Phenolics extract of Tetrapleura tetraptera fruit inhibits xanthine oxidase and Fe2+-induced lipid peroxidation in the kidney,liver,and lungs tissues of rats in vitro
Emmanuel Anyahukwu Ironi,Ganiyu Ooh,Samson Olalekan Agoola,Aline Augusti Boligon,Margareth Line Athaye
a Biochemistry Unit,Department of Biosciences and Biotechnology,Kwara State University,Malete,P.M.B.1530,Ilorin,Nigeria
b Functional Foods and Nutraceuticals Unit,Department of Biochemistry,Federal University of Technology,Akure,Nigeria,P.M.B.704,Akure 340001,Nigeria
c Department of Veterinary Physiology,Biochemistry and Pharmacology,University of Ibadan,Nigeria
d Phytochemical Research Laboratory,Department of Industrial Pharmacy,Federal University of Santa Maria,Building 26,Room 1115,Santa Maria CEP 97105-900,Brazil
Abstract
Keywords:Tetrapleura tetraptera;Phenolics;Xanthine oxidase;Hyperuricaemia;Lipid peroxidation
1.Intro duction
Experimental evidence has shown that elevated concentration of uric acid,otherwise known as hyperuricaemia,leads to the deposition of monosodium urate monohydrate crystals in tissue,especially joints,thereby resulting in gouty arthritis or uric acid nephrolithiasis[1,2].Gout is a chronic inflammatory arthritis characterized by elevated concentration of uric acid in body fluids,resulting from the over-activity of xanthine oxidase(XO)[3].It is also characterized with severe and episodic painful inflammation[4]:erythema and swelling[5].Epidemiological studies have shown that the overall burden of the disease is increasing globally[2].It is more prevalent in men above 30 years of age and in women older than 50 years[2,6].Moreover,it has the propensity to reduce the quality of life of these individuals[7].In addition to gouty arthritis,hyperuricaemia is also a well-established causative factor for uric acid kidney stones and acute kidney failure[8].Recent epidemiologic studies have also implicated chronic mild hyperuricaemia in the development of interstitial nephritis and progressive renal failure[9].Furthermore,it is an independent risk factor for metabolic syndrome,cardiovascular disease,hypertension,obesity,obstructive sleep apnea,stroke,vascular dementia,and preeclampsia[10].
Xanthine oxidase(XO)(EC 1.1.3.22)catalyzes the oxidation of hypoxanthine to xanthine and subsequently to uric acid[11]in the purine nucleotides catabolism.Its re-oxidation involves molecular oxygen which acts as electron acceptor,and during this reaction,superoxide radical(O2•−)and hydrogen peroxide(H2O2)are produced[12].The O2•−is transformed into H2O2and O2either spontaneously or by the catalytic action of superoxide dismutase.Thus,the over-activity of XO leads to the deposition of uric acid in the susceptible tissues,and this triggers the inflammatory pathways with a concomitant release of reactive oxygen species.Hence,gouty arthritis and other inflammatory diseases associated with hyperuricaemia are characterized by oxidative stress.The kidney,liver and lungs are three major organs in mammals involved in metabolism and excretion,and previous studies have reported XO activity in the tissues of these organs[6,13,14].Functionally,in these organs materials are chemically biotransformed and the metabolic wastes,such as carbon dioxide,water,salt,urea and uric acid,are removed from the body.
Clinically,anti-inflammatory agents are used to relieve the symptoms of gout,and XO inhibitors are used to block the synthesis of uric acid.These two approaches are common treatments of gout.Allopurinol,a purine analog,is the most common XO inhibitor that functions to reduce serum urate level[1].However,its use has some attendant side effects in patients,and for this reason it is usually contraindicated in patients with kidney or heart disease.The side effects include the risk of developing hypersensitivity syndrome that is characterized by renal impairment,hepatic dysfunction,fever,rashes,leucocytosis and eosinophilia[15].These limitations of allopurinol have necessitated research into alternative treatment strategies for gout that could be safer and effective.In this regard,medicinal plants are widely used to treat gout,as previous studies have demonstrated that several of them with high level of flavonoids and other phenolics compounds possess XO inhibitory activity[16–18].Plant-derived polyphenolics with antioxidant potential,including flavonoids and phenolic acids,can modulate the expression of pro-inflammatory signals and ameliorate inflammatory diseases such as arthritis[19,20].
Tetrapleuratetraptera,called“aidan”in the South-western part of Nigeria,and“ihokiriho”by the Ngwa people in the Southeastern part of Nigeria,is a deciduous tree belonging to the family Mimosaceae.It is generally distributed in the lowland forest of tropical Africa.The fruits,made up of a fleshy pulp with small,brownish-black seeds,are green when tender and dark brown when fully ripe and possess high nutritional value[21].When dry,they have a pleasant aroma,and therefore are used as spice in Central and West Africa[22].This spicy property makes them valuable for preparing soup for nursing mothers from the first day of birth to prevent post-partum contraction[23].Previous studies have demonstrated that different parts of the plant are used in ethnomedicine for the treatment of several ailments including diabetes mellitus,hypertension,intestinal parasites,malaria,asthma,epilepsy,schistosomiasis,wound healing and arthritis[24,25].Recent studies have also revealed that the pod possesses antioxidant and amylase inhibitory activities[21];the fruits and barks extracts also have antioxidant activities[22].
The aforementioned health benefits ofT.tetrapteramake it a promising functional food.Interestingly,functional foods of plant origin have continued to receive considerable research attention in the recent time due to their nutritional quality,therapeutic effects and presumed safety[26].Hence,to further explore the health benefits ofT.tetraptera,the present study characterized the phenolics ofT.tetrapterafruit,and evaluated the XO inhibitory activity of its phenolic extract in the kidney,liver and lungs tissues of ratsinvitro.
2.Materials and methods
2.1.Samples collection and preparation
Ripe fruits samples ofT.tetrapterawere harvested from the plant in Umueze area of Ekwereazu-Ngwa village in Obi-Ngwa local government area of Abia State,Nigeria,in November,2014.The fruits were botanically identified and authenticated at the herbarium of the Department of Botany,University of Ibadan,Nigeria.Subsequently,they were sun-dried for seven days,and the seeds were manually removed.The fruits were later milled into a fine particle size(0.5 mm)powder and packed in air-tight plastic vials,and stored at−4℃ until analysis.
Methanol,formic acid,gallic acid,chlorogenic acid,caffeic acid and ellagic acid purchased from Merck(Darmstadt,Germany).Catechin,epicatechin,quercetin,rutin,apigenin and luteolin;xanthine and allopurinol were acquired from Sigma Chemical Co.(St.Louis,MO,USA).All other chemicals used for analysis were of analytical grade.
2.2.Preparation of polyphenolics extract
Polyphenolics extract ofT.tetrapterafruits was prepared according to the method described by Kuo et al.[27].A portion ofT.tetrapterafruits powder(100 g)was extracted three successive times with 300 mL of methanol at 50℃ for 3 h,and the samples were filtered after each extraction with Whatman(No.2)filter paper.The combined extract was partitioned with 200 mL hexane in a separatory funnel to remove the lipids and some of the pigments.The aqueous phase was extracted three times with 180 mL ethyl acetate;after which it was evaporated to dryness at 45℃ under reduced pressure in a rotary evaporator.The residue from this step was redissolved in 250 mL water,and lyophilized to obtain approximately 5 g ofT.tetrapterafruits polyphenolics extract.The percentage yield of the extract was 5%.
2.3.Quantification of phenolic compounds using HPLC-DAD
High performance liquid chromatography(HPLC-DAD)was performed with a Shimadzu Prominence Auto Sampler(SIL-20A)HPLC system(Shimadzu,Kyoto,Japan),equipped with Shimadzu LC-20AT reciprocating pumps connected to a DGU 20A5 degasser with a CBM 20A integrator,SPD-M20A diode array detector and LC solution 1.22 SP1 software.
Reverse phase chromatographic analysis was carried out under gradient conditions using C18column(4.6 mm×150 mm)packed with 5μm diameter particles;the mobile phase was water containing 1% formic acid(A)and methanol(B),and the composition gradient was:13% of B until10 min,and changed to obtain 20%,30%,50%,60%,70%,20% and10%B at 20,30,40,50,60,70 and 80 min,respectively,following the method described by Menezes et al.[28]with slight modifications.TheT.tetrapterafruits extract and mobile phase were filtered through 0.45μm membrane filter(Millipore)and then degassed by ultrasonic bath prior to use.The extract was analyzed at a concentration of 15 mg/mL.The flow rate was 0.7 mL/min,and the injection volume was 40μL.The wavelength were 254 nm for gallic acid,280 nm for catechin and epicatechin,327 nm for chlorogenic,caffeic and ellagic acids,and 365 nm for quercetin,rutin,apigenin and luteolin.Stock solutions of standards references were prepared in the HPLC mobile phase at a concentration range of 0.030–0.450 mg/mL.The chromatography peaks were confirmed by comparing their retention time with those of reference standards and by DAD spectra(210–500 nm).The calibration curves were gallic acid:Y=11,964x+1283.5(r=0.9999);chlorogenic acid:Y=13,087x+1195.4(r=0.9998);caffeic acid:Y=11,962x+1273.8(r=0.9996);ellagic acid:Y=12,643x+1327.6(r=0.9995);catechin:Y=13,470x+1195.3(r=0.9999);epicatechin:Y=11,786x+1265.4(r=0.9997);quercetin:Y=12,762x+1373.8(r=0.9998);rutin:Y=13,194x+1197.0(r=0.9999);apigenin:Y=13,471x+1195.6(r=0.9994)and luteolin:Y=12,763x+1347.9(r=0.9999).All chromatography operations were carried out at ambient temperature and in triplicate.
The limit of detection(LOD)and limit of quantification(LOQ)were calculated based on the standard deviation of the responses and the slope using three independent analytical curves.LOD and LOQ were calculated as 3.3 and 10σ/S,respectively,whereσis the standard deviation of the response andSis the slope of the calibration curve[29].
2.4.Handling of experimental animals
Adult male Wistar strain albino rats weighing 200–250 gwere used in this study.The rats were procured from the experimental animal breeding unit of Department of Veterinary Medicine,University of Ibadan,Nigeria.The animals received humane care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Science and published by the National Institute of Health(USA)[30].The guidelines were followed to ensure the protection of the animals’welfare during the experiment.The rats were kept in a cage to acclimatize for 7 days,during which they were maintained at room temperature under the laboratory conditions and were fed with standard diet and wateradlibitum.
2.5.Preparation of Kidney,liver and lungs tissues homogenates for XO and lipid peroxidation inhibition assays
The kidney,liver and lungs tissues homogenates for XO and lipid peroxidation inhibition assays were prepared following the method described by Nakamura et al.[31].The kidney,liver and lungs were rapidly excised after decapitation of the rats under mild ether anesthesia.Each tissue was washed in cold 0.15 mol/L KCl,and blotted dry.Then a portion of 1 g of each tissue was homogenized in 9 volumes of ice-cold 50 mmol/L Tris–HCl buffer(pH 7.4)containing 1 mmol/L ethylene diamine tetraacetic acid(EDTA).A portion of the homogenate was centrifuged for 10 min at 1400×gto yield a low-speed supernatant that was used for the lipid peroxide assay.For the XO assay,another portion of the homogenate of each tissue was sonicated twice on ice for 30 sesonds and then centrifuged at 10,000×gfor 20 min at 4℃ to obtain the supernatant fraction used.
2.6.Xanthine oxidase inhibition assay
The ability of the extract to inhibit XO was determined following the method reported by Umamaheswari et al.[17]with slight modification.The reaction mixture consisted of 300μL of 50 mmol/L sodium phosphate buffer(pH 7.5),100μL of the extract at different concentrations(15,30,45 and 60 mg/mL)in dimethyl sulphoxide(DMSO),100μL of freshly prepared tissue enzyme preparation and 100μL of distilled water.The assay mixture was pre-incubated at 37℃ for 15 min.Then,200μL of 0.15 mmol/L of xanthine solution(substrate)was added to the mixture which was incubated at 37℃ for 30 min;this was followed by the addition of 200μL of 0.5 mol/L HCl to terminate the reaction.Allopurinol was used as a positive control for the assay.A reference test containing 100μL of DMSO instead of the extract was carried out in order to obtain the maximum uric acid formation.The absorbance was measured at 295 nm on a UV/VIS spectrophotometer against a blank prepared in the same way except that the enzyme solution was replaced with the phosphate buffer.One unit(U)of this enzyme is defined as the amount of enzyme required to form 1 mmol of uric acid per min at the reaction conditions.The percentage XO inhibitory activity of the extract was calculated thus:

whereA295referenceis the reference without the extract,andA295sampleis the absorbance of test containing the extract.
2.7.Lipid peroxidation inhibition assay
The ability of the extract to inhibit Fe2+-induced lipid peroxidation in kidney,liver and lungs tissues homogenates of rats was assayed according to the modified method of Ohkawa et al.[32].Briefly,to a reaction mixture containing 100μL of the homogenate supernatant,30μL of 0.1 mol/L Tris–HCl buffer(pH 7.4)and different concentrations of the extract,30μL of freshly prepared 25μmol/L ferric sulfate solution was added to initiate lipid peroxidation.The volume was made up to 300μL with deionized water before incubation at 37℃ for 1 h.The color reaction was initiated by adding 300μL of 81 g/L sodium duodecyl sulphate to the reaction mixture,followed by the addition of 600μL of acetic acid/HCl(pH 3.4)and 600μL of 0.8%(v/v)TBA(Thiobarbituric acid).This mixture was incubated at 100℃ for 1 h.The absorbance of thiobarbituric acid reactive species(TBARS)produced were measured at 532 nm in an UV–Visible spectrophotometer.A reference test without the plant extract was carried out in order to obtain the maximum TBARS formation.The decrease in the absorbance of test sample in relation to the reference test was used to calculate the% inhibition as follows:

whereA532referenceis the absorbance of the reference without the extract,andA532sampleis the absorbance of test containing the extract.
2.8.Statistical analysis
Results of replicate experiments were expressed as mean±standard deviation(SD).Analysis of variance(ANOVA)was carried out on the result data at 95% confidence level using SPSS statistical software package,version 17.IC50was calculated from the % inhibitionversusextract concentration non-linear regression curve of the extract.
3.Results
The HPLC chromatogram ofT.tetrapterafruits(Fig.1)revealed the presence of the gallic acid(tR=13.07 min;peak 1),catechin(tR=15.26 min;peak 2),chlorogenic acid(tR=20.11 min;peak 3),caffeic acid(tR=24.19;peak 4),ellagic acid(tR=29.87;peak 5),epicatechin(tR=35.46 min;peak 6),rutin(tR=41.08 min;peak 7),quercetin(tR=49.23 min;peak 8),luteolin(tR=55.16 min;peak 9)and apigenin(tR=62.73 min;peak 10).The phenolics composition of the fruits is shown in Table 1.The flavonoids were in the order of apigenin>quercetin>rutin>epicatechin>luteolin>catechin;whereas the phenolic acids were in the order of caffeic acid>ellagic acid>gallic acid>chlorogenic acid.
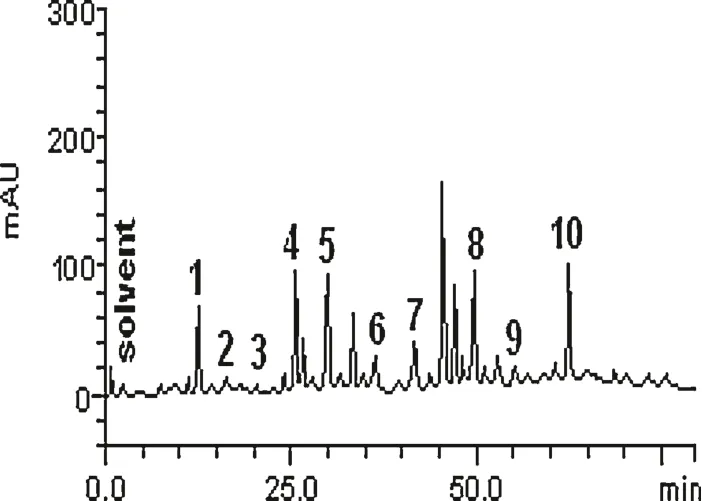
Fig.1.Representative high performance liquid chromatography profile of T.tetraptera fruit.Gallic acid(peak 1),catechin(peak 2),chlorogenic acid(peak 3),caffeic acid(peak 4),ellagic acid(peak 5),epicatechin(peak 6),rutin(peak 7),quercetin(peak 8),luteolin(peak 9)and apigenin(peak 10).
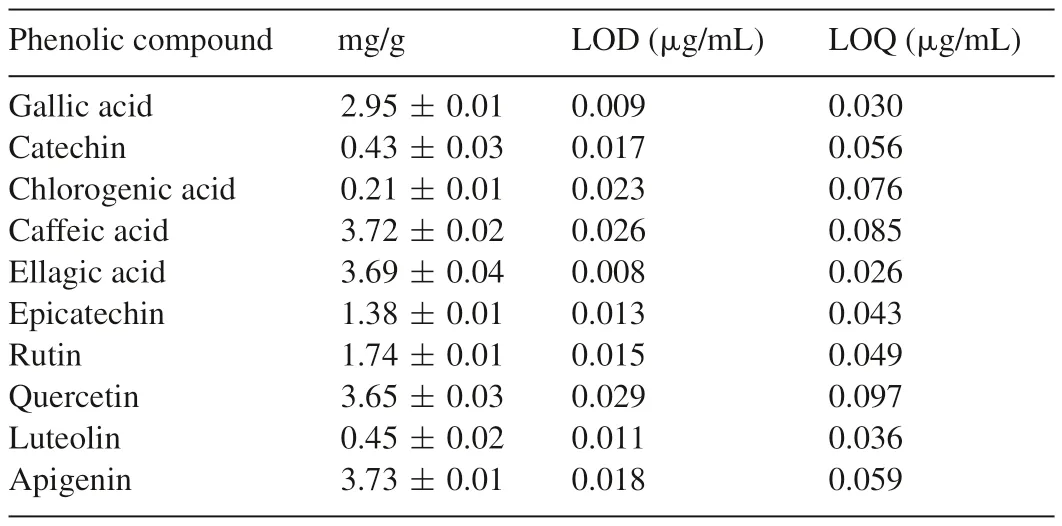
Table 1Phenolics composition of T.tetraptera fruits.
The ability of the extract to inhibit XO in the kidney,liver and lungs tissues homogenates of rats was tested,and the result is presented in Table 2 in terms of their half-maximal inhibitory concentration(IC50),in relation to allopurinol(positive control).The IC50of both the extract and allopurinol on the XO in the homogenates of the three tissues varied significantly(P<0.05)in the order of liver>kidney>lungs.The pattern of inhibition of XO by the extract was dose-dependent as depicted in Fig.2.Generally,allopurinol had a stronger inhibitory ability on the XO than the extract.

Table 2Half-maximal inhibitory concentration(IC50)of T.tetraptera fruits extract on xanthine oxidase activity in kidney,liver and lungs tissues of rats.

Fig.2.Xanthine oxidase activity% inhibition versus T.tetraptera fruit extract concentration curve in kidney,liver and lungs tissues of rats.
As shown in Table 3,the extract also inhibited Fe2+-induced lipid peroxidation in the kidney,liver and lungs tissues homogenates of rats.Its IC50values varied significantly(P<0.05)such that that of the liver(36.97±2.06μg/mL)>lungs(26.65±1.82μg/mL)>kidney(20.39±1.19μg/mL);and its pattern of inhibition was also dose-dependent as shown in Fig.3.
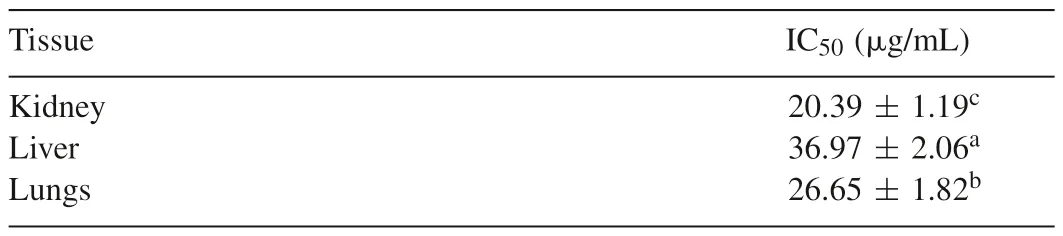
Table 3Half-maximal inhibitory concentration(IC50)of T.tetraptera fruits extract on Fe2+-induced lipid peroxidation in the kidney,liver and lungs tissues of rats.

Fig.3.Fe2+-induced lipid peroxidation % inhibition versus T.tetraptera fruit extract concentration curve in kidney,liver and lungs tissues of rats.
4.Discussion
Dietary spices are recognized as natural antioxidant agents and inhibitors of pro-oxidant enzymes such as XO.These beneficial activities are attributable to the flavonoids and other polyphenols present in the spices[33].Hence,we evaluated the inhibitory effect of the phenolic extract ofT.tetrapterafruits,a dietary spice,on XO and Fe2+-induced lipid peroxidation in the kidney,liver,and lungs tissues of ratsinvitro.The important roles the kidney,liver and lungs play as organs of metabolism and excretion in mammals,and the previously reported XO activity in these organs[6,13],informed the choice of their homogenates as the source of the enzyme.
The chromatographic analysis of phenolic compounds(Table 1)revealed thatT.tetrapterafruits are rich in flavonoids(apigenin,quercetin,rutin,epicatechin,luteolin and catechin)and phenolic acids(caffeic acid,ellagic acid,gallic acid and chlorogenic acid).These two classes of natural phenolic compounds are regarded to be of pharmacological importance[34],as they possess diverse health benefits,including antiinflammatory activity[35].They can modulate the expression of pro-inflammatory signals and ameliorate inflammatory diseases such as arthritis like other natural polyphenolics[19,20].As part of their anti-inflammatory activity,polyphenolics including the flavonoids and phenolic acids have been reported to inhibit XO activity by recent studies[33,36,37].Probably,like allopurinol,they inhibit XO by binding at its purine binding site[38],thereby blocking the ultimate formation of uric acid.In addition to their ability to inhibit XO,phenolics are well-known for their antioxidant activities[39],and ability to inhibit Fe2+-induced lipid peroxidation[34].The flavonoids exhibit antioxidant activity by acting as both electrons donors and terminators of chain reactions;and this is due the hydroxyl groups they possess,particularly at the 3'OH and 4'OH of their three-carbon chain[40,41].Phenolic acids,on the other hand,have a phenolic ring and an organic carboxylic acid function[42],which make them capable of stabilizing and delocalizing unpaired electrons[43].These distinguishing structural features make flavonoids and phenolic acids prominent antioxidant phenolic compounds.
Inhibitors of XO are used clinically for the treatment of hyperuricaemia and gouty arthritis;as they help in reducing the levels of uric acid in circulation,and vascular oxidative stress[44].In this regard,the efficacy of plant-derived polyphenolics in inhibiting XO and alleviating the resultant hyperuricaemia has been reported;and as natural components of food,they are regarded to be safer than synthetic XO inhibitors such as allopurinol[33].Their inhibitory effect against XO has been attributed to the possibility of the C-5 and C-7 hydroxyl groups of flavones and flavonols to replace the C-2 and C-6onesof xanthine in the XO active site[45,46],due to the mutual inter-convertibility of the carboxyl structures of xanthine to hydroxyl groups[47].In this study,the phenolic extract ofT.tetrapterafruits effectively inhibited XO in the kidney,liver and lungs tissues homogenates of rats.Interestingly,the IC50values of the extract on the XO from these three tissues(kidney:39.53±1.02μg/mL;liver:45.71±1.44μg/mL;lungs:33.87±0.96μg/mL)(Table 2)are in the range of the IC50values earlier reported forErythrina strictaleaves extract(21.2μg/mL)[17],andOleaeuropaealeaf extract(42μg/mL)[36]on XO.Furthermore,the order of the IC50values of the extract on the XO from these three tissues(liver>kidney>lungs)may suggest a higher activity of the XO in the liver homogenate than in the kidney and the lungs homogenates.This supports an earlier report that the distribution XO activity is highest in the liver followed by the kidney and other tissues,as demonstrated by Carro et al.[14].Similarly,Liu et al.[48]reported that XO is present in significant concentration in the liver.It is noteworthy that apigenin,the most abundant of the phenolics inT.tetrapterafruit from our HPLC-DAD result,was earlier reported to possess the strongest XO inhibitory activity compared with other phenolics inO.europaealeaf extract[36].
Elevated XO activity enhances lipid peroxidation due to the attendant excessive generation of reactive oxygen species(ROS),decreases in the levels of non-protein antioxidants including reduced glutathione(GSH),vitamin C and vitamin E;and the inflammation associated with neutrophil infiltration[31].Lipid peroxidation is the oxidative damage of lipids,especially the polyunsaturated fatty acids that are very susceptible to oxidative attack,by ROS and transition metal ions;and it is known to play a key role in cell injury[49].This is due to the diverse cytotoxic products,mostly aldehydes such as malondialdehyde(MDA)it yields[50].These cytotoxic aldehydes have been implicated in the pathogenesis of a number of oxidative stress-induced inflammatory diseases[51]such as gouty arthritis.Under such condition,the antioxidant status of the affected tissues is attenuated;thereby over-exposing the cells to oxidative damage,and aggravating the inflammatory condition.
The peroxidation of membrane lipids and the consequent oxidative damage to cell membrane may disrupt the membrane transport,ionic channels,proteins and deactivate membraneassociated enzymes;the membrane lipid bilayer itself may become more permeable due to oxidative damage[33].Hence,the ability of the extract to inhibit lipid peroxidation in the kidney,liver and lungs tissues homogenates of rats indicates its ability to prevent and/or ameliorate oxidative damage to the lipids in the membranes of the cells of these three important organs;thereby maintaining the integrity of their membrane structure and functionality.Furthermore,since the ROS generated by lipid peroxidation are involved in the pathogenesis of several other diseases such as diabetes mellitus,cardiovascular diseases and carcinogenesis,XO inhibitors,including polyphenolics,could also be useful for the prevention and management of many other diseases[44].This may explain whyT.tetrapterafruit has other medicinal uses as stated earlier.The lipid peroxidation inhibitory activity of theT.tetrapterafruit extract could be attributed to its flavonoids and phenolic acids.This is in agreement with our earlier report thatMangiferaindicaandMucunaurensseeds extracts rich in these two classes of phenolics inhibited Fe2+-induced lipid peroxidation in rat pancreas homogenate[34].
5.Conclusion
Phenolics extract ofT.tetrapterafruits inhibited xanthine oxidase and F2+-induced lipid peroxidation in the kidney,liver and lungs tissues of ratsinvitro.These activities could be attributed to the combined effect of the flavonoids and phenolic acids present in the fruits.Therefore,T.tetrapterafruits might be a promising functional food that could be explored for the prevention and management of hyperuricaemia and its associated disease conditions.
Conflict of interest statement
We declare that we have no conflict of interest regarding this study.
Acknowledgement
The authors acknowledge the Multi-Disciplinary Central Laboratory,University of Ibadan,Nigeria for giving access to the facilities in their laboratory to carry out the wet analyses.
- 食品科学与人类健康(英文)的其它文章
- Bioactive peptides on endothelial function
- Calcium intake,calcium homeostasis and health
- Characterization of volatiles Tribolium castaneum(H.)in flour using solid phase microextraction–gas chromatography mass spectrometry(SPME–GCMS)
- Nutritional composition,in vitro antioxidant and anti-diabetic potentials of Breynia retusa(Dennst.)Alston
- Effects of antimicrobial and antioxidant activities of spice extracts on raw chicken meat quality
- GUIDE FOR AUTHORS

