Bee pollen extract of Malaysian stingless bee enhances the effect of cisplatin on breast cancer cell lines
Bee pollen extract of Malaysian stingless bee enhances the effect of cisplatin on breast cancer cell lines
Wan Adnan Wan Omar*, Nur Asna Azhar, Nurdianah Harif Fadzilah, Nik Nur Syazni Nik Mohamed Kamal Advanced Medical and Dental Institute, Universiti Sains Malaysia, Bertam, 13200, Penang, Malaysia
Entomological research http://dx.doi.org/10.1016/j.apjtb.2015.12.011
Tel: +60 45622570
E-mail: wanadnan@usm.my
Foundation Project: Supported by Fundamental Research Grant Scheme, Ministry of Education Malaysia (Grant No: FRGS/2/2013/SKK01/USM/03/3).
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 10 Nov 2015
Received in revised form 30 Nov 2015
Accepted 8 Dec 2015
Available online 31 Dec 2015
Keywords:
Bee pollen extract
Antioxidant
Antiproliferative activity Synergistic effect
Malaysian stingless bee
ABSTRACT
Objective: To evaluate the antioxidant and antiproliferative effect of methanolic bee pollen extract (BPE) of Malaysian stingless bee [Lepidotrigona terminata (L. terminata)] and its synergistic effect with cisplatin (a chemotherapeutic drug) on MCF-7 cancer cell line.
Methods: The antioxidant activity of BPE from L. terminata was measured by using 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) assay. Antiproliferative activity at different concentrations of BPE and cisplatin was determined through using MTT assay on MCF-7 and L929 cell lines. An interaction effect (synergistic, additive and antagonistic) between BPE and cisplatin was determined by CompuSyn software based on MTT assay data.
Results: The EC50(50% decrement of DPPH inhibition) of BPE was 0.5 mg/mL. L. terminata BPE exhibited antiproliferative activity on both cancer and normal cell lines. The IC50(concentration of drug that was required for 50% of cell inhibition in vitro) of BPE on MCF-7 was 15 mg/mL whereas in normal cell line L929 was 26 mg/mL. The IC50for cisplatin on MCF-7 was 20 mmol/L. The combination effect of BPE and cisplatin on MCF-7 cells showed that BPE at 15 mg/mL was able to potentiate the inhibitory effect of cisplatin at all different concentrations (2.5-20.0 mg/mL). The average of cancer cells inhibition which was potentiated by BPE was around 50%. A combination index values of less than 1 reported in the CompuSyn software further proved the synergistic effect between BPE and cisplatin, suggesting that BPE was working synergistically with cisplatin.
Conclusions: Our study therefore suggested that BPE of Malaysian stingless bee, L. terminata is a potential chemopreventive agent and can be used as a supplementary treatment for chemotherapy drugs. BPE might be able to be used to potentiate the effect of chemotherapy drugs with the possibility to reduce the required dose of the drugs. The molecular mechanisms of how the BPE exerts antiproliferative activity will be a much interesting area to look for in future studies.
1. Introduction
Bee pollen is considered as a functional food due to its compositions of carbohydrates, proteins, amino acids, lipids, sugars,fibers, vitamins and mineral salts[1,2]. It is a collection of pollen grains collected by the bees from various botanical sources, mixed with nectar and secretion from the hypopharyngeal glands such as b-glycosidase enzymes. Its beneficial effect on health is due to the presence of phenolic compounds with high antioxidant activities[1,3].
Oxidative damage and carcinogenesis may gradually lead to the formation of tumors through several mechanisms. Polyphenols present in bee pollen and other bee products contain antioxidant and antiproliferative activities that can regulate cell proliferation and induce apoptosis [4]. Bee pollen has the antioxidative capacity and the ability to neutralize active oxygen species, extensively due to the action of polyphenolscombination. Previous studies have demonstrated that bee pollen can inhibit tumor growth and alleviate the pain of chemotherapy in cancer patients [5,6].
In China, bee pollen of Brassica campestris L. (B. campestris) has been widely used as food supplement to strengthen body resistance against cancer [7]. A steroid fraction of bee pollen chloroform extract of B. campestris was shown to strongly induce apoptosis in human prostate cancer PC-3 cells [7]. A study by Izuta et al. showed that bee pollen was able to suppress cells proliferation in in vitro model of vascular endothelial growth factor-induced human umbilical vein endothelial cells proliferation and migration [8]. Constituents of bee pollen such as polysaccharides were also shown by Wang et al. to have significant antiproliferative activity in colon cancer cell lines [5]. Thus the aim of the study is to evaluate the antiproliferative activity of bee pollen extract (BPE) from Malaysian stingless bee, Lepidotrigona terminata (L. terminata) and its effect when combined with cisplatin on MCF-7 cancer cell lines.
2. Materials and methods
2.1. Samples and reagents
Bee pollen or bee bread sample was acquired from a local stingless bee company, K.B meliponini. The source of bee pollen was from the species of L. terminata. The species was identified by the Entomology Section, Malaysian Agriculture and Research Development Institute, Serdang, Malaysia. Bee pollen sample was dried at 37°C for two consecutive days and once dried, was kept at 4°C until further use.
2.2. Bee pollen crude extract
The crude BPE was prepared by using modified method from LeBlanc et al. by extracting 10 g of bee pollen in 25 mL of 100% methanol [9]. The mixture was vortexed for 10 min and sonicated for 1 h at 41°C before kept overnight at 4°C. The mixture was then centrifuged at 6000 r/min for 10 min and the precipitation was discarded. The supernatant was filtered by using a 0.2 mm sterile filter unit (Millipore, USA). Once filtered, it was dried in the rotary evaporator at 41°C and freeze dried. The freeze dried BPE was kept at 4°C until further analysis.
2.3. Antioxidant activity
The free radical-scavenging capacity of bee pollen methanolic extract was determined by its ability to bleach the stable 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) and was expressed as the percentage of its neutralization. A modification of a method reported by Baharum et al. was employed[10]. The reaction mixture contained 150 mL of DPPH (600 mmol/L) with 7.5 mL of different concentrations of BPEs (3.125, 6.250, 12.500, 25.000, 50.000 and 100.000 mg/mL). The final concentrations of bee pollen were 0.15, 0.30, 0.60, 1.20, 2.40 and 4.80 mg/mL. After 30 min at room temperature, the reaction mixture was measured by the absorbance at λ= 517 nm. Trolox, a water-soluble derivative of vitamin E at concentration of 25-600 mmol/L, was used as standard. DPPH free radical scavenging activity was calculated through using the following formula:

2.4. Cell culture
MCF-7 and L929 cell lines used in this study were obtained from the Advanced Medical and Dental Institute, Universiti Sains Malaysia. The cell lines were cultured in a complete Dulbecco's modified Eagle's medium (DMEM) containing 4 mmol/L L-alanyl-glutamine (GlutaMAX™) and supplemented with 10% (v/v) fetal bovine serum, 100 IU/mL of penicillin and 0.1 mg/mL of streptomycin. The cells were grown in humidified atmosphere under the conditions of 37°C with 5% (v/v) CO2.
2.5. Cell counts
MCF-7 and L929 cells were harvested by removing the medium, washed with phosphate buffer saline (pH 7.45 without Ca2+and Mg2+) and then incubated with 1 mL of 0.25% trypsin for 2 min. The flask was gently tapped to detach them from the plastic surface. Five milliliters of medium was added to the cell suspension while the remaining cells were vigorously washed from the bottom of the culture vessel. The suspended cells were then collected in a 15 mL centrifuge tube and an aliquot of 10 mL was taken out prior centrifugation for cell count by using hemocytometer. The number of cells per milliliter and the total cell number were calculated. Subsequently after centrifugation, an appropriate volume of complete medium was added to the cell pellet.
2.6. Preparation of compound treatments
BPE stock solution (100 mg/mL) was prepared by diluting 0.1 g of crude extracts in 1 mL DMEM. The solution was then filtered by using a 0.2 mL sterile filter unit (Millipore, USA) and diluted with culture medium to the final concentration of 2.5 mg/ mL, 5 mg/mL, 10 mg/mL, 20 mg/mL and 40 mg/mL for treatments.
Cisplatin stock solution (10 mol/L) was prepared by dissolving 6 mg of cisplatin (molecular weight = 300.01 g/moL) in 2 mL DMEM. The stock solution was then diluted with culture medium to the final concentration of 2.5 mmol/L, 5 mmol/L, 10 mmol/L, 20 mmol/L and 40 mmol/L for treatments. The working solutions of the drug were freshly prepared prior usage.
2.7. Cytotoxicity determination by using MTT assay
Briefly, the cells were seeded at density of 1×104cells per well of flat-bottomed 96-well microplate. The cells were incubated at 37°C in a humidified 5% (v/v) CO2for 24 h to let the cells attach to the bottom of each well. The cultured cells were then treated with different concentrations of BPE and cisplatin, alone and with combination when the cells reached 70% confluence.
The cells were treated with 5 different concentrations of BPE (2.5, 5.0, 10.0, 20.0 and 40.0 mg/mL) and 5 different concentrations of cisplatin (2.5, 5.0, 10.0, 20.0 and 40.0 mmol/L). A total of 200 mL of medium containing compound treatments was added into each well. Treatments were carried out in triplicate. Well with only treatment solution (either BPE or cisplatin) was used as blank or background control, whereas well containing cells without treatment was used as negative control. Once treated, cells were incubated for 48 h.
The percentage of cell proliferation was determined by using MTT assay with the Vybrant®MTT cell proliferation assay kit (Thermo Fisher Scientific, USA). In brief, the fresh medium was replaced after 48 h of incubation. Ten microliters of the 12 mmol/L MTT stock solution were added into each well. The cells with MTT solution were incubated in the CO2incubator for 4 h. The medium was then removed from the well after the cells were labeled with MTT. DMSO (50 mL) was added to each well and mixed thoroughly with a pipette. The plate was incubated again at 37°C for 10 min. Finally the absorbance was read at 540 nm. The percentage of cell proliferation was calculated by using the formula below:

2.8. Analysis of synergistic effects
To determine the synergistic, additive or antagonistic effect, experiment with non-constant combination of BPE and cisplatin concentrations was done following the method by Qiao et al. with some modifications [11]. The cells were treated with cisplatin at concentrations of 2.5, 5.0, 10.0, 15.0, 20.0 mmol/L in combination with fixed concentration of IC50of BPE at 15 mg/mL. The data from this experiment were analyzed through using CompuSyn software to determine the synergistic, additive and antagonistic effect of the drugs combination. The statistical analysis was performed and the result was presented as combination index (CI). A CI value is a mathematical and quantitative representation of the pharmacological interplay of two drugs (CI>1: antagonism; CI = 1: additive; CI<1: synergism) [12].
2.9. Statistical analysis
All values were expressed as the mean±SD. The differences in the mean of treated and untreated cells were analyzed by using IBM SPSS Statistics 22 (US).
3. Results
3.1. Antioxidant capacity of BPE
In this study, the antioxidant activity of methanolic crude extract of L. terminata BPE measured by using DPPH assay showed a concentration dependent manner in the dose response curve (Figure 1). The EC50(50% decrement of DPPH inhibition) of BPE was 0.5 mg/mL.
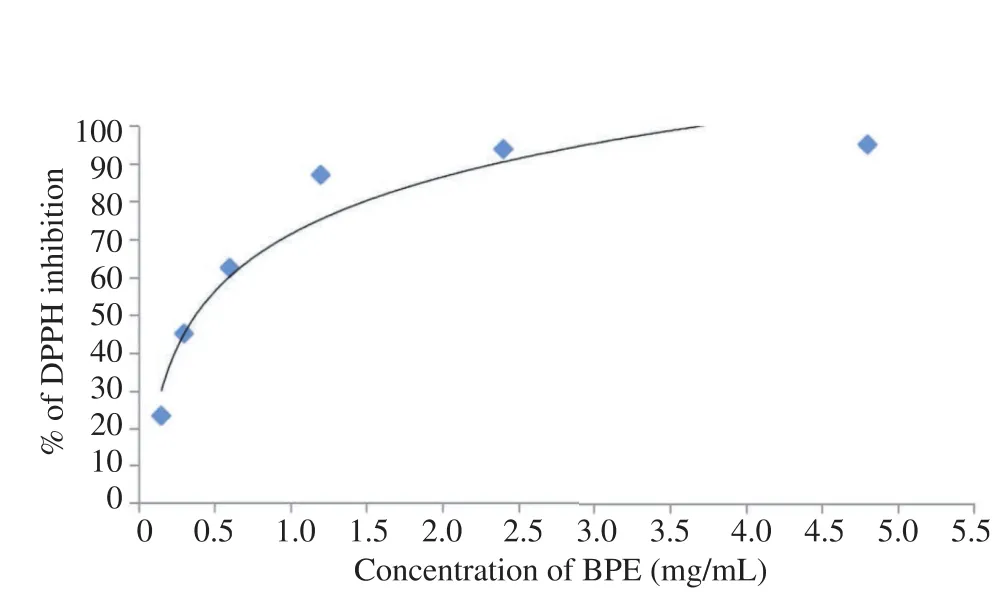
Figure 1. Scavenging effect of methanolic extract from L. terminata BPE at different concentrations (0.15, 0.3, 0.6, 1.2, 2.4 and 4.8 mg/mL) on the stable DPPH.Results were expressed as percentage of DPPH inhibition with respect to control; Each value represents the mean±SD.
3.2. Antiproliferative effect in vitro
The potential antiproliferative effect of BPE was assayed by using the surrogate cell viability MTT assay. Crude BPE was shown to inhibit the growth of both cancer and normal cell lines. The inhibition of cell growth on the breast cancer MCF-7 and normal L929 cell lines by BPE of L. terminata was in dosedependent manner (Figure 2). The IC50of BPE on MCF-7 was 15 mg/mL, whereas in normal cell line was 26 mg/mL. BPE at 20 mg/mL inhibited 70% of cell viability in the MCF-7 cells (Figure 2A) whereas in normal L929 cell lines, inhibition of 70% of cell viability was observed with much higher concentration of 40 mg/mL (Figure 2B).
3.3. Cytotoxic synergism of BPE with chemotherapy drug
To determine the combination effect of BPE and cisplatin (an anticancer chemotherapy drug), we have designed a nonconstant combination treatment of cisplatin and BPE on MCF-7 cells. Serial concentrations of cisplatin were combined with the fixed concentration of BPE at 15 mg/mL. Higher MCF-7 cells inhibition was seen in BPE (15 mg/mL) and cisplatin at all concentrations when compared to cisplatin alone. BPE combination with 2.5 mmol/L cisplatin inhibited 55% of MCF-7 cells proliferation when compared with 2.5 mmol/L cisplatin alone. Whereas BPE in combination with 20 mmol/L cisplatin could further reduce cell proliferation up to 76% compared to 52% reduction seen in treatment of 20 mmol/L cisplatin alone, although it was not statistically significant. The result showed that the BPE was able to potentiate the inhibitory effect of cisplatin in breast cancer cells when combined with cisplatin at all concentrations (Figure 3).
To further confirm the interaction between cisplatin and BPE, the data was computed in CompuSyn software. CI values of less than 1 were seen in all interactions between BPE and cisplatin. Reported results in the CompuSyn software were between 0.46 and 0.97 (Table 1). The lowest CI value was seen in combination of BPE and cisplatin at 10.0 mmol/L dose.
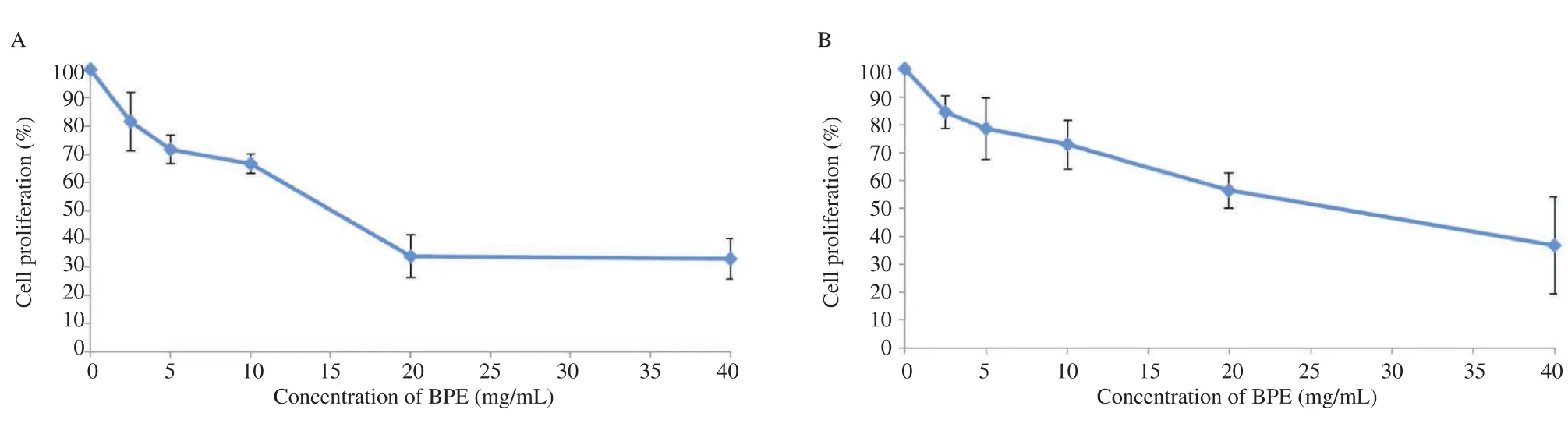
Figure 2. Antiproliferative effect of BPE at different concentrations on MCF-7 cell lines and L929 cell lines. A: MCF-7 cell lines; B: L929 cell lines; Each value represents the mean±SD from three separate experiments.

Figure 3. The effect of cisplatin alone and combination of cisplatin and BPE on MCF-7 cells.The percentage of cell proliferation was measured at 48 h post-treatment; Each value represents the mean±SD from three separate experiments; Statistical analysis was determined by using student's t-test with**: P<0.001;*: P<0.05.
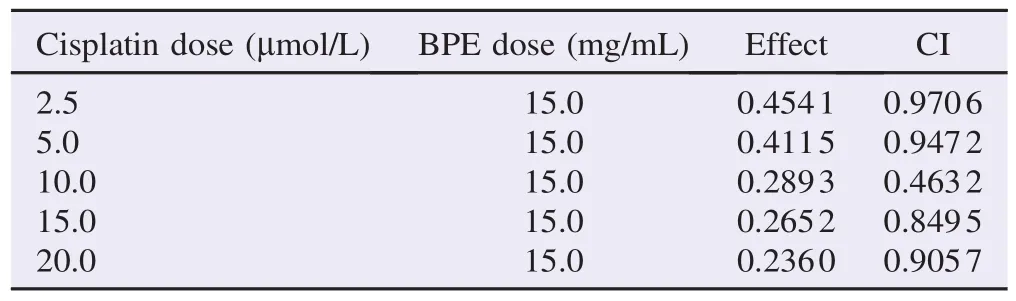
Table 1 CI data for combination effect of cisplatin and BPE on MCF-7 cells.
4. Discussion
Our study showed that the antioxidant activity of L. terminata BPE exhibited much higher percentage of DPPH inhibition than other Malaysian stingless bee species reported recently. BPE from species Trigona apicalis and Trigona itama at concentration of 1 mg/mL showed 39% and 19% of DPPH inhibition, respectively, compared to 70% DPPH inhibition of 1 mg/mL methanolic BPE from L. terminata[13]. However, the result of this study showed lower inhibition than that of Silva et al. which reported that crude BPE from the Brazilian stingless bee species Melipona rufiventris at concentration 0.1 mg/mL was shown to exhibit 50% DPPH inhibition [14].
The difference in antioxidant activity seen in our study could be due to the difference of the solvent used (methanol in this study vs. ethanol in the study by Silva et al.), which may give different compounds extraction efficacy, therefore contributed to the different chemical activities [14,15]. Furthermore specific pollen foraging activities, different pollen species and different diets of stingless bee itself may give different compounds activities found in BPE [14,16]. Previous studies have shown that bee pollen has good antioxidant properties related to the presence of phenolic compounds [2,17].
The antiproliferative activity of L. terminata BPE against MCF-7 and L929 cell lines was evaluated by using MTT assay. The concentration of BPE required to inhibit 50% of MCF-7 cells growth was 15 mg/mL. At 20.0 mg/mL, 70% inhibition of MCF-7 cells was seen. Whereas in normal L929 cell line, 26 mg/mL was required to exhibit 50% cells inhibition. The response different between normal and cancer cells may suggest that BPE can be relatively safe in normal cells and considered to be a useful agent for anticancer application in future. The much lower IC50of BPE in cancer cells suggests that BPE may be specifically targeting the MCF-7 cancer cells.
Similar antiproliferative effect of BPE had been reported in the study by Kustiawan et al. by using four stingless bee species [18]. The result revealed that all four species were cytotoxic to different cell lines tested although their antiproliferative activities were rather low compared to the chemotherapeutic drugs used. Another study by Wu and Lou also showed strong antiproliferative activity of B. campestris bee pollen chloroform extract in inducing apoptosis in human prostate cancer [7]. The observed cytotoxic activity exhibited by bee pollen varied with the different solvent used, bee species and different cell lines.
In cytotoxic synergism assay, the average of MCF-7 cells inhibition by BPE in combination of cisplatin was around 50%. This study shows that BPE is able to potentiate and enhance the cisplatin effect on MCF-7 cells at all concentrations.
Active compounds in the BPE might either enhance (synergistic) or decrease (antagonistic) the therapeutic activity of chemotherapy drugs. There was a great concern that some compounds present in the natural product might work antagonistically instead of synergistically with the therapeutic activity of drugs[19]. In this study, CI values reported in all interactions between BPE and cisplatin were less than 1. These CI values further proved the synergistic effect between the two compounds.
This study may suggest that BPE from Malaysian stingless bee L. terminata can be used to supplement the chemotherapeutic agents due to its antiproliferative activity and its ability to enhance the effect of chemotherapy agent, even at low concentration. In a recent review by Komosinska-Vassev et al., beepollen supplement can help to improve the condition of early prostate cancer when given together with chemotherapeutic drug [20]. Furthermore, bee pollen as supplement can be combined with chemotherapy treatment to treat side effects of cancer as reported in a recent study by Salles et al. which showed that bee pollen was able to improve muscle mass and metabolism in undernourished rats [21]. Decreasing muscle mass and poor metabolisms were commonly seen in patients with cancer [22]. The combination of chemotherapy drug and natural product such as BPE may be potentially effective in cancer treatment in future.
The data of this study suggest that bee pollen extract of Malaysian stingless bee is a potential chemopreventive agent and can be used as a supplement during chemotherapy treatment. The same data indicate that BPE works synergistically with chemotherapy drug, cisplatin and enhances the effect of cisplatin even at lower concentration. The molecular mechanisms of how the BPE exerts its antiproliferative and synergistic activities with cisplatin will be a promising area to further explore in future studies.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
We thank Dr. Hasni Arshad (Advanced Medical and Dental Institute, USM) for providing MCF-7 cell line and Dr. Rosliza Jajuli (Malaysian Agricultural Research and Development Institute) for stingless bee identification. This study was funded by Fundamental Research Grant Scheme, Ministry of Education Malaysia (Grant No: FRGS/2/2013/SKK01/USM/03/3).
References
[1] Carpes ST, Mourão GB, de Alencar SM, Masson ML. Chemical composition and free radical scavenging activity of Apis mellifera bee pollen from Southern Brazil. Braz J Food Technol 2009; 12: 220-9.
[2] Campos MGR, Bogdanov S, de Almeida-Muradian LB, Szczesna T, Mancebo Y, Frigerio C, et al. Pollen composition and standardisation of analytical methods. J Apic Res Bee World 2008; 47(2): 156-63.
[3] Graikou K, Kapeta S, Aligiannis N, Sotiroudis G, Chondrogianni N, Gonos E, et al. Chemical analysis of Greek pollen-antioxidant, antimicrobial and proteasome activation properties. Chem Cent J 2011; 5: 33.
[4] Premratanachai P, Chanchao C. Review of the anticancer activities of bee products. Asian Pac J Trop Biomed 2014; 4(5): 337-44.
[5] Wang B, Diao Q, Zhang Z, Liu Y, Gao Q, Zhou Y, et al. Antitumor activity of bee pollen polysaccharides from Rosa rugosa. Mol Med Rep 2013; 7(5): 1555-8.
[6] Gao YL, Hu FL, Zhu W, Li YH. [The comparison of anti-tumor function among propolis, bee pollen and royal jelly]. J Bee 2003; 7: 3-4. Chinese.
[7] Wu YD, Lou YJ. A steroid fraction of chloroform extract from bee pollen of Brassica campestris induces apoptosis in human prostate cancer PC-3 cells. Phytother Res 2007; 21(11): 1087-91.
[8] Izuta H, Shimazawa M, Tsuruma K, Araki Y, Mishima S, Hara H. Bee products prevent VEGF-induced angiogenesis in human umbilical vein endothelial cells. BMC Complement Altern Med 2009; 9: 45.
[9] LeBlanc BW, Davis OK, Boue S, DeLucca A, Deeby T. Antioxidant activity of Sonoran Desert bee pollen. Food Chem 2009; 115: 1299-305.
[10] Baharum Z, Akim AM, Taufiq-Yap YH, Hamid RA, Kasran R. In vitro antioxidant and antiproliferative activities of methanolic plant part extracts of Theobroma cacao. Molecules 2014; 19: 18317-31.
[11] Qiao H, Wang TY, Yan W, Qin A, Fan QM, Han XG, et al. Synergistic suppression of human breast cancer cells by combination of plumbagin and zoledronic acid in vitro. Acta Pharmacol Sin 2015; 36(9): 1085-98.
[12] Chou TC. Preclinical versus clinical drug combination studies. Leuk Lymphoma 2008; 49(11): 2059-80.
[13] Nurdianah HF, Ahmad Firdaus AH, Eshaifol Azam O, Wan Adnan WO. Antioxidant activity of bee pollen ethanolic extracts from Malaysian stingless bee measured using DPPH-HPLC assay. 2016 [Forthcoming].
[14] Silva TM, Camara CA, Lins AC, Agra Mde F, Silva EM, Reis IT, et al. Chemical composition, botanical evaluation and screening of radical scavenging activity of collected pollen by the stingless bees Melipona rufiventris (Uruçu-amarela). An Acad Bras Cienc 2009; 81(2): 173-8.
[15] Kuppusamy P, Yusoff MM, Parine NR, Govindan N. Evaluation of in-vitro antioxidant and antibacterial properties of Commelina nudiflora L. extracts prepared by different polar solvents. Saudi J Biol Sci 2015; 22(3): 293-301.
[16] Nagamitsu T, Inoue T. Foraging activity and pollen diets of subterranean stingless bee colonies in response to general flowering in Sarawak, Malaysia. Apidologie 2002; 33: 303-14.
[17] Leja M, Mareczek A, Wy.zgolik G, Klepacz-Baniak J, Czeko nska K. Antioxidative properties of bee pollen in selected plant species. Food Chem 2007; 100: 237-40.
[18] Kustiawan PM, Puthong S, Arung ET, Chanchao C. In vitro cytotoxicity of Indonesian stingless bee products against human cancer cell lines. Asian Pac J Trop Biomed 2014; 4(7): 549-56.
[19] HemaIswarya S, Doble M. Potential synergism of natural products in the treatment of cancer. Phytother Res 2006; 20(4): 239-49.
[20] Komosinska-Vassev K, Olczyk P, Ka zmierczak J, Mencner L, Olczyk K. Bee pollen: chemical composition and therapeutic application. Evid Based Complement Altern Med 2015; 2015: 297425.
[21] Salles J, Cardinault N, Patrac V, Berry A, Giraudet C, Collin ML, et al. Bee pollen improves muscle protein and energy metabolism in malnourished old rats through interfering with the Mtor signaling pathway and mitochondrial activity. Nutrients 2014; 6(12): 5500-16.
[22] Tisdale MJ. Biology of cachexia. J Natl Cancer Inst 1997; 89(23): 1763-73.
*Corresponding author:Wan Adnan Wan Omar, Advanced Medical and Dental Institute, Universiti Sains Malaysia, Bertam, 13200, Penang, Malaysia.
 Asian Pacific Journal of Tropical Biomedicine2016年3期
Asian Pacific Journal of Tropical Biomedicine2016年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Cyclical mastalgia: Prevalence and associated determinants in Hamadan City, Iran
- Quantitative determination of vitexin in Passiflora foetida Linn. leaves using HPTLC
- Screening and antibacterial efficacy of selected Indian medicinal plants
- Anti-herpes simplex virus activities of monogalactosyl diglyceride and digalactosyl diglyceride from Clinacanthus nutans, a traditional Thai herbal medicine
- Analgesic and anti-inflammatory potential of aerial parts of the Daphne mucronata Royle extract in mice: Opioid-independent action
- Sub-chronic effects of a Phthirusa pyrifolia aqueous extract on reproductive function and comparative hormone levels in male rats
