and incubated at 37℃ and 30℃,respectively,for 24 h.L.sakewas inoculated into a sterile MRS(Man-Rogosa-Sharpe)medium and incubated at 30℃ for 24 h.All media were purchased from Yongxin Biological Technology Co.,Ltd.(Yixing,Jiangsu,China).The bacterial population in all of the inoculated media was greater than 1×109CFU/mL after 24 h of incubation.
2.4.4.2.Antimicrobialactivitytestinginagarmedia.The antimicrobial activity of spice extracts was examined in triplicate using the well diffusion test[17]to detect the growth inhibition ofL.monocytogenes,E.coli,P.fluorescensandL.sake.The spice extracts were used singly or in combination.Tests for individual antimicrobials were carried out at the following diluted spice extract concentrations in 95% ethanol:80,40,20,10 and 5 mg/mL.A 95% ethanol solution was used as a control.The TSA-YE(tryptic soy agar-yeast extract)was used forL.monocytogenes,the agar medium(0.5% casein digest,0.25% yeast extract,0.1% dextrose and 1.5% agar)was used forE.coliandP.fluorescensand the MRS agar medium was used forL.sake.The inhibitory effect was assessed by measuring the disc diameter of the inhibition zone(clear zone)around the extract-containing steel cylinder using a vernier calliper[18].Sterile steel micro-cylinders(7.8 mm dia.×10 mm ht.)(Huanghai Medical Co.,Ltd.,Shanghai,China)were vertically set on the agar in the plates,and 0.1 mL of herb/spice extracts at each dilution was then aseptically transferred into the steel cylinders.The control was 0.1 mL of 95% ethanol alone.With the lid on,the plates were incubated at 30℃ for 24 h forP.fluorescensandL.sakeand at 37℃ for 24 h forL.monocytogenesandE.coli.
2.5.Sample preparation
Raw chicken breast meat fillets(70.1 g/100 g moisture,22.9 g/100 g protein,2.1 g/100 g fat content)were provided by a local poultry processing plant(Luoyang,China).They were placed in insulated polystyrene boxes on ice and transferred to the laboratory within 1 h of slaughtering.Breast fillets were then aseptically cut to 25 g portions[19].The samples were assigned to one of five treatments:C:control samples;PC:positive control with 0.02% BHT;T-CL:treatment with clove extract 1%(v/w);T-RO:treatment with rosemary extract 1%(v/w);or T-RO-CL:treatment with rosemary extract 0.5%(v/w)+clove extract 0.5%(v/w).Meat samples were aerobically packed in low-density polyethylene bags and stored at 4±1℃ for 15 days and analyzed for pH,instrumental colour attribute,microbial counts,thiobarbituric acid reactive substances(TBARS)and sensory attributes(taste and odour).The above-described experiment was carried out in triplicate.
2.6.Analysis of meat samples
2.6.1.pHdetermination
pH levels were determined according to AOAC(1995).Specifically,a 10.0 g sample of the meat muscle was homogenized in 100 mL distilled water,and the mixture was filtered.The pH of the filtrate was measured using a pH meter(Mettler Toledo 320-S,Shanghai Mettler Ltd.,China).
2.6.2.Colourvalues
The colour of the raw chicken breast meat fillets was determined using a Colour Difference Meter(WSC-S,Shanghai Physics and Optics Instrument Co.,Shanghai,China).Colour was described in terms of theL*(lightness),a*(redness),andb*(yellowness)colour space values.Measurements were made perpendicular to the fillet surface at five different locations per sample;mean values(L*,a*,andb*)from the samples were analyzed,and triplicate fillets were analyzed to obtain an average colourimetric value.
2.6.3.Microbiologicalanalysis
Samples were submitted to microbial analysis immediately after inoculation and again after 3,6,9,12,and 15 days of refrigerated storage.The following groups of microflora were examined:total viable counts(TVC),lactic acid bacteria(LAB),Enterobacteriaceae andPseudomonasspp.All microbiological analyses were performed using the standard procedures described in the Food and Drugs Administration(FDA)Bacteriological Analytical Manual(BAM),with some modifications(BAM,1998).Total viable counts(TVC)were determined using Plate Count Agar(PCA)after incubation for 48 h at 37℃.Enterobacteriaceae counts were determined using the plate counting method on Violet Red Bile Glucose Agar(VRBG)as a medium after 24 h of incubation at 37℃.Lactic acid bacteria were determined on de Man,Rogosa,and Sharpe(MRS)medium after 72 h incubation at 30℃.Pseudomonasspp.counts were determined using Glutamate Starch Phenol Red Agar(GSP)as a medium after 48 h of incubation at 30℃.All plates were examined visually for colony type and morphological characteristics associated with each growth medium.In addition,the selectivity of each medium was checked routinely by Gram staining and microscopic examination of smears prepared from randomly selected colonies from all of the media.Microbial colonies were counted and expressed as log 10 CFU(colony forming units)/g chicken meat.
2.6.4.Thiobarbituricacidreactivesubstances(TBARS)value
The TBARS value was determined according to Erkan and Özden[20],with some modifications.Approximately 5.0 g of meat was homogenized with 25 mL 7.5%(w/v)trichloroacetic acid(containing 0.1% EDTA)at 15,000 rev per minute.The mixture was centrifuged at 3600gfor 20 min at room temperature.The supernatant(5 mL)was mixed with 5 mL 0.02 mol/L TBA reagent.The mixture was heated in a boiling water bath for 30 min and cooled to room temperature.The absorbance of the resulting supernatant solution was measured using a UV spectrometer(2550,Shimadzu,Japan)at 532 nm against a blank prepared with 5 mL distilled water and 5 mL TBA solution.The amount of TBARS was expressed as mg of malondialdehyde(MDA)per kg meat sample.
2.6.5.Sensoryevaluation
Chicken meat samples(approximately 100 g)were cooked in a microwave oven at high power(700 W)for 4 min,including time for defrosting.A panel of seven judges experienced in chicken evaluation(laboratory-trained)performed sensory analysis.Panellists were asked to evaluate the taste and odour intensities of the cooked samples.Fresh chicken breast meat was served as a reference.Acceptability as a composite of odour and taste was estimated using a 9-point hedonic scale.Sensory evaluation was carried out in individual booths under controlled conditions.Water was provided for cleaning the palate between samples.The scale points were as follows:excellent,9;very good,8;good,7;acceptable,6;poor(first off-odour,off-taste development),6;a score of 6 was considered the lower limit of acceptability.A product was defined as unacceptable after the development of the first off-odour or off-taste.
2.7.Statistical analysis
Experiments were replicated twice using different batches of meat,and the specific analyses were conducted at least in duplicate.Data were subjected to analysis of variance(AOV)using the general linear model procedure to determine treatment effects.When a treatment was found to be significant(P<0.05),differences between sample means were identified using the least significance difference method.
3.Results and discussion
3.1.Extraction yield,antioxidant and antibacterial activity of spice ethanolic extracts
3.1.1.Extractionyield
There are numerous antioxidant methods and variations thereof for evaluating antioxidant activity.Among these,antioxidant activity,reducing power,and DPPH scavenging activity are most widely used for determining the antioxidant activity of extracts[21].Table 1 shows the yields and antioxidant activities of the spice ethanolic extracts tested.The results show that the extraction yields of ethanolic extracts from cloves was slightly higher than that from rosemary.The chemical composition of the spice extracts used in this work was previously determined.In the cloves extract,the main components were eugenol,caryophyllene and benzene,which are responsible for antimicrobial and antioxidant activity[22].In the rosemary extract,the predominant compounds were di-terpenes,volatile oils,and phenolic acids,which are responsible for antimicrobial and antioxidant activity[23].

Table 1Extraction yield,DPPH radical scavenging ability,ferrous ion-chelating ability,total phenolic content and total flavonoid content for ethanol extracts of cloves and rosemary.
3.1.2.Antioxidantactivity
DPPH is a free radical compound widely used to determine the free radical scavenging ability of various extracts[24].When DPPH is scavenged and transformed into DPPH-H,the colour of the solution changes from purple to yellow,and the degree of change can be detected by the decrease in absorbance at 517 nm[25].The results of the experiments are shown in Table 1.Cloves exhibited a higher DPPH radical scavenging activity than that of rosemary.Extracts of cloves have been reported in previous studies to be one of the strongest antioxidants,even stronger than synthetic antioxidants such as butylated hydroxytoluene or butylated hydroxyanisole[26].The strong activity of cloves may be caused by the presence of eugenol,the main constituent of cloves,which is known for its antioxidant activity.
Among the transition metals,iron is known as the most important lipid oxidation pro-oxidant.The ferrous state of iron accelerates lipid oxidation by breaking down hydrogen and lipid peroxides into reactive free radicals via the Fenton reaction[27].The ferrous ion-chelating activities of the extracts are shown in Table 1.With respect to the EC50values of the ferrous ion-chelating capacity,rosemary exhibited a higher ferrous ionchelating ability than cloves did(Table 1).The total phenolic and total flavonoid contents of rosemary were lower than those of cloves.However,the ferrous ion-chelating effect of rosemary was significantly higher than that of clove.Factors affecting the ion-chelating ability of spice extracts are complex.The main mechanism of ion-chelating activity is the ability to deactivate and/or chelate transition metals,which can promote the Fenton reaction and hydroperoxide decomposition.
3.1.3.Antibacterialactivityofspiceextracts
The antibacterial activity of spice extracts was measured using the agar well diffusion assay.Spice extracts showed various degrees of inhibition against the four meat pathogenic and spoilage organisms(Table 2).The results show thatL.monocytogenes(gram-positive)was the most sensitive among the four strains of bacteria used in the test.Listeriamonocytogeneshas emerged as a serious food-borne pathogen and is widely distributed in the environment.Because the pathogen survives and can grow over a wide temperature range,including refrigerator temperatures,L.monocytogenesposes a real threat as a post-process contaminant in ready-to-eat meat products.
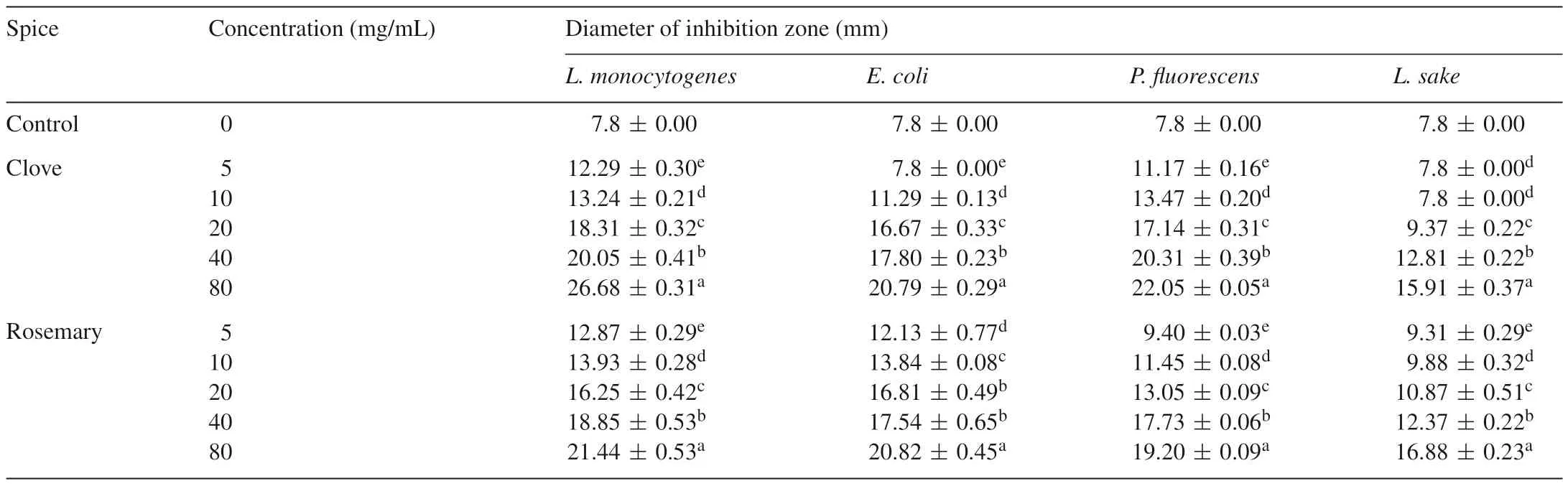
Table 2Antibacterial activities of ethanol extracts of spices for chosen bacterial strains.
It was been shown that essential oils and spice extracts are more active against gram-positive than gram-negative bacteria[28,29].Part of our results are consistent with those reported in previous studies.For example,L.monocytogenes(grampositive)was sensitive to the spices tested,whereasE.coli(gram-negative)was somewhat resistant.The sensitivity difference between the two groups of bacteria occurred because gram-negative bacteria possess an outer membrane and a unique periplasmic space not found in gram-positive bacteria[28].However,L.sake(gram-positive)was found to be the most resistant among the four strains of bacteria used in the test,which did not closely follow the trend described above.
3.2.Application of extracts in raw chicken meat samples
3.2.1.pH
Fig.1 shows the effect of spice extracts on the pH of raw chicken meat samples during refrigerated storage at 4℃ for 15 days.The initial pH value of the control and samples submitted to various treatments was found to be 5.65±0.05.The pH values(P<0.05)of the two control samples(C and PC)were found to increase from 5.65±0.05 to 6.66±0.02 and from 5.65±0.05 to 6.37±0.04,respectively,at the end of the storage period.Spice extract treatments inhibited the increase in pH to some extent during the storage period.Treatment with RO-CL had the best effect,which caused the pH to reach a level of only 5.48±0.06 compared with that measured for the control(C),6.66±0.02.No significant difference was measured between the samples treated with CL and RO(P>0.05),which reached final pH levels of 5.62±0.03 and 5.58±0.02,respectively.The pH increase(P<0.05)of the control samples may have been caused by the utilization of amino acids by bacteria,which are released during protein degradation because the stored glucose has been depleted.Accumulation of ammonia and the products of amino acid decomposition result in an increase in pH[30].The lower pH measured for chicken meat submitted to the CL,RO and RO-CL treatments is attributed to the inhibitory effect of antimicrobial ingredients found in natural spice extracts on the growth and proliferation of spoilage microorganisms that metabolize basic nitrogen compounds.

Fig.1.Effect of spice extracts on pH of raw chicken meat during storage at 4℃.
3.2.2.Colourvalues
The CIE colour values of raw chicken meat samples with/without spice extracts are shown in Figs.2–4.The lightness(L*)values(Fig.2)of the chicken meat samples was slightly affected by the addition of spice extracts.TheL*values(P<0.05)increased gradually over the storage period.However,the control samples showed decreasedL*values at the end of the storage period.TheL*values of samples treated with spice extracts were significantly(P<0.05)higher than those of the control over the entire storage period.Similarly,Valencia et al.[31]reported an increase in theL*value of fresh pork sausage containing green tea catechins and green coffee antioxidants relative to the value measured for a control.
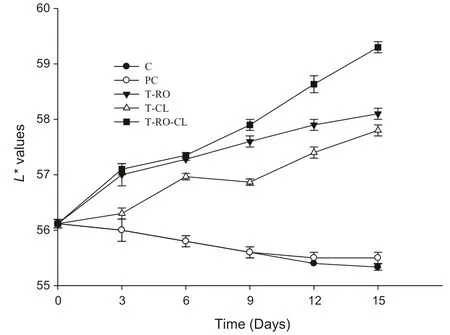
Fig.2.Effect of spice extracts on colour parameter L*in raw chicken meat during storage at 4℃.

Fig.3.Effect of spice extracts on colour parameter a*in raw chicken meat during storage at 4℃.
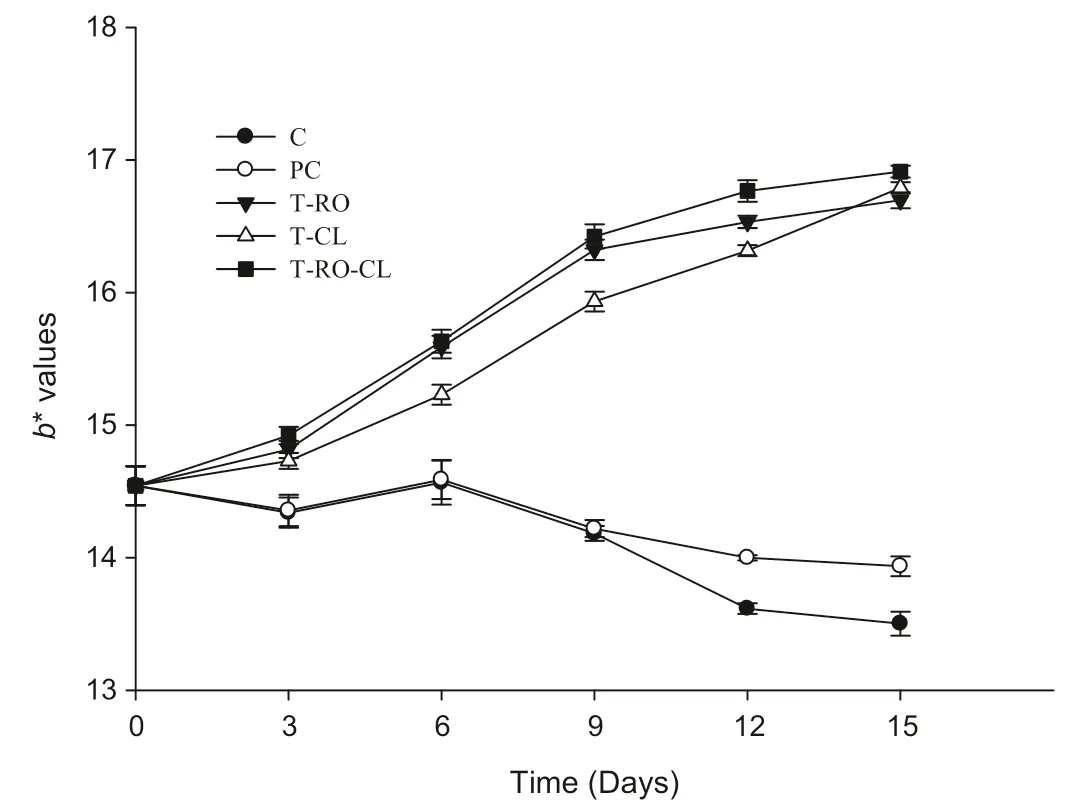
Fig.4.Effect of spice extracts on colour parameter b*in raw chicken meat during storage at 4℃.
As expected, chicken meat samples treated with spice extracts showed an intense red colour,and thereby higher values ofa*(Fig.3),than the control chicken meat samples because of the carotenoids in the spices.Similarly,Keokamnerd et al.[32]reported a decrease in thea*value of ground chicken during 12 days of storage.A significant(P<0.05)reduction in thea*value of all samples with increased storage time was observed in this study,although the control showed the lowesta*value at the end of the storage period.Tesoriere et al.[33]found that phenolic extracts from capers effectively inhibited the transition of myoglobin to its hypervalent state of ferrylmyoglobin,indicating a potential interaction between some phenolic compounds and heme protein redox reactions.The reduction in the intensity of red colour during storage could be caused by the interdependence between lipid oxidation and colour oxidation in meats[34].Pigment oxidation may facilitate lipid oxidation,and free radicals produced during oxidation may oxidase iron atoms or denature myoglobin molecules,adversely affecting the colour of meat products.
Samples treated with spice extracts also showed significantly increasedb*values(Fig.4)over the entire storage period.Theb*values of samples treated with spice extracts were higher than the value measured for the control throughout the entire storage period,which may have been caused by the presence of slight colour compounds in the spice extracts.It was also evident that the colour was significantly(P<0.05)stabilized by the inclusion of spice extracts,which significantly increased(P<0.05)the yellowness of the chicken meat samples.Maqsood et al.[35]similarly reported that the addition of kiam wood extract resulted in slightly darker fish emulsion sausages.Gibis and Weiss[36]also reported observing a discolouration of fried beef patties upon the addition of natural antioxidants such as grape seed extracts.Although the colour changes related to lipid oxidation are widely reported,the changes observed in this study cannot be definitively ascribed to lipid or protein oxidation because of the dark yellow colours of the extracts themselves.
3.2.3.Microbialanalysis
The changes in TVC,LAB,Enterobacteriaceae,andPseudomonasspp.of raw chicken meat with or without spice extracts and combinations thereof during refrigerated storage at 4℃ are shown in Fig.5.The initial TVC(P<0.05)for all samples was observed to be 5.41 log CFU/g.This value increased(P<0.05)steadily during storage,reaching 7.22 and 7.07 log CFU/g for the C and PC samples at the end of 15 days.ANOVA showed a significant effect of the antimicrobial treatments(CL,RO,RO-CL)and storage time on TVC(P<0.05)(Fig.5).Chicken samples reached or exceeded a TVC value of 7.0 log CFU/g,which was considered the upper acceptability limit for fresh meat[37]on days 12(C)and 12(PC),whereas the T-CL,TRO and T-RO-CL chicken samples never reached this limit after the 15-day storage period.TVC values were found to be 6.32,5.88,and 5.75 log CFU/g for the T-RO,T-CL and T-RO-CL samples,respectively,at the end of storage.The results show that the TVC values decreased significantly with the addition of the spice extracts,the effectiveness of which increased when the extracts were used in combination.
Fermentative LAB,which is able to grow both in the presence and absence of oxygen,constituted a substantial part of the natural microflora of chicken meat(Fig.5).LAB metabolism produces lactic acid,causing the development of sour flavours less offensive than putrid flavours formed through the metabolism of aerobic bacteria.Initial lactic acid bacteria(LAB)counts(P<0.05)were found to be 4.26 log CFU/g for all meat samples.The values increased in C and PC samples and reached 6.20 and 5.96 log CFU/g at the end of the storage period(Fig.5).LAB counts were found to be lower(P<0.05)in spice-treated samples relative to those measured for the control samples.During storage,chicken fillet samples treated with RO-CL showed a lower LAB count compared with the counts measured for the control and CL-and RO-treated samples.On day 15 of storage,LAB counts reached 5.47,5.43 and 5.08 log CFU/g for the RO,CL,and RO-CL samples,respectively.LAB are the most resistant bacteria among gram-positive bacteria against the antimicrobial action of EOs[38].Frangos et al.[39]reported that the high resistance of the LAB could be related to their ability to tolerate conditions of osmotic stress and respond effectively to the K+efflux caused by many EOs.Holley and Patel[40]also reported that the high tolerance of LAB toward the action of essential oils is attributed to their ability to generate ATP and to tolerate conditions of osmotic stress.

Fig.5.Antimicrobial activity of spice extracts against TVC,LAB,Pseudomonas spp.and Enterobacteriaceae counts in raw chicken meat during storage at 4℃.
Pseudomonads are gram-negative strict aerobic bacteria comprising the main spoilage microorganism of poultry meat stored under refrigeration([41],chap.2,4).Growth of Pseudomonads causes the development of highly unpleasant putrid flavours.Counts ofPseudomonasspp.(P<0.05)are presented in Fig.5.As expected,the RO extract showed a low inhibition effect.On the other hand,the CL and RO-CL samples showed considerable inhibition effects on the Pseudomonads.The combination of spice extracts was the most effective treatment in reducing the Pseudomonad population from 6.05(control samples)to 4.76 log CFU/g after 15 days of storage(P<0.05).In partial agreement with our results,Dabbah et al.[42]reported that citrus fruit oils completely eliminatedP.fluorescensin nutrient broth,whereas a 75% reduction occurred in the initial inoculum ofPseudomonasaeruginosa.Such differences may be attributed to the pure culture conditions used in the abovementioned study versus the growth conditions forPseudomonasspp.in ground chicken used in the present study.Food constituents such as fat usually exhibit a protective effect on microorganisms[43].Del Rio et al.[44]reported a decrease of 1.4 log CFU/g in Pseudomonads’population immediately after treatment of chicken legs with a solution of citric acid(2 mL/100 mL),whereas after 5 days of storage the authors reported a reduction of 0.7 log CFU/g relative to the value measured for a control sample.Emiro˘glu et al.[45]reported that an initialPseudomonasspp.count of 6.36 log CFU/g was reduced to 5.62 and 5.23 log CFU/g for ground beef patties coated with oregano and thyme EOs,respectively,at the end of storage.
Enterobacteriaceae,a hygiene indicator[46],were also part of the microflora of chicken meat(Fig.5).The initial population of Enterobacteriaceae(3.27 log CFU/g)is indicative of adequate hygiene conditions of production in the poultry plant.Enterobacteriaceae can grow under vacuum packaging and high pH values in meat and produce high levels of H2S,giving meat unpleasant odours.The growth pattern of Enterobacteriaceae,facultative anaerobic bacteria,was similar to that of LAB.The counts(P<0.05)obtained from spice treated samples were lower than those measured for the control samples.The combination of cloves and rosemary was the most effective in reducing the Enterobacteriaceae count.The final counts were 4.80,4.62,4.46,4.30 and 4.11 log CFU/g for C,PC,RO,CL,and RO-CL samples,respectively.
The mechanism of action for the antibacterial activity of spice extracts is not entirely clear;however,membrane disruption by phenolics and metal chelation by flavonoids are considered to inhibit the growth of microorganisms.Some researchers have reported that phenolic compounds from different plant sources could curb the effects of various food-borne pathogens,and total phenolic content has been strongly associated with antimicrobial activity[29].The antimicrobial activities of phenolic compounds may involve multiple modes of action.For example,phenolic compounds can break down the cell wall,disrupt the cytoplasmic membrane,cause leakage of cellular components,alter fatty acid and phospholipid constituents,influence the synthesis of DNA and RNA and destroy protein translocation[29].
The stronger antimicrobial activity of the mixed spice extracts was noticed when compared with the individual activities of the respective extracts.The strong antimicrobial effect of the combination of the clove and rosemary extracts observed in the present study could be a result of synergistic actions of specific compounds present in the mixed spices.Synergistic inhibitory effects on food-borne bacteria have been observed when spice extracts are combined.
3.2.4.TBARS
TBARS analysis determines the formation of secondary products of lipid oxidation,mainly malondialdehyde,which may contribute to the off-flavour of oxidized fat.Fig.6 illustrates the antioxidant effects of spice ethanolic extracts on the TBARS values of chicken fillets during storage(4℃)for 15 days.At day 0,the TBARS values(P<0.05)were found to be the same for all meat samples.The TBARS values of all samples increased significantly with the extension of the storage period.The TBARS values of all treated samples were significantly lower(P<0.05)than those of the negative(C)and positive controls(PC)for each sampling during storage,showing that the different spice ethanolic extracts had highly protective effects against lipid oxidation in chicken fillets.The inhibition effect was stronger(P<0.05)in the T-RO-CL samples than in the T-RO and T-CL samples at all storage times.Analysis of variance showed that the TBARS values were significantly affected(P<0.05)by both storage and treatment.These results suggest that these antioxidants delayed lipid oxidation during storage.
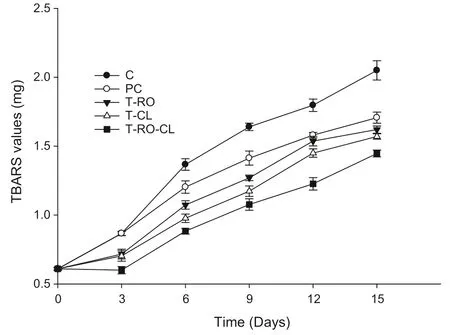
Fig.6.Effect of spice extracts on TBARS values of raw chicken meat during storage at 4℃.
Natural antioxidants are believed to interrupt free radical chains by offering hydrogen from the phenolic groups,result in the formation of a stable end product.This reasoning is in line with previous results showing that mustard leaf possesses antioxidant activity in foods because of its high content of phenolic compounds[47].Similarly,Tajik et al.[48],who examined the strong antioxidant effects of cloves and grape seed extracts,reported that the antioxidant potential of these extracts significantly inhibited TBA values in silver carp fillets.Negi and Jayaprakasha[49]reported a significant relationship between the phenolic content and antioxidant effect of pomegranate peel extract.Brannan[50]reported the antioxidant effects of grape seed extract in chicken patties.Although antioxidant activity is reported to be associated with phenolic compounds in fruits,a possible synergism between phenolic compounds and other compounds might be responsible for these observations.
3.2.5.Sensoryevaluation
The results presented in Table 3 show the score changes for odour and taste acceptance of cooked chicken samples submitted to different treatments during refrigerated storage.Sensory scores for the odour and taste of cooked chicken decreased with the duration of refrigerated storage(Table 3).Odour and taste scores for chicken,regardless of treatment,showed a similar pattern of decreasing acceptance(Table 3).Throughout the entire period of refrigerated storage,all spice-extract-treated(T-RO,T-CL and T-RO-CL)chicken samples received higher sensory scores(P<0.05)than the control(C)and positive control(PC)samples did,as judged by both odour and taste attributes.The limiting acceptability score of 6.0 for odour was reached after 6 days for the control samples and after 12 days for the samples treated with spice extracts.The same pattern of decreasing acceptability was observed for taste(Table 3).Both odour and taste proved to be equally sensitive sensory properties for chicken meat as indicated by the sensory scores in Table3.The appearance scores for all chicken samples decreased at a slower rate than did the odour and taste scores(results not shown).Higher microbial counts in samples without spice extracts might be the reason for their early spoilage.Moreover,lipid oxidation products and the production of ammonia from protein breakdown by microbes may have resulted in the production of off-odour,which may have been the cause for the poor score of samples not receiving treatment on the 15th day of storage.

Table 3Effect of spice extracts on sensory characteristics(odour and taste)of raw chicken meat stored at 4℃.
4.Conclusion
The results demonstrate the effectiveness of clove and rosemary extracts in inhibiting microbial growth,reducing lipid oxidation,maintaining or improving sensory characteristics and extending the shelf-life of raw chicken meat during storage at 4℃ for 15 days.The antioxidant properties of ethanolic spice extracts showed that cloves had good antioxidant activity with higher polyphenol and flavonoid contents and was significantly different from the rosemary extracts.The strongest preservative effect was achieved by combining the spice extracts(T-RO-CL)(shelf-life extension of ca.6 days compared with the shelf-life of control samples),indicative of the additive effect of combining the two.Because of the claimed salubrious attributes of these highly effective spice extracts,their application in the development of novel functional healthy meat products may be highly valuable and desirable.
- 食品科学与人类健康(英文)的其它文章
- Bioactive peptides on endothelial function
- Calcium intake,calcium homeostasis and health
- Phenolics extract of Tetrapleura tetraptera fruit inhibits xanthine oxidase and Fe2+-induced lipid peroxidation in the kidney,liver,and lungs tissues of rats in vitro
- Characterization of volatiles Tribolium castaneum(H.)in flour using solid phase microextraction–gas chromatography mass spectrometry(SPME–GCMS)
- Nutritional composition,in vitro antioxidant and anti-diabetic potentials of Breynia retusa(Dennst.)Alston
- GUIDE FOR AUTHORS

