Syncope and Early Repolarization: A Benign or Dangerous ECG Finding?
Matthew McKillop, MD, FACC, FHRS and William M. Miles, MD, FACC, FHRS
1Division of Cardiovascular Medicine, University of Florida, Gainesville, FL, USA
2North Florida/South Georgia VA Medical Center, Gainesville, FL, USA
Introduction
Early repolarization (ER) is a well-described electrocardiographic variant. It was initially felt to be a benign finding, but publications starting in the 1990s suggested a link between specific types of ER and sudden cardiac death [1, 2]. This association was later termed the J wave syndrome [3]. The odds of ER being associated with ventricular arrhythmias are exceedingly small. Nevertheless, the association of the fairly ubiquitous ECG finding of ER with the rare reported fatal or near fatal outcomes has raised alarm. Can we truly identify high-risk patients by ECG repolarization patterns? If a patient has a serious clinical event such as unexplained syncope with a concerning ECG repolarization pattern, what should be done next?
The best place to start is an understanding of the current de finition of ER and the particular patterns and clinical associations that may predict higher risk for serious ventricular arrhythmia. Past de finitions of ER and the related terminology have been widely variable. In an attempt to standardize the terminology,recent articles, including an expert consensus statement [4], de fine an ER pattern when the peak of the terminal QRS notch or the onset of the terminal QRS slur, also known as the J point or J peak, exceeds 1 mm in at least two contiguous inferior and/or lateral leads (Figure 1). The expert consensus statement [4]de fines the Brugada pattern separately as a characteristic ER change with 2 mm or greater J point elevation in a correctly placed V1or V2lead (Figure 2).
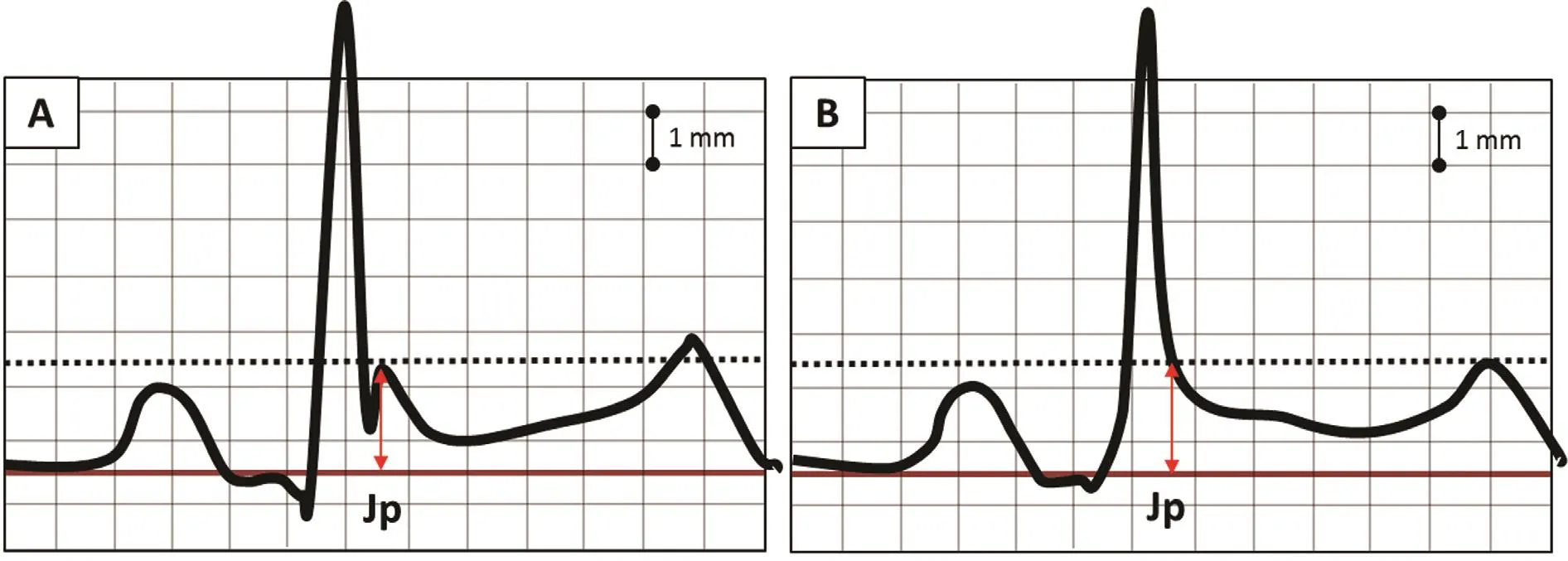
Figure 1 (A) This ECG tracing demonstrates a “notched QRS” and ST segment elevation consistent with early repolarization. The red double arrows represent the J point or peak (Jp). (B) This ECG tracing demonstrates a terminal QRS slur. The red double arrow represents the J point or peak (Jp). The hatched line represents the level to measure elevation above the baseline.The straight line represents the baseline reference level. Note the J point elevation in both tracings is close to 2 mm.
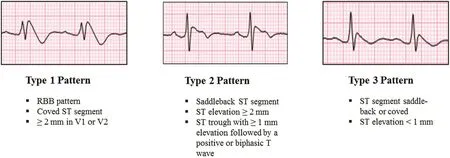
Figure 2 Brugada Patterns.
There are two specific inherited J wave syndromes involving these ER patterns that are associated with sudden cardiac death: ER syndrome and Brugada syndrome (BrS). Both are felt to arise from similar underlying mechanisms but originating from different regions of the ventricular myocardium. BrS involves ST segment elevation and T wave inversion in the right precordial leads as described above, re flecting repolarization heterogeneity in the ventricular out flow region The Brugada repolarization changes are further divided into three types ( Figure 2). The type 1 Brugada pattern has the highest association with sudden cardiac death [5].
ER syndrome is also manifested by ST segment elevation but in different ECG locations. These ER patterns are divided into three types on the basis of the ECG lead location: type 1 or lateral leads, type 2 or inferolateral leads, and type 3 or inferolateral plus anterior precordial leads. Types 2 and 3 are associated with the highest risk for ventricular arrhythmias[6]. In addition to location, certain morphologies of ST segment elevation within the ER pattern have been associated with higher risk for sudden death(analogously to BrS). specifically, the horizontal or descending ST segment portends a higher risk for sudden cardiac death as opposed to the rapidly ascending/upward sloping pattern (Figure 4) [7]. The rapidly ascending/upward sloping pattern is the most common ER pattern in adults and has had no established association with sudden death (Figure 3). A high-risk ER pattern, therefore, demonstrates signi fi-cant ST elevation (typically 2 mm or greater) in either a type 2 or a type 3 location and/or with a horizontal/descending ST segment. ER syndrome consists of a combination of a high-risk ER pattern and a clinical event such as survived sudden death, sustained ventricular arrhythmia, or unexplained syncope. BrS, on the other hand, is de fined simply by the presence of a spontaneous or provoked type 1 Brugada pattern.
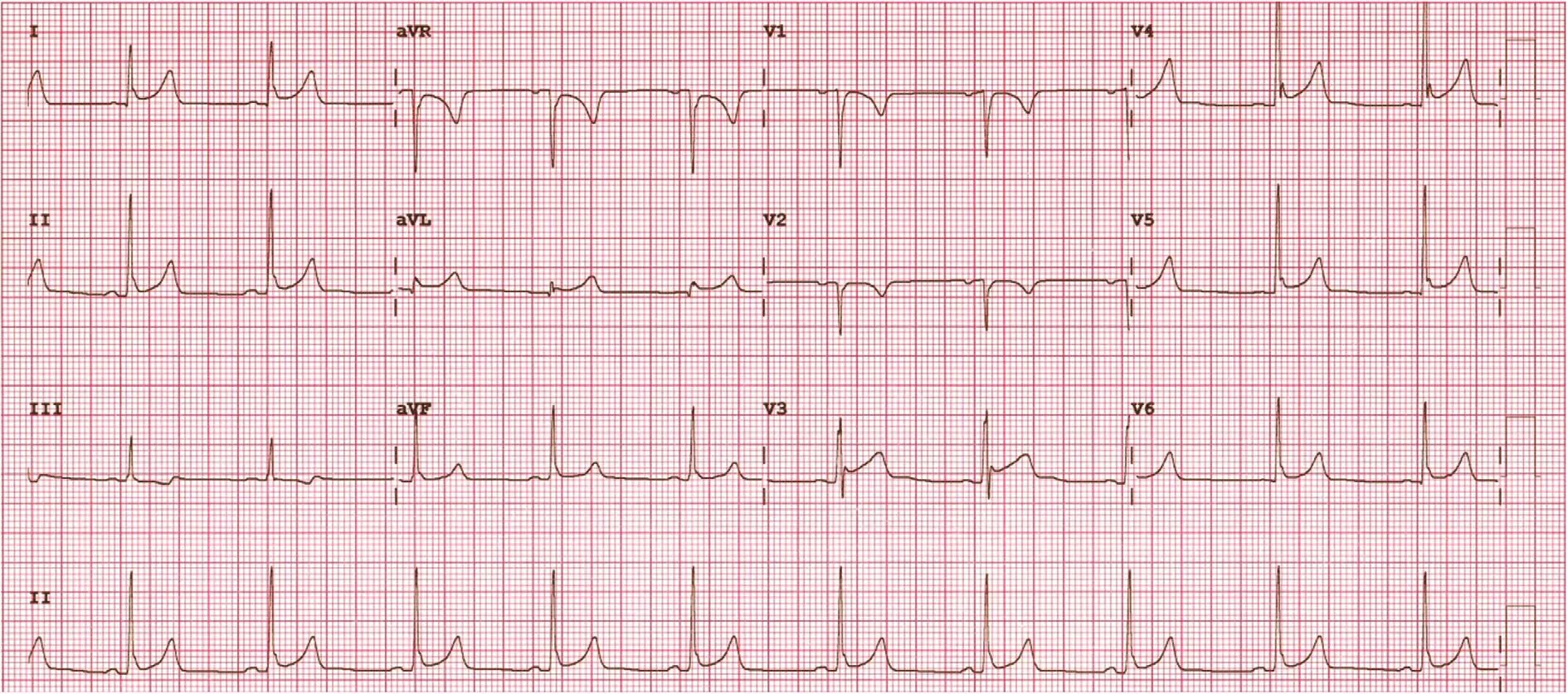
Figure 3 Early repolarization change in inferior and lateral leads or a type 2 early repolarization pattern. The morphology of the ST segment elevation is consistent with the common benign rapidly ascending/upward sloping pattern.
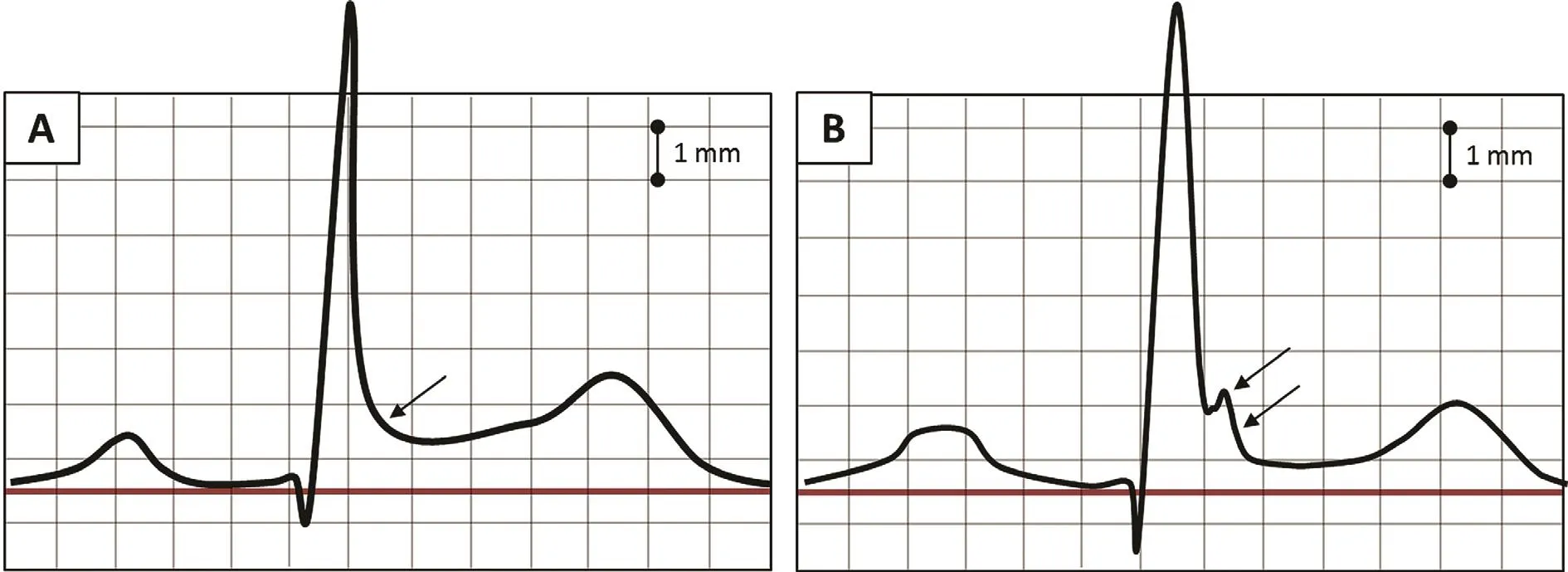
Figure 4 (A) ECG of early repolarization and a rapidly ascending/upward sloping ST segment morphology. Terminal QRS slurring is present (black arrow). This pattern is very common and is not associated with sudden death [7]. (B) ECG of horizontal/descending early repolarization. In the middle-aged general population, this was the only early repolarization that predicted arrhythmic death. Black arrows indicate terminal QRS notching and downward slurring [7].
Prevalence
The prevalence of both syndromes as it relates to sudden death is difficult to know exactly, but it is felt to be very low. The type 1 BrS pattern has a reported prevalence between one in 1000 and five in 10,000 in different population studies [8]. The difficulty with the non-Brugada ER pattern, however, is that it is not rare. In fact, ER patterns are seen in 5-13% of nonathletic adults and in up to 23-44% of young athletes[9]. However, despite J wave abnormalities being a common ECG finding, the overall odds of sudden death associated with any J wave abnormality remain extremely remote, estimated at one in 10,000 [10].
Mechanisms
The J wave and J point elevation causing these patterns is thought to be a consequence of a voltage gradient within the ventricular myocardium set up by dispersion in transient outward currents [11]. The mechanism of arrhythmogenesis associated with ER patterns is still debated, with two currently prevailing explanations. The depolarization hypothesis asserts the presence of slow conduction through principal locations in the right ventricular out flow tract for BrS or in the left inferolateral wall for ER syndrome leads to ventricular tachycardia (VT) or ventricular fibrillation (VF). Electrical mapping of these areas has shown late potentials and fractionated bipolar electrograms, and ablation in the right ventricular out flow tract at these sites has led to clinical improvement in the arrhythmia burden of BrS patients [12]. The repolarization hypothesis, on the other hand, maintains that the outward shift in the balance of transient outward currents causes repolarization abnormalities that, in turn, create phase 2 reentry and resultant tightly coupled premature ventricular beats capable of initiating VT or VF [10]. In a recent study [13], the BrS and ER syndrome channelopathies were pharmacologically mimicked in a coronary perfused canine wedge model with demonstration of both high-frequency late potentials and low-voltage fractionated electrograms. Delayed conduction, however, was not observed anywhere in the model, and instead, the origin of the high-frequency late potential was felt to be due to concealed phase 2 reentry and the electrogram fractionation was felt to be due to regional dysschronization of action potentials. It was speculated that ablation at these sites would also lead to improved clinical outcomes by destruction of the cells principally involved in creating and maintaining the repolarization abnormalities, and these findings were offered as evidence supporting the repolarization hypothesis [13].
Genetics
A multitude of genetic mutations have been identified and associated with both BrS and ER syndrome[10]. These mutations typically lead to a loss of function in sodium or calcium channel currents or a gain of function in the transient outward or the ATP-sensitive potassium currents. Despite many mutations having been identi fied by genetic analysis,its role in the diagnosis and management of these syndromes should be approached with caution. It is clear that we have identi fied only a small percentage of the genetic mutations responsible for these clinical syndromes, and as of yet, there are no reliable data on the predictive nature of the genetic mutations already identi fied. Further work is needed to correlate the known genotypes with phenotypic presentations [10]. Therefore, if genetic testing fails to identify a known genetic mutation, then this does not exclude the presence of a syndrome. Furthermore, genotyping should not be used to predict those at higher risk for sudden cardiac death. Genetic analysis is best suited for the family planning of relatives of an already identi fied proband and as an adjunct test in a clinically suspected or established syndrome providing con firmatory information.
Diagnosis and Risk Strati fication
The diagnosis of both BrS and ER syndrome requires first the establishment of a high-risk pattern on ECG. BrS is diagnosed solely on the basis of the type 1 pattern occurring either spontaneously or provoked by drug challenge (e.g., the use of sodium channel blocker procainamide or flecainide)and does not require a coinciding clinical event [4].ER syndrome has a stricter diagnosis requiring evidence of augmentation of the ER pattern immediately before a VT or VF event. ER syndrome can be strongly considered in the presence of a high-risk ER pattern and a history of sudden cardiac arrest while at rest or sleep [14]. It can also be considered in sudden cardiac death victims with negative autopsy findings, no cause identi fied on medical record review, and a high-risk ER pattern [4].
In most patients who have a concerning ER pattern and have survived sudden cardiac arrest, risk strati fi-cation and subsequent management is relatively clear(Figure 4). However, there are additional historical and ECG findings beyond the above-described highrisk patterns that may provide prediction of higher risk in other individuals. These features include a history of syncope, observed pause-dependent augmentation of the J wave accompanied by T wave inversion, and prominent J point elevation throughout all ECG leads, including a type 1 Brugada pattern [10]. There are still other more obscure findings that investigators have proposed might play a role in negative outcomes, but data for these findings are less robust [10]. Aside from syncope, the presence of any of these additional findings is not enough to warrantimplantable cardioverter-de fibrillator(ICD) placement, but these findings may inform how closely one should follow-up a patient and what future monitoring is considered.
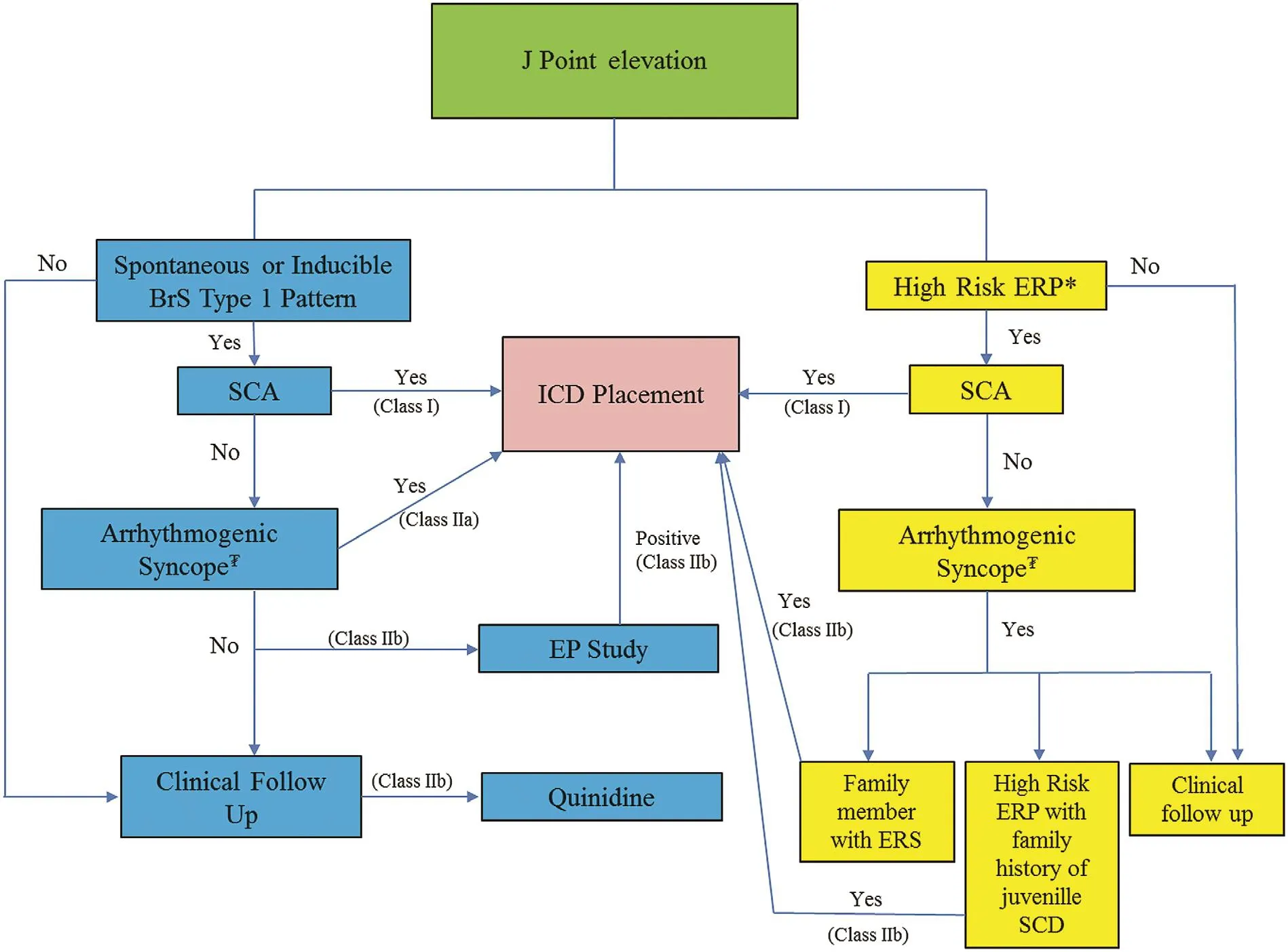
Figure 5 Management of J Point Elevation.BrS, Brugada syndrome; EP, electrophysiology, ERP early repolarization pattern; ERS, early repolarization syndrome; ICD,implantable cardioverter-de fibrillator; SCA, sudden cardiac arrest; SCD, sudden cardiac death; asterisk, a high-risk early repolarization pattern is de fined as 2 mm or greater J point elevation in either inferior leads (type 2) or inferolateral plus right precordial leads (type 3) or any type with horizontal/downward sloping ST segment change; tugrik sign, syncope felt to be likely related to ventricular tachycardia or ventricular fibrillation. Levels of recommendation cited from [4].
Management
ICD Placement
ICD implantation is recommended in patients who have survived sudden cardiac arrest with evidence of BrS or ER syndrome [4]. In the absence of cardiac arrest or sustained ventricular arrhythmias, decision making regarding ICD placement becomes more nuanced and differs slightly between the two syndromes (Figure 5). In BrS, ICD implantation is reasonable with a con firmed spontaneous or induced type 1 Brugada ECG pattern and a history of syncope judged likely to be related to VT or VF[4]. ICD placement in BrS patients regardless of the pattern is not recommended on the basis of family history alone [4]. ICD placement, however, can be considered in BrS patients with inducible VT or VF by programmed stimulation during electrophysiology study. The utility of electrophysiology study is still debated. However, in a recent study outlining their last 20 years of experience, Brugada and coworkers [15] showed the presence or absence of inducible arrhythmia during programmed electrical stimulation had both a high positive and a high negative predictive value, respectively. The protocol used a single right ventricular apical site of stimulation with three basic pacing cycles (600, 500, and 400 ms) and the introduction of one, two, and three ventricular premature beats down to a minimum of 200 ms. Most patients, however, had no inducible ventricular tachyarrhythmias (81.9%), and there were still a number of VT events in these noninducible patients. Consequently, the absence of inducible arrhythmia does not eliminate the risk entirely.
For ER syndrome, ICD placement can be considered in a patient with unexplained syncope who has a family member already identi fied with ER syndrome. An ICD may also be considered in patients with a high-risk ER pattern (de fined as a J point of 2 mm or greater with horizontal/downward sloping morphology) and a strong family history of juvenile sudden death [4]. Electrophysiology study in patients with a non-Brugada ER pattern has not been shown to have a bene ficial role for risk strati fication, and ICD implantation is not recommended in patients with an isolated ER pattern and no other historical or clinical events [4].
Syncope
In both syndromes, unexplained syncope is a poor prognostic indicator. For BrS, suspected arrhythmogenic syncope in combination with a type 1 Brugada pattern should prompt consideration of ICD placement directly. If only a type 2 or type 3 Brugada pattern is present, then drug provocation with sodium channel blockers should be considered to see if a type 1 pattern can be elicited. Patients with syncope and a non-Brugada ER pattern must be quali fied further by the presence of an already identi fied relative with ER syndrome or by a family history of early sudden death combined with a highrisk pattern before ICD placement can be considered [4]. Essentially, patients with syncope who do not have a relative with ER syndrome, a high-risk ER pattern, or a spontaneous/induced type 1 Brugada pattern should be investigated and managed on the basis of clinical assessment independent of the ER pattern [14]. The role of electrophysiology study and programmed ventricular stimulation for induction of ventricular arrhythmias in risk-stratifying syncope patients with these ECG patterns is limited but potentially helpful in those suspected of having BrS as already discussed but has not been not established for ER syndrome. However, electrophysiology study may be useful in selected syncope patients to help exclude other bradyarrhythmic or tachyarrhythmic causes of syncope.
In patients with syncope who do not meet a clear indication for ICD implantation, the use of implantable loop recorders could be considered to provide surveillance rhythm information, allowing a higher degree of con fidence in classifying future clinical events as nonarrhythmogenic. In one small study,11 patients that fell into this category were monitored for 33 months. Eight patients had recurrent syncope. In none of these patients was ventricular arrhythmia recorded during clinical episodes, in half of these patients bradycardia mechanisms were identi fied, and the remaining patients had vasovagal spells or epileptic seizures [16].
Recurrent VT and VT/VF storm
In patients with an ICD already in place, recurrent arrhythmia can be a problem. As the mechanism of both syndromes is thought to be related to voltage gradients exacerbated by transient outward current dispersion, giving a transient outward channel blocker has been proven to be clinically bene ficial[17]. Quinidine is the only commercially available oral agent with transient outward current blocking properties, and it is reasonable to use it in BrS and ER syndrome patients with recurrent VT or VF as a pharmacologic adjunct to ICD therapy. It is also reasonable to use quinidine in BrS patients who have a contraindication for or who refuse ICD placement, and can even be considered in asymptomatic patients with a type 1 Brugada pattern [4].The phosphodiesterase inhibitor cilostazol has also been reported to suppress ventricular arrhythmias associated with J wave syndromes [18].
For patients who are admitted following multiple appropriate ICD therapies for VT or VF in a 24-h period (VT/VF storm), the use of the beta agonist isoproterenol should also be considered for both syndromes [4]. Isoproterenol has been shown to reduce the dispersion in transient outward current in this population, and limited data suggest it can reduce VT/VF episodes during storm [17, 19]. The use of isoproterenol in this patient population is distinctly unique because conventional therapy in all other causes of VT storm involves beta blockade and/or chemical or surgical sympathectomy. Consequently, the use of conventional therapy for VT storm in J wave syndromes may actually exacerbate arrhythmia.
Summary and Take-Home Messages
1. Electrocardiographic ER patterns are common in the normal adult population, especially in young athletes. However, the most frequently observed pattern, the rapidly ascending/upward sloping variety, is not associated with negative outcomes.
2. There are uncommon morphologies, including the type 1 Brugada pattern and type 2 or 3 ER pattern with horizontal/descending ST segments, that have associations with sudden cardiac death. The event rate in such patients is still very low. Therefore, ICD placement is indicated for those patients with high-risk ER patterns and clinical events, specifically those who survived sudden cardiac arrest or sustained ventricular arrhythmias.
3. Unexplained syncope felt to be arrhythmogenic should also raise concern but should not prompt ICD implantation unless a type 1 Brugada pattern is observed spontaneously or with drug provocation. Patients with syncope and a high-risk ER pattern, along with a family member with documented ER syndrome or juvenile relatives with sudden death, can also be considered for an ICD.
4. Genetic screening may be helpful for family planning but has only limited utility for clinical decision making in our current state of knowledge.
5. The use of programmed stimulation for induction of ventricular arrhythmias at electrophysiology study has a limited role in Brugada pattern patients but no documented bene fit in ER syndrome, and implantable loop recorders can provide clinical bene fit allowing more exact classification of future clinical events in patients who do not meet ICD placement criteria.
6. Asymptomatic patients with no signi ficant family history and ER, regardless of the pattern,have good outcomes. Management and continued follow-up of these patients should fall to clinical discretion, and in most cases, no further interventions are needed.
7. In symptomatic patients, additional attention should be paid to whether the ER pattern is associated with higher risk along with family history. Additional interventions in this population may be reasonable, including electrophysiology study, clinical monitoring, and possibly ICD implantation.
Conflict of Interest
The authors declare no Conflict of interest.
REFERENCES
1. Haissaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, de Roy L, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med 2008;358(19):2016-23.
2. Garg A, Finneran W, Feld GK.Familial sudden cardiac death associated with a terminal QRS abnormality on surface 12-lead electrocardiogram in the index case. J Cardiovasc Electrophysiol 1998;9(6):642-7.
3. Antzelevitch C, Yan GX, Viskin S.Rationale for the use of the terms J-wave syndromes and early repolarization. J Am Coll Cardiol 2011;57(15):1587-90.
4. Priori SG, Wilde AA, Horie M,Cho Y, Behr ER, Berul C, et al.HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES,and AEPC in June 2013. Heart Rhythm 2013;10(12):1932-63.
5. Antzelevitch C, Brugada P,Borggrefe M, Brugada J, Brugada R, Corrado D, et al. Brugada syndrome: report of the second consensus conference:endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 2005;111(5):659-70.
6. Antzelevitch C, Yan GX. J wave syndromes. Heart Rhythm 2010;7(4):549-58.
7. Tikkanen JT, Junttila MJ, Anttonen O, Aro AL, Luttinen S,Kerola T, et al. Early repolarization: electrocardiographic phenotypes associated with favorable long-term outcome. Circulation 2011;123(23):2666-73.
8. Hermida JS, Lemoine JL, Aoun FB,Jarry G, Rey JL, Quiret JC. Prevalence of the Brugada syndrome in an apparently healthy population.Am J Cardiol 2000;86(1):91-4.
9. Adler A, Rosso R, Viskin D, Halkin A, Viskin S. What do we know about the “malignant form” of early repolarization? J Am Coll Cardiol 2013;62(10):863-8.
10. Antzelevitch C, Yan GX. J-wave syndromes: Brugada and early repolarization syndromes. Heart Rhythm 2015;12(8):1852-66.
11. Yan GX, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation 1996;93(2):372-9.
12. Nademanee K, Veerakul G, Chandanamattha P, Chaothawee L, Ariyachaipanich A, Jirasirirojanakorn K, et al. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation 2011;123(12):1270-9.
13. Szel T, Antzelevitch C. Abnormal repolarization as the basis for late potentials and fractionated electrograms recorded from epicardium in experimental models of Brugada syndrome. J Am Coll Cardiol 2014;63(19):2037-45.
14. Obeyesekere MN, Klein GJ, Nattel S, Leong-Sit P, Gula LJ, Skanes AC, et al. A clinical approach to early repolarization. Circulation 2013;127(15):1620-9.
15. Sieira J, Conte G, Ciconte G, de Asmundis C, Chierchia GB, Baltogiannis G, et al. Prognostic value of programmed electrical stimulation in Brugada syndrome: 20 years experience. Circ Arrhythm Electrophysiol 2015;8(4):777-84.
16. Kubala M, Aissou L, Traulle S,Gugenheim AL, Hermida JS.Use of implantable loop recorders in patients with Brugada syndrome and suspected risk of ventricular arrhythmia. Europace 2012;14(6):898-902.
17. Dakkak M, Baxi K, Patel A. Bene ficial effects of isoproterenol and quinidine in the treatment of ventricular fibrillation in Brugada syndrome. Case Rep Cardiol 2015;2015:753537.
18. Shinohara T, Ebata Y, Ayabe R,Fukui A, Okada N, Yufu K, et al.Combination therapy of cilostazol and bepridil suppresses recurrent ventricular fibrillation related to J-wave syndromes. Heart Rhythm 2014;11(8):1441-5.
19. Bernard A, Genee O, Grimard C,Sacher F, Fauchier L, Babuty D.Electrical storm reversible by isoproterenol infusion in a striking case of early repolarization.J Interv Card Electrophysiol 2009;25(2):123-7.
 Cardiovascular Innovations and Applications2016年1期
Cardiovascular Innovations and Applications2016年1期
- Cardiovascular Innovations and Applications的其它文章
- Implantable Cardiac De fibrillators: Who Needs Them and Who Does Not?
- The Subcutaneous Implantable Cardioverter-De fibrillator: A Practical Review and Real-World Use and Application
- Changing the Way We “See” Scar:How Multimodality Imaging Fits in the Electrophysiology Laboratory
- Stroke Prevention in Atrial Fibrillation:Current Strategies and Recommendations
- Principles of Arrhythmia Management During Pregnancy
- Current Management of Ventricular Tachycardia:Approaches and Timing
