The Subcutaneous Implantable Cardioverter-De fibrillator: A Practical Review and Real-World Use and Application
Mark E. Panna Jr, MD, FACC, FHRS and William M. Miles, MD, FACC, FHRS
1Division of Cardiology, Department of Medicine, North Florida/South Georgia Veterans Affairs Hospital, Gainesville, FL, USA
2Section of Electrophysiology, Division of Cardiology, Department of Medicine, University of Florida, FL, USA
Abbreviations
ATP antitachycardia pacing
CHD congenital heart disease
CIED cardiac implantable electronic device
ESRD end-stage renal disease
HCM hypertrophic cardiomyopathy
ICD implantable cardioverter-de fibrillator
IDE investigational device exemption
LVAD left ventricular assist device
MRI magnetic resonance imaging
S-ICD subcutaneous implantable cardioverterde fibrillator
TV-ICD transvenous implantable cardioverterde fibrillator
VF ventricular fibrillation
VT ventricular tachycardia
Introduction
Sudden cardiac death remains a signi ficant public health problem and is a leading cause of cardiovascular death, with approximately half of all cardiovascular deaths in the United States occurring suddenly[1]. Implantable cardioverter- de fibrillators (ICDs)have been shown to decrease the risk of sudden cardiac death in both primary prevention trials [2, 3]and secondary prevention trials [4]. The subcutane-ous ICD (S-ICD; Boston Scienti fic, Marlborough,MA, United States) was developed as an alternative therapy to transvenous ICD (TV-ICD) systems, as it is a fully subcutaneous system that does not require leads within (endovascular) or on the epicardial surface of the heart [5]. TV-ICD implantation carries immediate complication risks of pneumothorax,cardiac perforation, and lead dislodgement [6]; as well as long term risks of lead malfunction [7, 8],device-related infection, and venous occlusion. The S-ICD was developed to decrease periprocedural implantation risks, eliminate venous access diffi-culties, and reduce endovascular mechanical stress on leads. In addition, transvenous lead extraction is increasing in frequency because of the increasing number of cardiac implantable electronic devices(CIEDs), their risk of infection, and recent ICD lead recalls [7-9]. The S-ICD decreases the risk of future extraction-related morbidity associated with nonfunctional and infected TV-ICD systems.
The S-ICD system may be considered for patients meeting ICD guideline criteria [10] for either primary or secondary prevention who do not have a pacing and/or cardiac resynchronization therapy indication. In addition, the S-ICD cannot deliver antitachycardia pacing (ATP) [5]. Therefore patients with known pace-terminable ventricular tachycardia(VT) and/or recurrent monomorphic VT who may bene fit from ATP therapies should be considered for a TV-ICD system rather than an S-ICD system.
S-ICD Components/Implantation Procedure
The S-ICD system consists of a pulse generator(130 g) positioned over the sixth rib between the mid to anterior left axillary line and a single (45 cm long) lead containing two sensing electrodes positioned adjacent to either end of an 8 cm shock coil.The lead is tunneled across the chest wall and positioned parallel to and 1 to 2 cm to the left of the sternal midline, with the distal sensing electrode secured adjacent to the manubriosternal junction,and the proximal sensing electrode secured adjacent to the xiphoid process [5]. Standard implantation involves three incisions: one lateral pocket incision and two parasternal incisions. However, reports of a two- incision technique eliminating the superior parasternal incision appear safe, and may simplify future implantation [11]. A submuscular approach to S-ICD generator placement has also been described that may reduce pocket-related complications in select patients [12, 13] but it needs further clinical validation. Care should also be taken not to introduce subcutaneous air, as it can insulate the sensing electrodes, causing inadequate sensing and low- amplitude signals, leading to oversensing due to auto gain and potential inappropriate shocks [14].
S-ICD Advantages
The S-ICD may be implanted strictly by anatomical landmarks without the use of fluoroscopy, limiting radiation exposure of the patient and providers [5].It does not require favorable venous anatomy, which may be bene ficial in patients with limited venous access such as patients undergoing dialysis and with congenital heart disease (CHD). It can preserve vascular access for future use and avoids the risks of future transvenous lead extractions. There is a potentially lower risk of systemic infection and a decreased risk of procedural complications, including pneumothorax and cardiac perforation [15]. There is also less biomechanical stress on a subcutaneous lead as it is not exposed to the dynamics of cardiac motion and/or subclavian crush forces. Procedure times are more predictable, with an average procedure time of 69±27 min in the EFFORTLESS (Boston Scienti fic postmarket S-ICD) registry [16].
S-ICD Disadvantages
The major disadvantage of the S-ICD system is its lack of pacing capabilities, including ATP therapies. This not only excludes some patients at the time of implantation, but may also be problematic in a minority of patients who develop symptomatic bradyarrhythmias after S-ICD implantation.The generator is larger than that of TV-ICDs, and the S-ICD has no impedance monitoring for surveillance of congestive heart failure patients. The S-ICD is currently not magnetic resonance imaging (MRI) compatible and it should be used with caution in patients with a history of “slow” VT, as the lowest programmed detection rate is 170 bpm.The S-ICD does not have a programmable monitor zone, which can be useful in detecting the pres-ence of arrhythmias with rates occurring below the programmed detection intervals. The S-ICD also has a higher frequency of T-wave oversensing compared with TV-ICD systems [15, 16].
System Characteristics
The S-ICD can deliver only 80 J biphasic shocks (no other energy is programmable). It may deliver up to five shocks per tachycardia episode, and can reverse shock polarity if an initial shock fails. As previously noted, the S-ICD has no baseline pacing capabilities.It can deliver postshock demand pacing at 50 bpm only for up to 30 s [5]. After 30 s, no further pacing will occur regardless of the underlying rhythm.Battery longevity of first-generation devices was approximately 5 years [17]. Second-generation devices are expected to have an average longevity of 7.3 years [18]. The S-ICD can store data on more than 40 arrhythmic events (treated and untreated).The S-ICD detects changes in ventricular rates by using modi fied subsurface electrocardiography via a primary (proximal electrode to can), a secondary (distal electrode to can), or an alternate (distal electrode to proximal electrode) sensing vector(Figure 1). It automatically determines the optimal sensing vector on the basis of an R wave to T wave ratio that attempts to avoid double QRS counting or T-wave oversensing [19]. It measures rate as a rolling average of four consecutive beats, with VT/ventricular fibrillation (VF) detection indicated when 18 of 24 consecutive intervals fall within the detection zone. If this criterion is met, the S-ICD can charge capacitors and deliver a shock (Figure 2)[5, 19]. All device settings are automated except for shock therapy (on/off), postshock pacing (on/off),and conditional discrimination of supraventricular tachycardia (on/off) [5]. The device is programmable as either a single or a dual zone device. In single-zone configurations, there is a shock-only zone that relies solely on heart rate. Dual-zone programming incorporates an additional conditional zone that uses a unique morphology-based discrimination algorithm to classify rhythms as either shockable or nonshockable [20].
The mean time to therapy in the EFFORTLESS registry for induced ventricular arrhythmia (de fi-brillation testing) was 15.1 s (7-37 s), and for spontaneous ventricular events was 17.5 s (6-29 s).Because of a longer time to therapy, S-ICD shocks were withheld for 46% of recorded episodes [16].An early report raised concerns of syncope due to delays in therapy secondary to arrhythmia undersensing [21]. However, in pooled analysis of the investigational device exemption (IDE) trial and the EFFORTLESS registry, syncope was reported in only 1.7% of patients [22]. Bardy et al. [5] evaluated de fibrillation threshold testing in patients in whom both an S-ICD and a TV-ICD has been implanted. The S-ICD required higher energy on average (36.6±19.8 J vs 11.1±8.5 J).
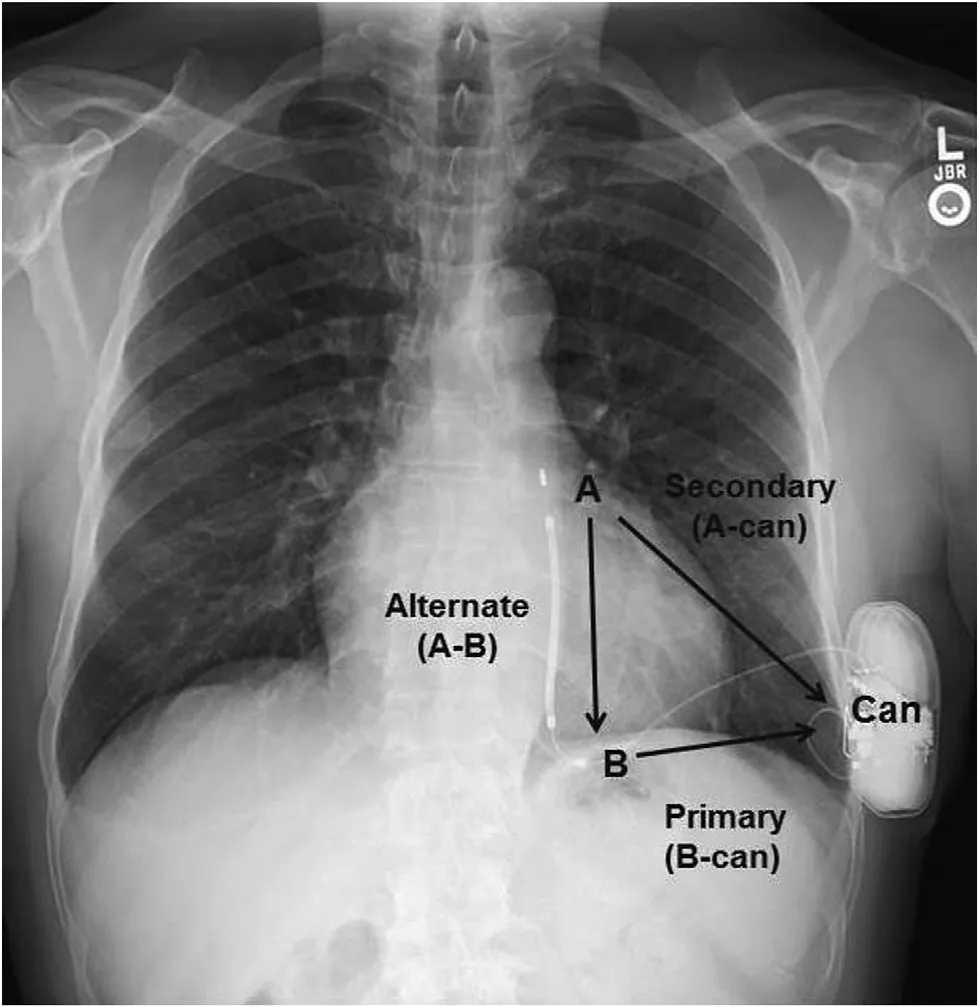
Figure 1 Subcutaneous Implantable Cardioverter-De fibrillator Sensing: Primary Vector B-can, Secondary Vector A-can, and Alternate Vector A-B.A, Distal electrode; B, proximal electrode.
S-ICD ECG Vector Screening
All patients should undergo preprocedure S-ICD screening. This ensures that an adequate R wave to T wave ratio is present, attempting to avoid T-wave oversensing. ECG leads are positioned to mimic S-ICD sensing vectors. Evaluation of all three vectors with the patients in both the supine position and the standing position is performed for 10 s,with use of an ECG screening tool. Patients qualify for an S-ICD if all beats in any screening vector pass inboththe supine position and the standing position [23]. Approximately 7-8% of patients fail the screening test [23, 24]. Reported clinical predictors of failed screening include hypertrophic cardiomyopathy (HCM), heavy weight, and prolonged QRS duration [24]. ECG predictors of failed screening include simultaneous T wave inversions in leads I, II, and aVF [23], and an R wave to T wave ratio of less than 3 in the ECG lead with the largest T wave [24]. Conversion of an ECG screening failure to a pass has been reported with a right parasternal electrode configuration [25], but safety needs to be evaluated further. If patients pass only in the alternate vector, additional testing with the patient bending/ flexing forward should be considered as reports of oversensing in this position (due to low R wave amplitude), leading to triple counting of the P/QRS/T waves and inappropriate shocks,have been reported [26].
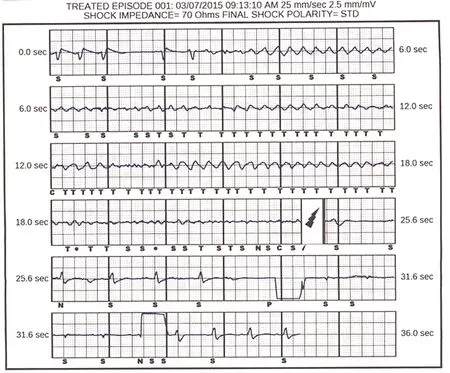
Figure 2 Subcutaneous Implantable Cardioverter-De fibrillator (S-ICD): Conversion of Clinical Ventricular Fibrillation with a Single S-ICD Shock Back To Normal Sinus Rhythm.Mild undersensing of fine ventricular fibrillation is noted. C, capacitor charging; P, paced event; S, sensing of an event not classi fied as tachycardia; T, sensed event classi fied as tachycardia; lightning bolt, shock.
S-ICD IDE Trial and the EFFORTLESS Registry (VT/VF Conversion and Infection Rates)
The Food and Drug Administration IDE trial [15]enrolled 330 patients, 74% male with a mean age of 51.9±15.5 years and mean left ventricular ejection fraction of 36.1±15.9%. Seventy-nine percent had a primary prevention indication. Patients had to be older than 18 years, meet the ICD guideline criteria,and pass ECG vector screening. Epicardial patches,unipolar pacers, severe renal dysfunction, and patients with pace-terminable VT were excluded.The system complication-free rate at 180 days was 99%. The primary efficacy end point, de fined as two consecutive de fibrillations at 65 J out of a possible four attempts, was 100%. An additional substudy repeated de fibrillation testing at 6 months in 78 patients, with a 96% conversion rate with one 65 J shock and a 100% conversion rate with shock of up to 80 J. One hundred nineteen episodes of VT/VF (81 during VT storms and 38 discrete episodes)had 100% spontaneous conversion or termination with an 80 J shock, with no arrhythmic deaths. There were 18 device infections (5.6%), with four (1.3%)requiring explanation. There were increased episodes of infections early in the trial that decreased with operator experience.
The EFFORTLESS registry [16] is an observational nonrandomized multicenter standard-of-care registry of 472 patients, with interim analysis available. The mean age was 49±18 years (younger than typical TV-ICD trials), with a signi ficant proportion of nonischemic cardiomyopathy (31%), channelopathies (13%), and CHD (7%), which differed from the IDE trial population. Sixty-three percent of devices were placed for primary prevention compared with 79% in the IDE trial. Only 29% of patients had a diagnosis of congestive heart failure,and the mean level ventricular ejection fraction was 42%. The complication-free procedure rate at 360 days was 94%. Successful de fibrillation (de fined as successful conversion at 80 J or less) was 99.7%.Ninety-one episodes of VT/VF (40 during VT storm and 51 discrete episodes) occurred. Ninetysix percent of discrete episodes terminated with one to five shocks, and single-shock efficacy was 88%.One episode spontaneously terminated after the fifth shock. Only one patient died of an arrhythmic death, a patient with Löf fler’s syndrome receiving high-dose steroids who previously had successful de fibrillation threshold testing.
In pooled analysis (IDE trial and EFFORTLESS registry), only 17 devices needed removal/revision because of infection (1.7%) [22]. In patients in whom an S-ICD had been implanted after TVICD extraction, the risk of S-ICD infection is low even in patients in whom the TV-ICD was removed because of prior TV-ICD infection [27].
S-ICD and Inappropriate Shocks
S-ICD inappropriate shocks are more commonly caused by double counting of cardiac signals(most frequently T-wave oversensing) rather than supraventricular arrhythmias [15]. The system has shown excellent discrimination of supraventricular arrhythmias from ventricular arrhythmias [15,28, 29]. Early clinical experience raised concern of inappropriate shocks, with rates up to 15% [30,31]. In the IDE trial, 41 patients (13.1%) received inappropriate shocks (22 from T-wave oversensing). Of these, 32 patients underwent device reprogramming that solved the issue but nine patients required reoperation [15]. The addition of dualzone programming signi ficantly decreased the rate of inappropriate shocks in the IDE study [15]. In the EFFORTLESS registry the inappropriate shock rate was much improved at 7% [16], but was slightly higher than in recent TV-ICD trials [32]. In pooled analysis (IDE trial and EFFORTLESS registry),the 3-year Kaplan-Meier estimated inappropriate shock rate was 11.7% for dual-zone programming and 20.5% for single-zone programming [22]. Data support the use of dual-zone programming as a standard setting for S-ICD patients [20, 22].
The initial step in avoiding inappropriate shocks is proper preprocedure screening. Once the S-ICD has been implanted, dual-zone programming should be considered [20]. Inappropriate shocks may be managed after implantation by reprogramming of the sensing vector and/or therapy zones of the device with use of a template acquired during exercise. Exercise-optimized programming may be considered to prevent future inappropriate shocks and should be considered in patients who are felt to be at high risk of T-wave oversensing [33] such as HCM patients. A new S-ICD discrimination algorithm has been developed to reduce T-wave oversensing but will need to be evaluated prospectively [29].Reevaluation of ECG screening should be considered with any change in QRS morphology, such as new bundle branch blocks. Inappropriate shocks due to myopotentials [34] and electromagnetic interference [15, 35] have rarely been reported.
Real-World Populations and Clinical Scenarios
S-ICD and Pacemakers
Patients with pacing indications should be considered for TV-ICD systems rather than an S-ICD. However,S-ICDs have been implanted in patients with preexisting pacemakers and vascular access difficulties.In pooled analysis (IDE trial and EFFORTLESS registry), 2.2% of patients (n=19) had preexisting pacemakers at the time of S-ICD implantation [22].Pacemaker devices were programmed to be bipolar,as unipolar pacing is contraindicated (owing to inappropriate sensing related to the large unipolar pacing artifact) [15]. Patients have the same ECG screening as nonpaced patients. If intrinsic rhythm is present,both paced and intrinsic rhythms should be evaluated and pass in the same vector. If atrial arrhythmias are present, the stored conditional zone morphology template should re flect the native intrinsic rhythm(if present) rather than the ventricular paced rhythm.Once the devices have been implanted, interference between both devices should be evaluated, as pacing spikes could be counted independently from R waves [36]. Porter field et al. [36] reported placement of an S-ICD in a patient with preexisting pacemaker and complete atrioventricular block whofailedECG screening. S-ICD arrhythmia detection was programmed at more than two times the upper rate limit of the pacemaker to prevent inappropriate shocks from T-wave oversensing (double counting). They also evaluated sensing at the maximum pacer output to ensure the S-ICD did not mark pacer spikes.
Some pacemakers have backup safety modes which may switch them to unipolar mode, and this feature should be disabled if possible [36]. Akin et al. [37] reported de fibrillation threshold testing in a pacemaker-dependent patient in whom the pacemaker was programmed DOO. During testing the patient received a shock that failed to convert VF, but switched the pacemaker to unipolar mode.The larger unipolar pacing signals were sensed by the S-ICD, causing undersensing of VF, requiring external de fibrillation. Consideration can also be given for programming postshock pacing off, as it could inhibit intrinsic pacemakers [36]. There are also reports of successful placement of S-ICDs in patients with bipolar epicardial leads [38, 39].
S-ICD and End-Stage Renal Disease
Because of the risk of venous occlusion and device infections with TV-ICD systems, S-ICD systems may be considered for use in patients with end-stage renal disease (ESRD). However, data in ESRD patients are limited. Patients with a glomerular filtration rate less than 29 mL/min were excluded from the Food and Drug Administration IDE trial[15] and only 9% of patients in the EFFORTLESS registry [16] had renal disease, with a pooled analysis showing only 34 patients (3.9%) with glomerular filtration rates less than 45 mL/min [22]. However,reports from two large university facilities suggest a higher percentage of S-ICD implantation in ESRD patients (28 of 92 [40] and 17 of 74 [41]) than seen in clinical trials. In ESRD an S-ICD can be used in patients with recurrent thrombosis (related to transvenous leads), in patients with preexisting stenosis preventing transvenous device placement, and in patients at risk of developing intravascular stenosis and/or infection [42].
El-Chami et al. [40] reported a single-center experience of S-ICD implantation in 79 patients(27 with ESRD undergoing dialysis). Patients with HCM and channelopathies were excluded from the analysis. ESRD patients were older and likelier to have diabetes. The annual rate of the combined primary end point (death, heart failure, hospitalization, or appropriate S-ICD shock), although not signi ficant, was higher in the dialysis cohort (23.8%/year vs 10.9%/year,P=0.317) driven by a higher incidence of appropriate shocks (17.0%/year vs 1.4%/year,P=0.021). There were no S-ICD-related infections in the ESRD patients. The rate of inappropriate shocks was similar (6.0%/year of dialysis vs 6.8%/year for no dialysis,P=0.509). The low inappropriate shock rate is notable, as concerns exist for T-wave oversensing in patients with fluctuating potassium levels and QRS/T wave amplitudes. Kiamanesh et al. [43] reported an episode of hyperkalemia (K+7.0 mmol/L) in an ESRD patient leading to T-wave oversensing and an inappropriate shock that initiated VF, requiring four additional shocks before VF termination. However, despite the potential for transient potassium level fluctuations with QRS/T wave amplitude changes, inappropriate shocks due to hyperkalemia have rarely been reported.
S-ICD and HCM
Pooled analysis (IDE trial and EFFORTLESS registry) included 96 patients with HCM (75% male),88.5% with a primary prevention indication. Oneyear complication rates including inappropriate shocks were equivalent between HCM and non-HCM patients, with no observed deaths. Only four HCM patients experienced VT, all of which were appropriately sensed and de fibrillated [44]. Careful preprocedure ECG screening is important in HCM to prevent T-wave oversensing and inappropriate shocks. HCM has been suggested as an independent predictor for ECG screening failure [24] S-ICD screening failure rates of 7% [45] to 16% [46] have been reported, with failure rates of 36% in patients considered at high-risk of sudden cardiac death[46]. Primary causes of screening failure were high T-wave voltage in 25% of vectors, T-wave inversions in more than two surface leads, and prior myectomy [46]. Exercise ECG screening should be considered as it can unmask unsuitable HCM patients formerly thought eligible at rest [45]. Postimplant exercise-based optimization should also be considered [33, 45]. This should include examination of all three sensing vectors during exercise as well as during acquisition of subcutaneous ECG templates [47]. Right parasternal chest electrode position could also be assessed if screening fails with the traditional electrode position [45].
A single-center experience in 18 patients with HCM reported T-wave oversensing in seven patients(39%), with four patients (22%) having inappropriate shocks. Reprogramming of the sensing vector eliminated T-wave oversensing in three of the four patients. A low R wave to T wave ratio was a major risk factor for the occurrence of oversensing in this patient cohort [47].
S-ICD with Left Ventricular Assist Devices and Other Implantable Devices
S-ICDs have been placed in conjunction with several CIEDs and noncardiac implantable devices.Saeed et al. [48] reported placement of a HeartWare(Framingham, MA, United States) left ventricular assist device (LVAD) in a patient with preexisting S-ICD. After implantation, noise was noted in the primary and secondary sensing vectors, both of which incorporate the pulse generator close to the LVAD. No inappropriate therapies were noted;however, the device could have withheld appropriate therapy in the setting of true arrhythmia. The alternate vector was not affected. Another patient had a preexisting HeartMate II LVAD (Thoractec Corporation; Pleasanton, CA, United States), and an S-ICD was implanted in that patient. Sensing in all three vectors was found to be appropriate without any interference from the continuous- flow LVAD.The S-ICD system initially chose the secondary vector, later programmed to the primary vector. There was no interference in sensing or shock delivery from the S-ICD. LVAD readings were unchanged.The HeartMate II functions at higher speeds that appeared less likely to cause interference [49].Reevaluation of S-ICD sensing after LVAD implantation or after LVAD speed change should be considered to exclude device-device interaction.
Kuschyk et al. [50] reported S-ICD implantation in six patients with cardiac contractility modulation devices and one vagus nerve stimulator.Cardiac contractility modulation devices deliver high-voltage biphasic electrical impulses during the absolute refractory period which enhance the contractile strength of failing myocardium. S-ICD implantation in patients with a cardiac contractility modulation device appeared safe during an intermediate follow-up period (mean 17 months) [50].Intraoperative and postprocedure cross talk testing in patients with both a CIED and an S-ICD should be performed, and ergonomic testing should be considered. The sensing vector with the clearest result should be programmed. Bader et al. [51] reported S-ICD implantation in a patient with a preexisting deep brain stimulator. The deep brain stimulator should be programmed to be bipolar if possible;however, in the short term no artifact was seen with either unipolar mode or bipolar mode, likely due to S-ICD signal filtering.
S-ICD in Children and CHD
TV-ICDs have been effective in managing malignant arrhythmias in selected pediatric and adult CHD patients [52]. Potential transvenous leadrelated problems are of particular concern in the young because of increased physical activity, ongoing growth, and longer life expectancy, allowing more time for potential lead complications [53].Younger patients are likelier to undergo implantation for HCM and ion channelopathies, disorders likelier to present with VF, making lack of ATP capabilities less worrisome. Limited experience exists with S-ICDs in children. Patients (younger than 18 years) were excluded from the IDE trial. Early experience raised concern for wound dehiscence and threatened generator erosion in young patients[21]. An observational case-control series evaluated patients (younger than 20 years, range 10-18 years) receiving an S-ICD (n=9) and case-matched TV-ICD patients (n=8). Three patients received appropriate S-ICD shocks. In limited follow-up,the S-ICD may offer similar survival bene fit to the TV-ICD but with a lower incidence of complications requiring reoperations [53]. Future evaluation of a submuscular approach may decrease concern for skin erosion in children. Alternative generator and coil configurations have been reported in small patients [54].
Patients with CHD often have complex cardiac and venous anatomy, making TV-ICD placement challenging. S-ICD placement may therefore be considered, but limited experience exists. In the EFFORTLESS registry only 7% of patients (n=33)had CHD [16]. In pooled analysis (IDE trial and EFFORTLESS registry), the inappropriate shock rate for CHD patients was similar to that for non-CHD patients (10.5% vs 10.9%,P=0.96) and successful de fibrillation threshold testing (at 65 J)was comparable in CHD versus non-CHD patients(88.2% vs 94.6%,P=0.26). No spontaneous ventricular arrhythmias were noted on follow-up [55].Zeb et al. [56] evaluated ECG vector screening in 30 patients with CHD, with 86% having suitable screening vectors. The alternate and primary vectors were more often suitable. In addition, screening of six versus two postures did not signi ficantly affect S-ICD eligibility in CHD patients (83% vs 87%).
Future Directions/Investigations S-ICD and Leadless Pacemakers
A signi ficant limitation of the S-ICD is its inability to provide backup pacing and ATP therapies.Mondésert et al. [57] reported implantation of a leadless pacemaker in a patient with complete heart block and preexisting S-ICD. No oversensing was seen by the S-ICD during pacing, and the leadless pacemaker functioned normally after an S-ICD shock. Feasibility was also evaluated in a bovine model, with successful implantation of S-ICDs and leadless pacemakers without safety or performance issues in either device after multiple S-ICD and external shocks [58]. The development of a leadless pacemaker that could communicate with the S-ICD and provide both backup pacing and deliver ATP therapies would be a major advance, allowing a fully functional leadless system.
S-ICD and MRI
The compatibility of MRI scanning with cardiac implantable devices is an evolving field, with recent approval of MRI-compatible pacemakers and de fi-brillators. MRI scanning isnotcurrently approved for use with the S-ICD system. However, Keller et al. [59] reported the safety of MRI scanning in 15 patients with S-ICDs undergoing 22 scans (5 brain,3 cardiac, 12 lumber, and 1 knee scan) at 1.5 T.Device therapies were disabled, and a temperature probe was placed over the S-ICD pocket during scanning. As the S-ICD electrode is extravascular,device heating does not affect the myocardium, but heating of the system/generator may cause discomfort. Four patients noted discomfort due to heating over the S-ICD generator (all during lumbar spine examinations). Two of these patients were rescanned after removal of the external temperature probe,without recurrent symptoms, suggesting heating of the temperature probe may have led to their discomfort. Imaging of the lumbar spine, brain, and knee showed no device artifact. Cardiac imaging showed adequate right ventricular visualization, but S-ICD generator artifact limited visualization of parts of the left ventricle. No delayed enhancement imaging was performed, and no device malfunctions were observed. Early experience with MRI scanning is promising and will need to be con firmed in larger trials.
Conclusions and Take-Home Message
The S-ICD system appears safe to implant and is effective at detecting and treating induced and spontaneous VT/VF episodes. It may be considered in patients having an ICD indication who do not have a pacing and/or cardiac resynchronization therapy indication and who are unlikely to bene fit from ATP therapy.
The ideal S-ICD candidate is still to be determined. Young patients and patients with poor venous access, indwelling catheters, channelopathies, and CHD seem the most suitable. However, limited data exist in many of these patient cohorts, and further studies and/or registry data will be needed to determine the long-term suitability of the S-ICD in these populations.
Initial concerns of inappropriate shocks can be mitigated by appropriate preprocedure screening,dual-zone programming, and sensing optimization during exercise testing. Advances in leadless pacing may make the device more applicable to a larger population.
Disclosure
Dr. Panna: No disclosures.
Dr. Miles: Consultant, Medtronic, Inc; Fellowship funding, Medtronic, St. Jude, Boston Scienti fic,Biosense-Webster.
REFERENCES
1. Goldberger JJ, Basu A, Boineau R,Buxton AE, Cain ME, Canty JM Jr,et al. Risk strati fication for sudden cardiac death: a plan for the future.Circulation 2014;129:516-6.
2. Moss AJ, Zareba W, Hall WJ,Klein H, Wilber DJ, Cannom DS,et al. Prophylactic implantation of a de fibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877-83.
3. Bardy GH, Lee KL, Mark DB,Poole JE, Packer DL, Boineau R,et al. Amiodarone or an implantable cardioverter-de fibrillator for congestive heart failure. N Engl J Med 2005;352:225-37.
4. The Antiarrhythmics Versus Implantable De fibrillators (AVID)Investigators. A comparison of antiarrhythmic-drug therapy with implantable de fibrillators in patients resuscitated from nearfatal ventricular arrhythmias. N Engl J Med 1997;337:1576-83.
5. Bardy GH, Smith WM, Hood MA,Crozier IG, Melton IC, Jordaens L,et al. An entirely subcutaneous implantable cardioverter-de fibrillator. N Engl J Med 2010;363:36-44.
6. van Rees JB, de Bie MK, Thijssen J, Borleffs CJ, Schalij MJ,van Erven L. Implantation-related complications of implantable cardioverter-de fibrillators and cardiac resynchronization therapy devices:a systematic review of randomized clinical trials. J Am Coll Cardiol 2011;58:995-1000.
7. Ellenbogen KA, Wood MA,Shepard RK, Clemo HF, Vaughn T,Holloman K, et al. Detection and management of an implantable cardioverter de fibrillator lead failure:incidence and clinical implications.J Am Coll Cardiol 2003;41:73-80.
8. Zeitler EP, Pokorney SD, Zhou K,Lewis RK, Green field RA, Daubert JP, et al. Cable externalization and electrical failure of the Riata family of implantable cardioverterde fibrillator leads: a systematic review and meta-analysis. Heart Rhythm 2015;12:1233-40.
9. Birgersdotter-Green UM, Pretorius VG. Lead extractions: indications,procedural aspects, and outcomes.Cardiol Clin 2014;32:201-10.
10. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2013;61:e6-75.
11. Knops RE, Olde Nordkamp LR,de Groot JR, Wilde AA. Twoincision technique for implantation of the subcutaneous implantable cardioverter-de fibrillator. Heart Rhythm 2013;10:1240-3.
12. Migliore F, Bottio T, Iliceto S,Bertaglia E. Submuscular approach for subcutaneous implantable cardioverter de fibrillator: a potential alternative technique. J Cardiovasc Eletrophysiol 2015;26:905.
13. Willner JM, Miller MA, Singh A,Sharma D, Palaniswamy C, Kuk S,et al. Chronic safety and efficacy of submuscular implantation of a subcutaneous ICD. Heart Rhythm 2015;12:(5 Suppl):S336.
14. Sing-Chien Y, Bhagwandien RE,Szili-Torok T, Theums DA. Air entrapment causing early inappropriate shocks in a patient with a subcutaneous cardioverterde fibrillator. Heart Rhythm Case Rep 2015;1:156-8.
15. Weiss R, Knight BP, Gold MR,Leon AR, Herre JM, Hood M, et al.Safety and efficacy of a totally subcutaneous implantable-cardioverter de fibrillator. Circulation 2013;128:944-53.
16. Lambiase PD, Barr C, Theuns DA,Knops R, Neuzil P, Johansen JB,et al. Worldwide experience with a totally subcutaneous implantable de fibrillator: early results from the EFFORTLESS S-ICD Registry.Eur Heart J 2014;35:1657-65.
17. Theuns DA, Crozier IG, Barr CS,Hood MA, Cappato R, Knops RE,et al. Longevity of the subcutaneous implantable de fibrillator: long-term follow-up of the European regulatory trial cohort. Circ Arrhythm Electrophysiol 2015;8:1159-63.
18. Boston Scienti fic EMBLEMTDS-ICD pulse generator user’s manual, 359278-002 EN US 2015-02.
19. Aziz S, Leon AR, El-Chami MF.The subcutaneous de fibrillator: a review of the literature. J Am Coll Cardiol 2014;63:1473-9.
20. Gold MR, Weiss R, Theuns DA,Smith W, Leon A, Knight BP, et al.Use of a discrimination algorithm to reduce inappropriate shocks with a subcutaneous implantable cardioverter-de fibrillator. Heart Rhythm 2014;11:1352-8.
21. Jarman JW, Lascelles K, Wong T,Markides V, Clague JR, Till J.Clinical experience of entirely subcutaneous implantable cardioverter-de fibrillators in children and adults: cause for caution. Eur Heart J 2012;33:1351-9.
22. Burke MC, Gold MR, Knight BP,Barr CS, Theuns DA, Boersma LV, et al. Safety and efficacy of the totally subcutaneous implantable de fibrillator: 2-year results from a pooled analysis of the IDE study and EFFORTLESS registry. J Am Coll Cardiol 2015;65:1605-15.
23. Groh CA, Sharma S, Pelchovitz DJ,Bhave PD, Rhyner J, Verma N,et al. Use of an electrocardiographic screening tool to determine candidacy for a subcutaneous implantable cardioverter-de fibrillator. Heart Rhythm 2014;11:1361-6.
24. Olde Nordkamp LR, Warnaars JL, Kooiman KM, de Groot JR,Rosenmöller BR, Wilde AA, et al.Which patients are not suitable for a subcutaneous ICD: incidence and predictors of failed QRS-T-wave morphology screening. J Cardiovasc Electrophysiol 2014;25:494-9.
25. Chan NY, Yuen HC, Mok NS.Right parasternal electrode configuration converts a failed electrocardiographic screening to a pass for subcutaneous implantable cardioverter-de fibrillator implantation.Heart Lung Circ 2015;24:e203-5.
26. Sharma D, Sharma PS, Miller MA,Singh SM, Kalahasty G,Ellenbogen KA. Position and sensing vector-related triple counting and inappropriate shocks in the subcutaneous implantable cardioverter-de fibrillator system. Heart Rhythm 2015;12:2458-60.
27. Boersma L, Burke MC, Neuzil P,Lambiase P, Friehling T,Theuns DA, et al. Infection and mortality after implantation of a subcutaneous ICD after transvenous extraction. Heart Rhythm 2016;13:157-64.
28. Gold MR, Theuns DA, Knight BP,Sturdivant JL, Sanghera R,Ellenbogen KA, et al. Head-tohead comparison of arrhythmia discrimination performance of subcutaneous and tranvenous ICD arrhythmia detection algorithms: the START study. J Cardiovasc Electrophysiol 2012;23:359-66.
29. Brisben AJ, Burke MC, Knight BP,Hahn SJ, Herrmann KL, Allavatam V, et al. A new algorithm to reduce inappropriate therapy in the S-ICD system. J Cardiovasc Electrophysiol 2015;26:417-23.
30. Jarman JW, Todd DM. United Kingdom national experience of entirely subcutaneous implantable cardioverter-de fibrillator technology: important lessons to learn.Europace 2013;15:1158-65.
31. Olde Nordkamp LR, Dabiri Abkenari L, Boersma LV, Maass AH, de Groot JR, van Oostrom AJ,et al. The entirely subcutaneous implantable cardioverter-de fibrillator: initial clinical experience in a large Dutch cohort. J Am Coll Cardiol 2012;60:1933-9.
32. Moss AJ, Schuger C, Beck CA,Brown MW, Cannom DS, Daubert JP, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 2012;367:2275-83.
33. Kooiman KM, Knops RE,Olde Nordkamp L, Wilde AA,de Groot JR. Inappropriate subcutaneous implantable cardioverterde fibrillator shocks due to T-wave oversensing can be prevented:implications for management.Heart Rhythm 2014;11:426-34.
34. Corzani A, Ziacchi M, BiffiM,Diemberger I, Martignani C,Boriani G. Inappropriate shock for myopotential over-sensing in a patient with subcutaneous ICD.Indian Heart J 2015;67:56-9.
35. Frommeyer G, Reinke F, Eckardt L,Wasmer K. Inappropriate shock in a subcutaneous ICD due to interference with a street lantern. Int J Cardiol 2015;198:6-8.
36. Porter field C, DiMarco JP,Mason PK. Effectiveness of implantation of a subcutaneous implantable cardioverter-de fibrillator in a patient with complete heart block and a pacemaker. Am J Cardiol 2015;115(2):276-8.
37. Akin I, Röger S, Borggrefe M,Kuschyk J. Impeding sudden cardiac death despite subcutaneous implantable de fibrillator due to fatal crosstalk. J Cardiovasc Electrophysiol 2015. doi:10.1111/jce.12871.
38. Hong PS, Callinan P, Amit G,Healey JS. Successful implant of a subcutaneous ICD system in a patient with an ipsilateral epicardial pacemaker. Indian Pacing Electrophysiol J 2015;15:62-4.
39. Gemein C, Haj M, Chasan R,Weipert K, Abaci G, Helmig IM,et al. Combining a S-ICD and a pacemaker with abdominal device location and bipolar epicardial LV-lead: first-in-man approach. Heart Rhythm 2015;12(5 Suppl):S534.
40. El-Chami MF, Levy M, Kelli HM,Casey M, Hoskins MH, Goyal A,et al. Outcome of subcutaneous implantable cardioverter de fibrillator implantation in patients with end-stage renal disease on dialysis. J Cardiovasc Electrophysiol 2015;26:900-4.
41. Koman E, Gupta A, Subzposh F,Tiwari A, Fnataine J, Kusmirek SL, et al. Safety and efficacy of subcutaneous implantable cardioverter de fibrillator implantation in patients on hemodialysis. Heart Rhythm 2015;12(5 Supp):S249.
42. Dhamija RK, Tan H, Philbin E,Mathew RO, Sidhu MS, Wang J,et al. Subcutaneous implantable cardioverter de fibrillator for dialy-sis patients: a strategy to reduce central vein stenoses and infections.Am J Kidney Dis 2015;66:154-8.
43. Kiamanesh O, O’Neill D,Sivakumaran S, Kimber S, Kimber S. Inappropriate shocks by subcutaneous implantable cardioverterde fibrillator due to T-wave oversensing in hyperkalemia leading to ventricular fibrillation. Heart Rhythm Case Rep 2015;1:257-9.
44. Lambiase PD, Hood M, Boersma L,Theuns D, Burke M, Weiss R,et al. Combined analysis of subcutaneous ICD outcomes in hypertrophic cardiomyopathy patients from the Effortless registry and IDE study. Heart Rhythm 2015;12(5 Suppl):S15.
45. Francia P, Adduci C, Palano F,Semprini L, Serdoz A,Montesanti D, et al. Eligibility for the subcutaneous implantable cardioverter-de fibrillator in patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol 2015;26:893-9.
46. Maurizi N, Olivotto I, Olde Nordkamp LR, Baldini K,Fumagalli C, Brouwer TF, et al. Prevalence of subcutaneous implantable cardioverter- de fibrillator candidacy based on template ECG screening in patients with hypertrophic cardiomyopathy. Heart Rhythm 2016.Doi:10.1016/j.hrthm.2015.09.007.
47. Frommeyer G, Dechering DG,Zumhagen S, Löher A, Köbe J,Eckardt L, et al. Long-term follow-up of subcutaneous ICD systems in patients with hypertrophic cardiomyopathy: a single-center experience. Clin Res Cardiol 2016;105:89-93.
48. Saeed D, Albert A, Westenfeld R, Maxhera B, Gramsch-Zabel H, O’Connor S, et al. Left ventricular assist device in a patient with a concomitant subcutaneous implantable cardioverter de fibrillator. Circ Arrhythm Electrophysiol 2013;6:e32-3.
49. Gupta A, Subzposh F, Hankins SR,Kutalek SP. Subcutaneous implantable cardioverter-de fibrillator implantation in a patient with a left ventricular assist device already in place.Tex Heart Inst J 2015;42:140-3.
50. Kuschyk J, Stach K, Tülümen E,Rudic B, Liebe V, Schimpf R, et al.Subcutaneous implantable cardioverter-de fibrillator: first singlecenter experience with other cardiac implantable electronic devices.Heart Rhythm 2015;12:2230-8.
51. Bader Y, Weinstock J. Successful implantation of a subcutaneous cardiac de fibrillator in a patient with a preexisting deep brain stimulator. Heart Rhythm Case Rep 2015;1:241-4.
52. Alexander ME, Cecchin F, Walsh EP,Triedman JK, Bevilacqua LM,Berul CI. Implications of implantable cardioverter de fibrillator therapy in congenital heart disease and pediatrics. J Cardiovasc Electrophysiol 2004;15(1):72-6.
53. Pettit SJ, McLean A, Colquhoun I,Connelly D, McLeod K. Clinical experience of subcutaneous and transvenous implantable cardioverter de fibrillators in children and teenagers. Pacing Clin Electrophysiol 2013;36(12):1532-8.
54. Reeves J, Kim J, Mitchell M,McCanta A. Implantation of the subcutaneous implantable cardioverter-de fibrillator with retroperitoneal generator placement in a child with hyoplastic left heart syndrome. Heart Rhythm Case Rep 2015;1:176-9.
55. D’Souza BA, Kim YY, Burke M,Morgan JM, Leon AR, Patton KK,et al. Outcome in patients with congenital heart disease receiving the subcutaneous implantable de fibrillator (S-ICD): results from a pooled analysis from IDE study and EFFORTLESS registry. Heart Rhythm 2015;12(5 Suppl):S225.
56. Zeb M, Curzen N, Veldtman G,Yue A, Roberts P, Wilson D, et al.Potential eligibility of congenital heart disease patients for subcutaneous implantable cardioverterde fibrillator based on surface electrocardiogram mapping. Europace 2015;17(7):1059-67.
57. Mondésert B, Dubuc M, Khairy P, Guerra P, Gosselin G, Thibault B. Combination of a leadless pacemaker and subcutaneous de fi-brillation: first in-human report.Heart Rhythm Case Rep 2015;1(6):469-71.
58. Tjong F, Smeding L, Kooiman K,Olde Norkamp, De Groot J,Wilde A. Combined implantation of a subcutaneous cardioverterde fibrillator (S-ICD) and a leadless pacemaker: evaluation of feasibility, safety, and performance in an ovine model. Heart Rhythm 2015;12(5 Suppl):S64.
59. Keller J, Neužil P, Vymazal J, Janotka M, Brada J, Žácˇek R, et al. Magnetic resonance imaging in patients with a subcutaneous implantable cardioverter- de fibrillator. Europace 2015;17(5):761-6.
 Cardiovascular Innovations and Applications2016年1期
Cardiovascular Innovations and Applications2016年1期
- Cardiovascular Innovations and Applications的其它文章
- Implantable Cardiac De fibrillators: Who Needs Them and Who Does Not?
- Syncope and Early Repolarization: A Benign or Dangerous ECG Finding?
- Changing the Way We “See” Scar:How Multimodality Imaging Fits in the Electrophysiology Laboratory
- Stroke Prevention in Atrial Fibrillation:Current Strategies and Recommendations
- Principles of Arrhythmia Management During Pregnancy
- Current Management of Ventricular Tachycardia:Approaches and Timing
