HBV合并HEV感染导致慢加急性肝衰竭HBV-PC区变异及临床特征分析
信亮亮 李冰 荣义辉
101100 北京 通州区新华医院消化内科(信亮亮,李冰);解放军第三〇二医院肝脏肿瘤诊疗与研究中心(荣义辉)
·论著·
HBV合并HEV感染导致慢加急性肝衰竭HBV-PC区变异及临床特征分析
信亮亮李冰荣义辉
101100北京通州区新华医院消化内科(信亮亮,李冰);解放军第三〇二医院肝脏肿瘤诊疗与研究中心(荣义辉)
【摘要】目的分析乙型肝炎病毒合并戊型肝炎病毒导致的慢加急性肝衰竭的HBV-PC区变异及临床特征。方法回顾性分析HBV感染的慢加急性肝衰竭(ACLF)患者69例,其中单独HBV感染患者39例,HBV合并HEV感染患者30例;比较2组患者肝功能、HBV DNA水平、凝血功能、MELD评分以及预后情况;PCR扩增HBV-PC区序列,测序与ACLF相关的变异位点A1762T、G1764A、C1766T、T1768A、G1896A、A1762T+G1764A、G1764A+C1766T+T1768A,比较两组患者之间的差异。分析HBV合并HEV感染存活与死亡患者,Logistic回归分析重叠感染患者预后相关因素。结果与单纯 HBV感染组比较,合并HEV感染组患者的TBil[(216.4 ± 12.1) μmol/L对(364.2 ± 170.24) μmol/L]、肝性脑病发生率(17.9%对33.3%)、MELD评分(21.26 ± 6.65对28.26 ± 8.65 )均呈不同程度的升高;PTA [(33.3±22.4)%对(24.5±20.1)%]明显降低,差异均有统计学意义(P<0.05);2组患者HBV-PC变异分析比较位点A1762T(66.7%对76.7%)、G1764A(69.2%对80.0%)、A1762T+G1764A(59.0%对70.0%)和G1764A+C1766T+T1768A(2.6%对10.0%) 差异有统计学意义(P<0.05)。HBV合并HEV感染患者存活组与死亡组比较,MELD评分和肝性脑病发生率明显升高,PTA明显降低,差异均有统计学意义(P<0.05); HBV-PC变异分析比较G1764A+C1766T+T1768A 三联变异差异有统计学意义(P<0.05);Logistic回归分析显示,TBil(P=0.006,OR=2.672)、PTA(P=0.036,OR=2.115)、MELD评分(P=0.003,OR=1.682)、肝性脑病并发症(P=0.001,OR=3.631)和G1764A+C1766T+T1768A 三联变异(P=0.043,OR=2.081)因素与预后有关。结论单独HBV与合并HEB感染导致的ACLF患者病情更加严重,预后更差。TBil、MELD评分、肝性脑病并发症和HBV-PC区G1764A+C1766T+T1768A 三联变异发生率越高。PTA越低,ACLF患者预后越差。
【关键词】乙型肝炎病毒;戊型肝炎病毒;慢加急性肝衰竭;HBV-PC区变异
HBV感染可引起多种临床表现,包括无症状HBV携带状态、轻中度和重度慢性乙型肝炎以及慢加急性肝衰竭(ACLF)等。在我国,乙型肝炎相关的ACLF患者占所有ACLF患者80%以上,致死率高达60%~80%[1]。研究显示,病毒和宿主因素在HBV慢性感染的严重程度上可能起着一定作用,HBV 重叠感染其他病毒会使乙型肝炎症状加重,临床以HBV合并HEV感染较为常见。 HBV基本核心启动子(BCP)/前C区的突变可增加病毒复制力,影响HBeAg的表达和分泌,导致HBeAg阴性慢性乙型肝炎,从而影响病情进展[2]。本研究回顾性分析69例ACLF患者,筛选出其中30例HBV合并HEV感染的ACLF患者,对其临床资料和HBV-PC变异作比较分析。
资料和方法
一、一般资料
收集2011年6月至2014年6月北京通州区新华医院住院的 ACLF患者69例,其中单独HBV感染导致ACLF患者39例为对照组;HBV合并HEV感染导致的ACLF患者30例为观察组。HEV感染和ACLF诊断符合2009年《戊型病毒性肝炎诊疗规范》[3]及《肝衰竭诊治指南(2012年版)》标准[4]。排除妊娠期女性、药物性肝损伤、自身免疫性肝病、合并HAV、HCV和HIV感染、肝硬化、肝癌等。
二、观察指标
以诊断患者为ACLF的时间点为基线,收集基线时患者肝功能、HBV DNA水平、凝血功能、MELD评分(MELD评分=3.8×ln[TBil(mg/dL)]+11.2×ln(INR)+9.6×ln[肌酐(mg/dL)]+6.4)。HBV合并HEV感染导致ACLF患者病情好转且稳定24周为存活组,病情恶化、死亡和转院失访的患者为死亡组。
三、患者血清HBV DNA的提取以及HBV-PC区PCR扩增
血清中HBV DNA提取方法参照文献[5],套式PCR扩增BCP/前C区基因片段,产物大小695 bp。 对PCR产物直接测序,结果用Vector NTI 10.0软件进行比对,重点分析5个位点的变异,包括:1762、1764、1766、1768 和1896位点。
四、统计学处理

结果
一、观察组与对照组的临床资料和HBV-PC变异率比较
两组患者性别、年龄、白蛋白、ALT、HBV DNA载量差异无统计学意义,HBV-PC C1766T、T1768A和G1896A位点变异率差异无统计学意义(P>0.05);与对照组相比,观察组在TBil、PTA、MELD评分、合并肝性脑病和24周死亡率升高,HBV-PC A1762T、G1764A、A1762T+G1764A和G1764A+C1766T+T1768A位点变异率升高(P<0.05)。见表1。
二、观察组中存活者与死亡者的临床资料和HBV-PC变异率比较
存活与死亡患者资料单因素分析显示,年龄、TBil、PTA、MELD评分、合并肝性脑病和HBV-PC三联变异G1764A+C1766T+T1768A差异有统计学意义(P<0.05);多因素Logistic回归分析显示,存活组与死亡组在TBil、PTA、MELD评分、合并肝性脑病和HBV-PC三联变异G1764A+C1766T+T1768A等5种风险因素与预后相关,见表2。
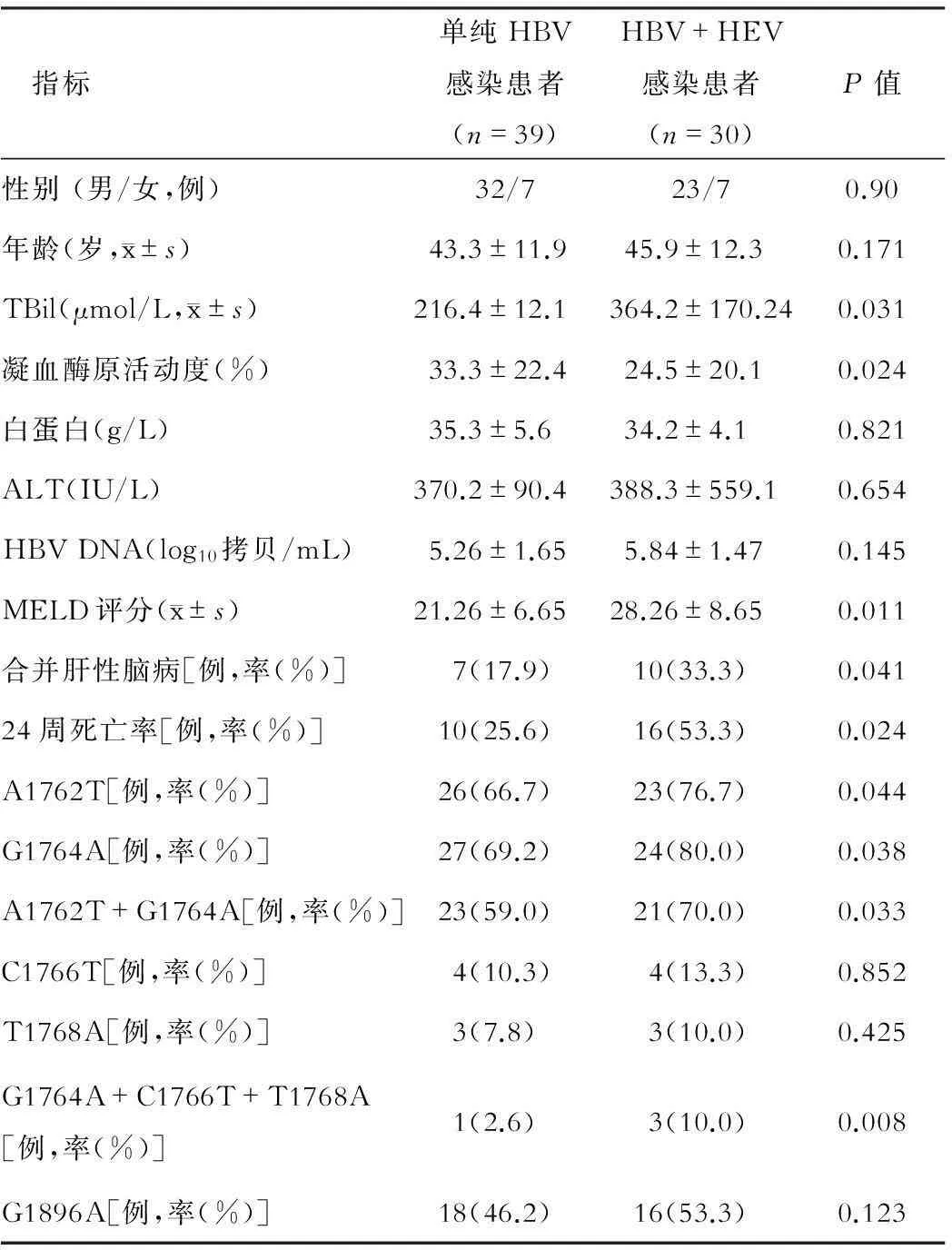
表1 两组患者临床资料和HBV-PC变异位点比较
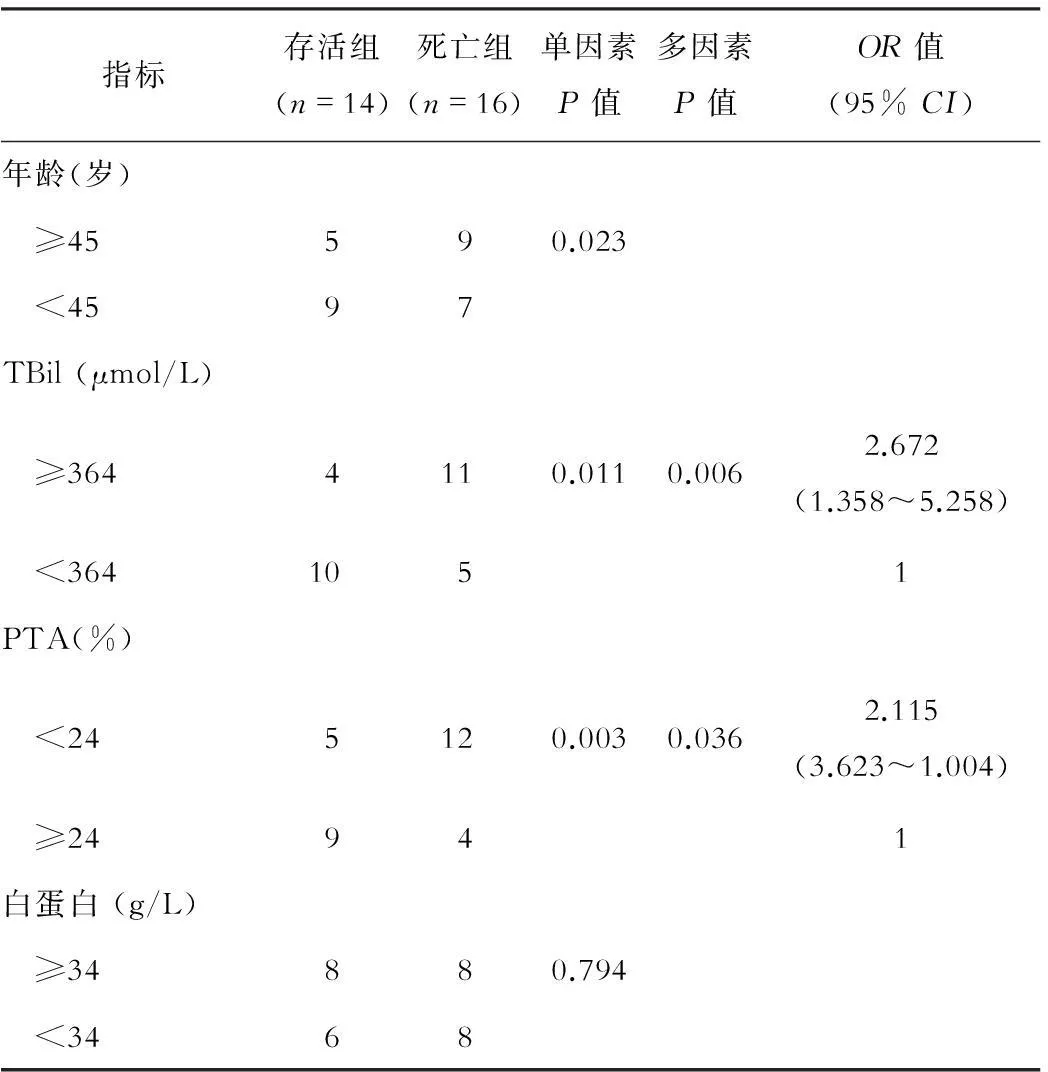
表2 HBV合并HEV感染患者存活组与死亡组临床资料比较
续表2
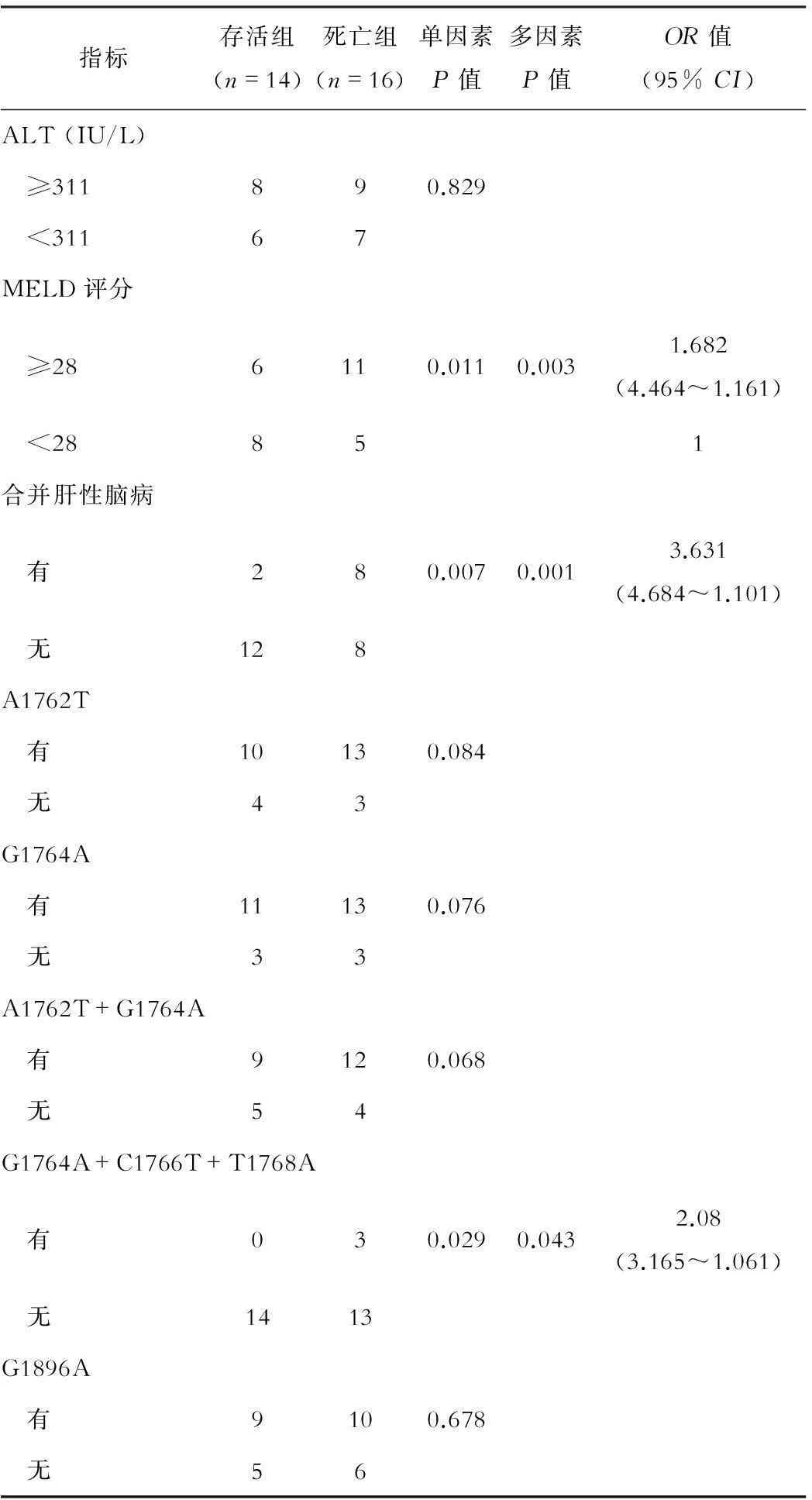
指标存活组(n=14)死亡组(n=16)单因素P值多因素P值OR值(95%CI)ALT(IU/L) ≥311890.829 <31167MELD评分 ≥286110.0110.0031.682(4.464~1.161) <28851合并肝性脑病 有280.0070.0013.631(4.684~1.101) 无128A1762T 有10130.084 无43G1764A 有11130.076 无33A1762T+G1764A 有9120.068 无54G1764A+C1766T+T1768A 有030.0290.0432.08(3.165~1.061) 无1413G1896A 有9100.678 无56
讨论
我国HBV感染人群约 9300万例[4],全球每年新发戊肝约3400 万例,其中死亡30万例[6]。由于各型嗜肝病毒之间没有交叉免疫性, 在慢性乙型肝炎患者病程中仍可以重叠合并感染HEV。慢性乙型肝炎重叠急性戊型肝炎患者与单独慢性乙型肝炎患者相比TBil、并发症发生率、重型肝炎发生率和病死率均明显升高, 而 ALT、Alb和 PTA均明显降低[7]。已有研究提示PC 区突变可能是影响HBV感染疾病进展的重要因素之一。本研究回顾性分析了HBV合并HEV感染导致 ACLF 患者临床资料,发现与单纯HBV感染导致ACLF患者比较,HBV合并HEV感染导致的ACLF患者TBil、MELD评分、合并肝性脑病发生率和病死率明显升高,PTA则明显降低;A1762T、G1764A、A1762T+G1764A和G1764A+C1766T+T1768A位点变异率升高。以上分析结果提示HBV合并感染HEV相关的ACLF患者病情更为严重,预后更差。
ACLF发病较为复杂,而慢性乙型肝炎合并HEV感染病情加重目前机制还不清楚。急性戊型肝炎的发病主要与细胞免疫有关,慢性乙型肝炎患者合并HEV感染后,HEV直接破坏并诱发免疫反应对肝细胞造成损伤[8];也会产生一些诱发因子,刺激免疫细胞,介导TNF-α、IL-6等已知与凋亡相关的细胞信号途径,使肝细胞进一步损伤[9]。HBV 基因变异导致的病毒生物学特性改变也是导致ACLF的重要因素之一[10]。慢性乙型肝炎患者合并HEV感染以后,可能加速了肝病的发展进程,导致患者病情加重。而HEV分泌蛋白可能直接或间接与HBV调控区相互作用,使HBV向病程加重的方向选择性变异。HBV合并HEV导致ACLF患者预后多因素分析显示,存活者和死亡者TBil、PTA、MELD评分、合并肝性脑病和HBV-PC三联变异G1764A+C1766T+T1768A等5种风险因素与预后相关。研究表明,MELD评分可用于急性肝衰竭和ACLF的预后评估[11]。合并肝性脑病等并发症在肝衰竭患者中经常出现,是影响ACLF患者预后的重要因素,G1764A+C1766T+T1768A变异也有报道,该三联变异是ACLF患者HBV序列特有,具有激活下游基因调控的功能[11-12]。
总之,HBV合并HEV感染导致的ACLF患者病情更重,预后更差,并且HBV-PC的调控区变异也向使ACLF加重的方向发展,但本研究样本量较小,今后将在增大样本量的基础上进一步分析。
参考文献
1 Ozasa A, Tanaka Y, Orito E, et al. Influence of genotypes and precore mutations on fulminant or chronic outcome of acute hepatitis B virus infection. Hepatology, 2006,44:326-334.
2 Chen MT, Billaud JN, Sallberg M, et al. A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen.Proc Natl Acad Sci USA, 2004, 101: 14913.
3 中国医师协会感染科医师分会. 戊型病毒性肝炎诊疗规范. 中华临床感染病杂志, 2009, 2: 260-263.
4 中华医学会感染病学分会肝衰竭与人工肝学组,中华医学会肝病学分会重型肝炎与人工肝学组. 肝衰竭诊治指南(2012年版). 中华临床感染病杂志,2012, 5(6): 321-327.
5 Xu ZH, Ren XQ, Liu Y, et al. Association of hepatitis B virus mutations in basal core promoter and precore regions with severity of liver disease: an investigation of 793 Chinese patients with mild and severe chronic hepatitis B and acute-on-chronic liver failure. J Gastroenterol, 2011, 46: 391-400.
6 WHO. Viral hepatitis in the WHO south-east Asia region. India: regional office for south-east Asia, 2011.
7 Acharya SK, Sharma PK, Singh RA, et al. Hepatitis E virus(HEV) infection in patients with cirrhosis associated with rapid decompen sation and death.J Hepatol, 2007, 46: 387-394.
8 Wedemeyer H, Pischke S, Manns MP. Pathogenesis and treatment of hepatitis E virus infection. Gastroenterology,2012,142:1388.
9 Salam GD, Kumar A, Kar PA, et al. Serum tumor necrosis factor-alpha level in hepatitis E virus-related acute viral hepatitis and fulminant hepatic failure in pregnant women.Hepatol Res, 2013,43:826-835.
10Gérolami R, Henry M, Borentain P, et al. Fulminant hepatitis B associated with a specific insertion in the basal core promoter region of hepatitis B virus DNA after immunosuppressive treatment. Clin Infect Dis, 2005, 40: 24.
11Sun QF, Ding JG, Xu DZ, et al. Prediction of theprognosis of patients with acute-on-chronic hepatitis B liver failure using the model for end-stage liver disease scoring system and a novel logistic regression model. J Viral Hepat,2009,16: 464-470.
12丁剑波,罗晓岚,田一梅,等. 慢性乙型重型肝炎预后影响因素 Logistic回归分析.传染病信息,2012,25:161-163.
13王耀,刘妍,许智慧,等.G1764A/C1766T/T1768A三联突变对HBV核心启动子活性的上调作用.解放军医学杂志,2010,35: 954-957.
(本文编辑:钱燕)
Analysis of HBV PC mutations in ACLF patients infected with HBV combined HEV and their clinical characteristics
XINLiang-liang,LIBing,RONGYi-hui.
ThedigestiveinternalmedicineofXin-HuahospitalinTongzhoudistrict,Beijing101100,China
【Abstract】ObjectiveTo study hepatitis B virus (HBV) basic core promoter and pre-C (PC) nucleotides mutations in patients coinfected with hepatitis E virus (HEV) related acute on chronic liver failure (ACLF) , and to analyze the clinical characteristics. MethodsSixty-nine cases with ACLF caused by HBV infection were retrospectively analyzed, of which 39 patients suffered single HBV infection, and the other 30 patients suffered HBV and HEV coinfection. Liver function, HBV DNA level, blood coagulation function, model for end stage liver disease (MELD) score and prognosis of the two groups were compared respectively. Polymerase chain reaction (PCR) was used for assessing HBV PC region amplification. Sequencing analysis was applied to detect reported ACLF-related mutation sites A1762T, G1764A, C1766T, T1768A, G1896A, A1762T + G1764A and G1764A + C1766T + T1768A for comparative analysis between the two groups. The survival rates and mortality of patients with HBV and HEV coinfection were evaluated for related prognostic factors by logistic regression analysis. ResultsIn the coinfection group, total bilirubin (TBiL), incidence of hepatic encephalopathy and levels of the MELD score were significantly increased (216.4 ± 12.1 vs 364.2 ± 170.24, 17.9% vs 33.3%, 21.26 ± 6.65 vs 28.26 ± 8.65, respectively) when compared with those in single infection group, while prothrombin time activity (PTA) was obviously decreased (33.3 ± 22.4 vs 24.5 ± 20.1) (P<0.05). The comparative analysis of HBV-PC mutation sites A1762T (66.7% vs 76.7%), G1764A (69.2% vs 80.0%), A1762T+G1764A (59.0% vs 70.0%) and G1764A+C1766T+T1768A (2.6% vs 10.0%) showed statistically differences between the two groups (P<0.05). Additionally, among HBV and HEV coinfection patients, the MELD score and incidence of hepatic encephalopathy in death group increased significantly when compared to those in survival group, but the PTA decreased obviously (P<0.05). The comparative analysis of HBV-PC mutation site G1764A + C1766T + T1768A triplet mutation also showed significant difference (P<0.05) between the two groups in patients with coinfection. The logistic regression analysis suggested that TBiL (P=0.006, OR=2.672), PTA (P=0.036, OR=2.115), MELD score (P=0.003, OR=1.682), hepatic encephalopathy incidence (P=0.001, OR=3.631) and G1764A + C1766T + T1768A triplet mutation (P=0.043, OR=0.043) were all related to the prognosis. Conclusion The prognosis is poor in ACLF patients caused by HBV and HEV coinfection, which could be indicated from high TBiL level, MELD score, incidence of hepatic encephalopathy and HBV-PC area G1764A+C1766T+T1768A triplet mutation rate. Furthermore, lower PTA level always predicts a worse prognosis.
【Key words】Hepatitis B virus; Hepatitis E virus; Acute-on-chronic liver failure; HBV-PC nucleotides mutations
(收稿日期:2015-11-01)
Corresponding author:XIN Liang-liang, Email: xinliangliangwz_1980@sina.com
通信作者:信亮亮,Email:xinliangliangwz_1980@sina.com

