Anti-herpes simplex virus activities of monogalactosyl diglyceride and digalactosyl diglyceride from Clinacanthus nutans, a traditional Thai herbal medicine
Anti-herpes simplex virus activities of monogalactosyl diglyceride and digalactosyl diglyceride from Clinacanthus nutans, a traditional Thai herbal medicine
Sirada Pongmuangmul1,2, Supaporn Phumiamorn3, Phanchana Sanguansermsri1, Nalin Wongkattiya4, Ian Hamilton Fraser5, Donruedee Sanguansermsri1,6*1Faculty of Medical Science, Naresuan University, Phitsanulok 65000, Thailand
2Regional Medical Sciences Center Nakhonswan, Department of Medical Sciences, Ministry of Health, Nakhonswan 60130, Thailand
3Institute of Biological Products, Department of Medical Sciences, Ministry of Health, Nonthaburi 11000, Thailand
4Faculty of Science, Maejo University, Chiang Mai 50290, Thailand
5School of Chemistry, Monash University, Victoria 3800, Australia
6Centre of Excellence in Medical Biotechnology, Faculty of Medical Science, Naresuan University, Phitsanulok 65000, Thailand
Floral research http://dx.doi.org/10.1016/j.apjtb.2015.12.014
Tel: +66 55 964798
E-mail: donruedees@nu.ac.th
Foundation Project: Supported by Department of Medical Sciences, Ministry of Public Health, Thailand (Grant No. RMSc-3Nk-RD-27-2011).
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 19 Nov 2015
Received in revised form 1 Dec 2015
Accepted 8 Dec 2015
Available online 6 Jan 2016
Keywords:
Glycoglycerolipids
Monogalactosyl diglyceride
Digalactosyl diglyceride
Clinacanthus nutans
Herpes simplex virus type 1
Herpes simplex virus type 2
Antiviral activity
ABSTRACT
Objective: To evaluate the monogalactosyl diglyceride (MGDG) and digalactosyl diglyceride (DGDG) from Clinacanthus nutans (C. nutans) for their in vitro antiviral activities against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) by plaque reductionassay.
Methods: MGDG and DGDG were extracted with chloroform from C. nutans leaves. MGDG and DGDG were separated from chloroform crude extract using column chromatography, characterized by thin layer chromatography and quantified by high performance liquid chromatography. The anti HSV-1 and 2 activity against pre-treatment and posttreatment of the compounds was evaluated using plaque reduction assay. The cytotoxicity of the extract and the compounds on Vero cells were performed by MTT assay.
Results: MGDG and DGDG obtained by column chromatography showed identical profiles as standard MGDG and standard DGDG using thin layer chromatography and high performance liquid chromatography. MGDG and DGDG from C. nutans showed 100% inhibition of HSV-1 replication at the post step of infection at noncytotoxic concentration with IC50values of 36.00 and 40.00 mg/mL, and HSV-2 at 41.00 and 43.20 mg/mL, respectively.Moreover,MGDGand DGDGfrom C.nutansweredemonstratedtohaveantiherpes simplex activity at the same level as standard synthetic compounds. In contrast, pretreatment of Vero cells with MGDG and DGDG before HSV-1 and HSV-2infectiondid not show inhibitory effect against these viruses. MGDG and DGDG exhibited antiviral activity against HSV-1 with selectivity index of 26.00 and 23.00 and HSV-2 of 23.30 and 21.30.
Conclusions: MGDG and DGDG from C. nutans, a traditional Thai herbal medicine illustrated inhibitory activity against HSV-1 and HSV-2, probably by inhibiting the late stage of multiplication, suggesting their promising use as anti-HSV agents.
1. Introduction
Herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) infections are distributed worldwide in humans with different degree of severity. It may lead to life-threatening conditions, especially in immunocompromised patients. HSV-1 is more frequently associated with orofacial mucocutaneous lesions. HSV-2 is more commonly associated with genital herpes. Herpes viruses can cause primary, and recurrent infections upon viral reactivation through stimuli such as sunlight, stress and weakened immunity[1,2]. Thedrug of choice of the prophylaxis and treatment of HSV infection is acyclovir, a nucleoside analog, which selectively inhibits HSV replication with low host cell toxicity [3,4]. However, the emergence of resistant viruses to commonly used antiviral drugs is a problem in the treatment, particularly in immunocompromised patients and infants [5-7]. Consequently, there is a need to search for new and more effective antiviral agents that can substitute or complement currently used antiviral drugs[8].
Medicinal plants are known to be a source of abundant of chemical compounds and traditionally used in healthcare in many countries. A large number of plants, either as extracts or as pure compounds, have been demonstrated to exhibit antiviral activity[9-11].Clinacanthusnutans(Burm.f.)Lindau(C.nutans)isamedicinal plant in family Acanthaceae which is native to many tropical Asia countries including Thailand and often cultivated. C. nutans has been traditionally used as a medicine for topical treatment of skin rashes, insect and snake bite, HSV, and varicella-zoster virus lesions [12,13]. Clinical trials have reported the successful use of C. nutans topical treatment for relief of skin inflammation and insect bites, genital herpes and varicella-zoster lesions[14]. Crude extract of C. nutans leaves was shown to inhibit HSV-1 and HSV-2 activity[15]. In phytochemical investigation,flavonoids, steroids, triterpenoids, cerebrosides, two glycoglycerolipids, glycerides, sulfur-containing glycerides were isolated from this plant [16,17]. Trigalactosyl and digalactosyl diglycerides isolated from C. nutans have been shown to exhibit antiviral activity [18]. Synthetic glycoglycerolipids has also been shown to inhibit HSV[19].
In this study, the inhibition of HSV-1 and HSV-2 by two glycoglycerolipids: monogalactosyl diglyceride (MGDG) and digalactosyl diglyceride (DGDG), isolated from C. nutans and chloroform crude extract of C. nutans leaves were investigated using plaque reduction assay. Furthermore, the mode of the inhibitory activities was also determined by adding the compound in the different step of virus replication.
2. Materials and methods
2.1. Plant material and extraction
C. nutans plants were collected from Uthai Thani, Thailand by Warayu Leeprasert, Thai medicinal plant expert. Dried leaves were extracted with chloroform using a Soxhlet extraction apparatus at 60°C for 8 h. Whole extracts were collected and the solvent evaporated to dryness with a rotary evaporator. The crude extracts were stored at−20°C until used in tests.
2.2. Preparation of MGDG and DGDG
The crude extracts were partially purified on a column of silica gel 60 by exhaustive elution with 350 mL of each of the following elution steps: chloroform: acetone (9:1, 3:1 and 1:1), pure acetone and acetone: methanol (3:1) [20]. And 50 mL of each fraction was collected and evaporated for further identification of MGDG and DGDG.
Each fraction was verified for MGDG and DGDG by thin layer chromatography (TLC) and compared with standard MGDG and DGDG (Larodan AB, Sweden). The phase for MGDG identification consisted of chloroform: acetone: distilled water (30:60:2). Mobile phase for DGDG identification consisted of chloroform: acetone: methanol: glacial acetic acid: distilled water (100:40:20:30:10) [21].
Fractions which contained MGDG or DGDG were pooled and evaporated using rotary evaporator. Confirmation of MGDG was performed using TLC with 2 systems of mobile phase, chloroform: acetone: distilled water (30:60:2) and acetone: glacial acetic acid: distilled water (100:2:1) [22]. Confirmation of DGDG was performed using TLC with 2 systems of mobile phase, chloroform: methanol: glacial acetic acid: distilled water (85.0:15.0:10.0:3.5) [22] and chloroform: acetone: methanol: glacial acetic acid: distilled water (100:40:20:30:10)[21].
The quantity of MGDG and DGDG was observed and determined by high performance liquid chromatography (HPLC) using an Agilent 1100 equipped with photo diode array detector at 254 nm. MGDG and DGDG were separated at 30°C using a silica column, size 200.0 mm×4.6 mm, particle size 5 mm (hypersil silica). The flow rate was 1 mL/min and the mobile phase was hexane: iso-propanol (97.5:2.5) [23].
2.3. Viruses and cell lines
Vero cells (African green monkey kidney cells) were grownin monolayer cultured with Dulbecco's modified Eagle medium, supplemented with 5% fetal bovine serum. HSV-1 and HSV-2 were provided by Dr. Panadda Dhepakson, Medical Biotechnology Center, Department of Medical Science, Thailand. Virus stocks were prepared from infected cultures in Vero cells with maintenance medium (Dulbecco's modified Eagle medium, 2% fetal bovine serum and 1% antibiotic), propagated at 37°C, 5% CO2and virus titer was determined by plaque assay in Vero cells.
2.4. Cytotoxicity assay
Cytotoxicity was evaluated by the MTT assay. A volume of 100 mL of Vero cells at concentration 2.5×105cell/mL were seededin96-wellplateandpropagatedat37°Cwith5%CO2for1 day. The cultured medium was replaced by fresh maintenance medium containing different concentrations of chloroform crude extract, extracted and purified MGDG or DGDG. The cells were incubated at 37°C with 5% CO2for 2 days, and the MTT reagent (20mL)wasaddedtoeachwell.After4hoffurtherincubation,the formazan was solubilized by adding diluted HCl (0.04 mol/L) in isopropanol, and the absorbance was determined at 490 nm by Biotek ELISA plate reader with a reference wavelength of 620 nm. The 50% cytotoxic concentration (CC50) causing visible morphologicalchangesin50%of Verocellsweredetermined[24].
2.5. Antiviral activity assay
The antiviral activity of glycoglycerolipid from Phaya Yaw was evaluated by plaque reduction assay as previously described [25]. The compounds were added at different stage during viral infection cycle in order to trace the mode of antiviral action.
2.6. Pre-treatment
The Vero cell monolayer was pretreated with chloroform crude extract, MGDG, DGDG, standard MGDG, standard DGDG and acyclovir at 37°C for 24 h prior to virus infection. After washing, cells were infected with HSV-1 or HSV-2 at a multiplicity of infection of 0.1 at 37°C for 1 h. The infected cells were washed, and overlaid with 3 mL of 1.2% nutrient methylcellulose and incubated at 37°C for 48 and 96 h for HSV-1 and HSV-2, respectively. The infected cells were fixed with 5% formaldehyde, stained with 0.5% crystal violet and then the number of plaques was counted. The percentage of inhibitory activity concentration was determined and the IC50was determined from the curve relating the percentage of inhibitory activity concentration to the concentration of the samples.
2.7. Post-treatment
Confluent Vero cells were infected with 100 plaque forming unit of HSV-1 or HSV-2. After adsorption at 37°C for 1 h, the infected cells were washed and overlaid with 3 mL of 1.2% nutrient methylcellulose containing chloroform crude extract, MGDG, DGDG, standard MGDG, standard DGDG and acyclovir in various concentration and incubated at 37°C for 48 and 96 h for HSV-1 and HSV-2, respectively. The cells were then treated as described earlier for the viral plaque number reduction assay.
3. Results
3.1. Isolation and characterization of crude extract and MGDG and DGDG from C. nutans
Crude extract of C. nutans dried leaves was obtained after chloroform extraction with yield of 7%. The crude extract was then run through a silica gel column and sequentially eluted with 5 different eluants. Fractions of 50 mL were collected as per the method described by Diehl et al. [20]. After running the crude extract, MGDG was eluted from fractions 12-15 and DGDG was eluted from fractions 26-28. The pooled portions of each fraction of MGDG and DGDG were preliminary identified by TLC (Figure 1) when using different mobile phase systems. The MGDG from C. nutans showed similar Rfas the standard MGDG (Figure 1a,b) as well as the DGDG from C. nutans and the standard DGDG (Figure 1c,d). The identification of MGDG and DGDG extracts were confirmed by HPLC. HPLC profiles obtained from MGDG and DGDG extracts were compared well to that of standard MGDG and DGDG (Figure 2).
3.2. Cytotoxicity
To examine the possible cytotoxic effects of chloroform extract,extractedandpurified MGDGand DGDG from C.nutans on cell viability, Vero cells were incubated with different concentrations (100-15000 mg/mL) of the compounds for 48 h and cell viability was measured by MTT assay. As shown in Figure 3, extractedandpurified MGDGand DGDGfrom C.nutansshowed a lower toxic effect than chloroform crude extract. In Table 1, the CC50for these compounds were reported. In this study, chloroform extract, extracted and purified MGDG and DGDG from C. nutans were investigated for the effectiveness against HSV-1 and HSV-2 infected Vero cells. In Vero cells, the CC50of chloroform extract, extracted and purified MGDG and DGDG from C. nutans was between 520 and 960 mg/mL. Activity of the extractedandpurified MGDGand DGDGfrom C.nutanswasless toxic than the chloroform crude extract.
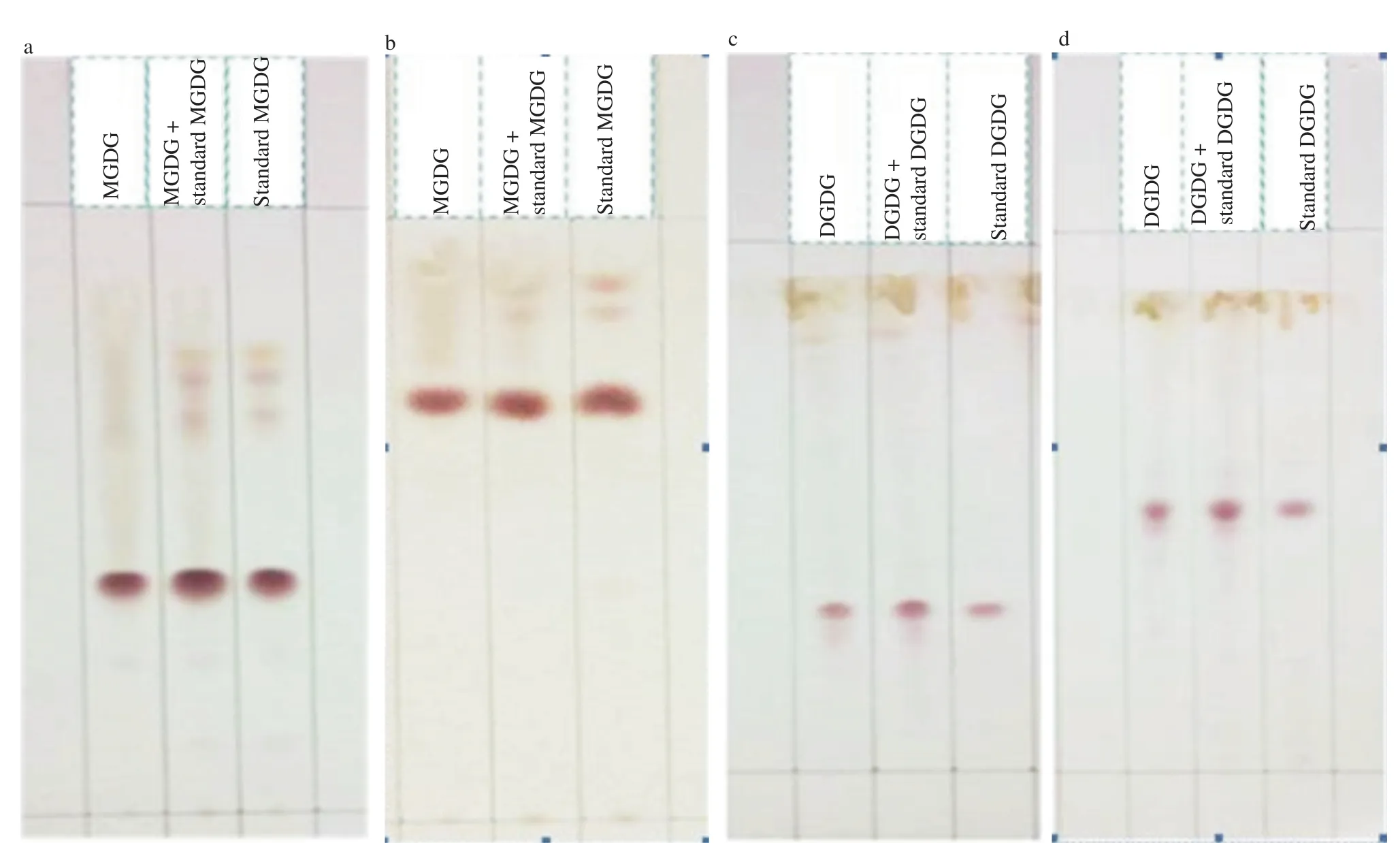
Figure 1. TLC chromatogram of extracted and purified MGDG from C. nutans and standard MGDG in chloroform: acetone: water (a) and in acetone: glacial acetic acid: water (b), extracted and purified DGDG from C. nutans and standard DGDG in chloroform: acetone: water (c) and in acetone: glacial acetic acid: water (d).
3.3. Anti HSV-1 and 2 activity
The effect of chloroform extract, MGDG and DGDG from C. nutans on HSV was evaluated for inhibition at different stepsof infection. Vero cells were pre-treated with chloroform crude extract, extracted and purified MGDG, extracted and purified DGDG, standard MGDG and standard DGDG in different concentrations at subtoxic levels. For post-treatment, the compounds were added to the cells after viral infection. Acyclovir was also used as a drug control in anti-HSV study. At the maximum non-cytotoxic concentrations of all compounds, the anti HSV-1 and HSV-2 activities with post-treatment showed 100% inhibition of plaque formation whereas pre-treatment showed less than 50% inhibition of plaque formation (Figure 4). The result indicated that chloroform extract, extracted and purified MGDG and DGDG from C. nutans, standard MGDG and standard DGDG predominantly affected anti HSV-1 activities in post viral infection with IC50value of 115.00, 36.00, 40.00, 31.00 and 33.60 mg/mL, respectively and anti HSV-2 activities with IC50value of 140.00, 41.00, 43.20, 33.40 and 36.80 mg/mL, respectively. In addition, acyclovir expressed the IC50value on post-treatment at 0.64 and 0.80 mg/mL for HSV-1 and HSV-2 (Table 1). In particular, extracted and purified MGDG and DGDG from C. nutans showed antiviral activity with selectivity indexes (CC50/IC50) on HSV-1 of 26.0 and 23.1 and on HSV-2 of 23.3 and 21.3, respectively (Table 2).

Figure 2. HPLC chromatogram. A: Extracted and purified MGDG from C. nutans; B: Standard MGDG; C: Extracted and purified DGDG from C. nutans; D: Standard DGDG.
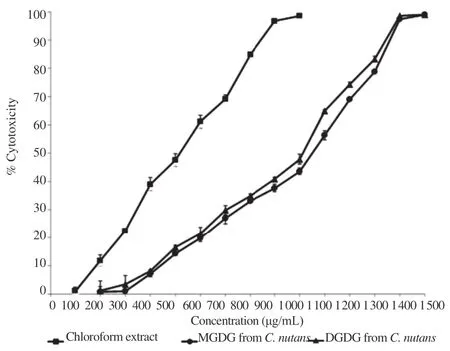
Figure 3. Determination of CC50of chloroform crude extract, extracted and purified MGDG and DGDG from C. nutans on Vero cells.
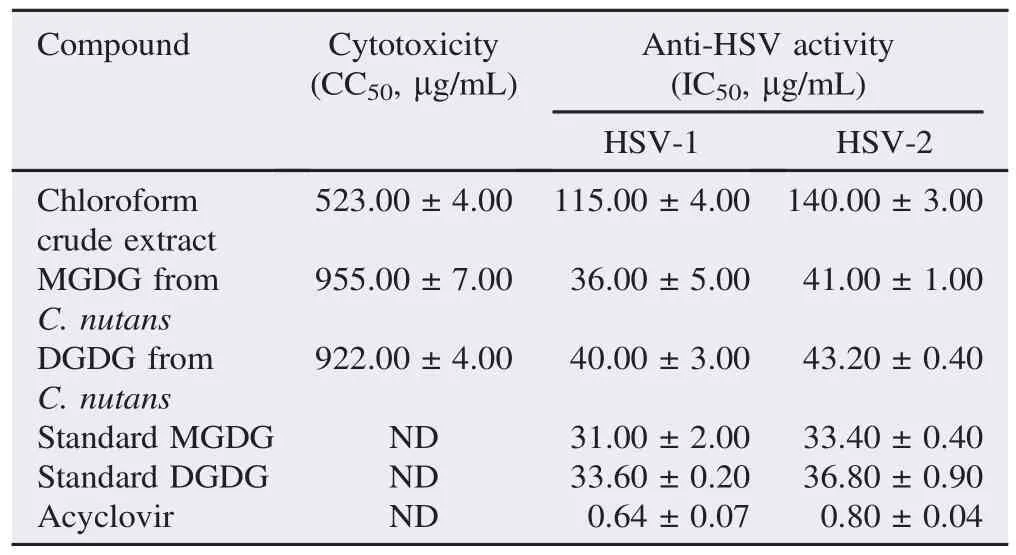
Table 1 Cytotoxicity, anti-HSV activity on post-treatment, and selectivity index of chloroform crude extract, extracted and purified MGDG and DGDG from C. nutans, standard MGDG, standard DGDG and acyclovir.
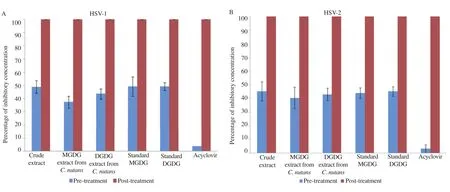
Figure 4. Mode of inhibitory activities of chloroform crude extract, extracted and purified MGDG and DGDG from C. nutans against HSV-1 (A) and HSV-2 (B) during different stages of the viral infection.Viruses were treated with the maximal subtoxic concentration of the compounds. Acyclovir was used as a control in each experiment. Data represented the percentage of virus inhibition when compared to untreated controls as mean±SD.
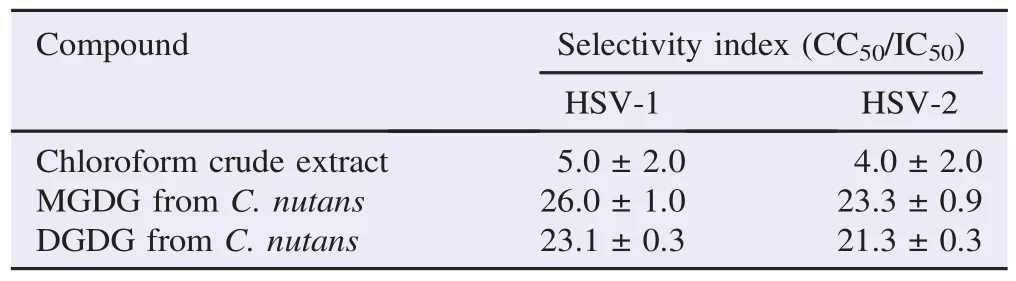
Table 2 Selectivity index of crude extract, extracted and purified MGDG and DGDG from C. nutans on the replication of HSV-1 and HSV-2 on posttreatment.
4. Discussion
For many generations, medicinal plants have been used by indigenous people for the treatment of many infectious diseases including HSV infection. Modern medicine currently uses acyclovir and other nucleoside derivatives have been used in the treatment of HSV infections. However, these are expensive and a large number of people, especially from developing countries, may not be able to afford those expensive drugs. Thus, some medicinal plants which can be found locally would be an alternative treatment.
In this study, the antiviral activity of chloroform crude extract, extracted and purified MGDG and DGDG from C. nutans were investigated for their antiviral activity against HSV-1 and HSV-2 on Vero cells. Cytotoxicity assay is an important part for evaluation of a potential antiviral agent because an extract or compounds should be selective for virus specific processes with little or no effects on host cellular metabolism.
Pre-treatment of Vero cells with chloroform extract, extracted and purified MGDG and DGDG for 24 h before viral infection demonstrated that these samples showed 50% protective effect against the HSV-1 and HSV-2 infection process. In contrast, the results showed that extracted and purified MGDG and DGDG from C. nutans demonstrated 100% inhibition of HSV-1 and HSV-2 replication at the post-infection step at subtoxic concentration levels. Moreover, extracted and purified MGDG and DGDG from C. nutans exhibited anti-HSV activity at the same level as the standard MGDG and DGDG samples. The extracted and purified compounds displayed moderate anti HSV-1 and HSV-2 activity with IC50values ranging from 36.00 to 43.20 mg/mL. In addition, the chloroform crude extract of C. nutans has shown less effective against HSV-1 and HSV-2 than the extracted and purified MGDG and DGDG. It may postulate that MGDG and DGDG are one of the main contributors towards the inhibition of herpes virus replication. The selectivity index shows that extracted and purified MGDG and DGDG from C. nutans are selective for the inhibition of HSV-1 and HSV-2 infection. Similar to the acyclovir, extracted and purified MGDG and DGDG inhibit HSV-1 and HSV-2 replication. Acyclovir specifically inhibits the viral DNA polymerase during the replication cycle when new viral DNA is synthesized [26]. From this study, it could be said that the inhibitory activities against HSV-1 and HSV-2 of extracted and purified MGDG and DGDG from C. nutans are the most effective in the post step of infection. Even though the mechanism of anti-HSV activity of MGDG and DGDG is still not clear, a report showed that some monoglycerides showed antiviral activities against enveloped viruses by the destruction of viral envelopes [27].
Study of the synthetic monoglycosyl diglycerides also showed inhibitory activity against HSV-1 and HSV-2 in Vero cells[19]. This is in accordance with previous study that reported the inhibitory effect of C. nutans extract against HSV-1 and HSV-2 infection before viral entry to the host cells [15]. The active constituent from C. nutans, chlorophyll a and chlorophyll b, were also found to have anti-HSV activity before viral entry step[16]. Other investigators have also reported anti-HSV activities of hexane, dichloromethane and methanol extracts from C. nutans at the post step of infection [15]. It is interesting to note that this differs to a study where water and organic solvent extracts of C. nutans did not inhibit HSV replication in Vero cells [28,29].
In conclusion, this study has discovered the inhibitory activities of extracted and purified MGDG and DGDG from C. nutans against HSV-1 and HSV-2 probably in post viral entry step. C. nutans is a Thai herbal medicine used for herpes infection for many generations and the herb is generously available throughout Southeast Asia. MGDG and DGDG from C. nutans have been demonstrated to have anti-herpes simplexactivity at the same level as commercially available synthetic compounds.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
We would like to thank Department of Medical Sciences, Ministry of Public Health, Thailand for their financial support (Grant No. RMSc-3Nk-RD-27-2011).
References
[1] Johnston C, Corey L. Current concepts for genital herpes simplex virus infection: diagnostics and pathogenesis of genital tract shedding. Clin Microbiol Rev 2016; 29(1): 149-61.
[2] Garland SM, Steben M. Genital herpes. Best Pract Res Clin Obstet Gynaecol 2014; 28(7): 1098-110.
[3] Canadian Agency for Drugs and Technologies in Health. CADTH rapid response reports. In: Acyclovir versus valacyclovir for herpes virus in children and pregnant women: a review of the clinical evidence and guidelines. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2014.
[4] Sawleshwarkar S, Dwyer DE. Antivirals for herpes simplex viruses. BMJ 2015; 351: h3350.
[5] L´opez-Labrador FX, Berenguer M, Navarro D. Overcoming drug resistance in HSV, CMV, HBV and HCV infection. Future Microbiol 2015; 10: 1759-66.
[6] De SK, Hart JC, Breuer J. Herpes simplex virus and varicella zoster virus: recent advances in therapy. Curr Opin Infect Dis 2015; 28(6): 589-95.
[7] James SH, Prichard MN. Current and future therapies for herpes simplex virus infections: mechanism of action and drug resistance. Curr Opin Virol 2014; 8: 54-61.
[8] Hornig J, McGregor A. Design and development of antivirals and intervention strategies against human herpesviruses using highthroughput approach. Expert Opin Drug Discov 2014; 9(8): 891-915.
[9] Hassan ST, Masarˇcikov´a R, Berchov´a K. Bioactive natural products with anti-herpes simplex virus properties. J Pharm Pharmacol 2015; 67(10): 1325-36.
[10] Son M, Lee M, Sung GH, Lee T, Shin YS, Cho H, et al. Bioactive activities of natural products against herpesvirus infection. J Microbiol 2013; 51(5): 545-51.
[11] Marathe SA, Datey AA, Chakravortty D. Herbal cocktail as antiinfective: promising therapeutic for the treatment of viral diseases. Recent Pat Antiinfect Drug Discov 2012; 7(2): 123-32.
[12] Kongkaew C, Chaiyakunapruk N. Efficacy of Clinacanthus nutans extracts in patients with herpes infection: systematic review and meta-analysis of randomised clinical trials. Complement Ther Med 2011; 19(1): 47-53.
[13] Siew YY, Zareisedehizadeh S, Seetoh WG, Neo SY, Tan CH, Koh HL. Ethnobotanical survey of usage of fresh medicinal plants in Singapore. J Ethnopharmacol 2014; 155(3): 1450-66.
[14] Charuwichitratana S, Wongrattanapasson N, Timpatanapong P, Bunjob M. Herpes zoster: treatment with Clinacanthus nutans cream. Int J Dermatol 1996; 35(9): 665-6.
[15] Kunsorn P, Ruangrungsi N, Lipipun V, Khanboon A, Rungsihirunrat K, Chaijaroenkul W. The identities and anti-herpes simplex virus activity of Clinacanthus nutans and Clinacanthus siamensis. Asian Pac J Trop Biomed 2013; 3(4): 284-90.
[16] Sakdarat S, Shuyprom A, Pientong C, Ekalaksananan T, Thongchai S. Bioactive constituents from the leaves of Clinacanthus nutans Lindau. Bioorg Med Chem 2009; 17(5): 1857-60.
[17] Tu SF, Liu RH, Cheng YB, Hsu YM, Du YC, El-Shazly M, et al. Chemical constituents and bioactivities of Clinacanthus nutans aerial parts. Molecules 2014; 19(12): 20382-90.
[18] Satakhun S. Chemical constituents of Clinacanthus nutans leaves [dissertation]. Bangkok: Chulalongkorn University; 2001.
[19] Janwitayanuchit W, Suwanborirux K, Patarapanich C, Pummangura S, Lipipun V, Vilaivan T. Synthesis and anti-herpes simplex viral activity of monoglycosyl diglycerides. Phytochemistry 2003; 64(7): 1253-64.
[20] Diehl BWK, Herling H, Riedl I, Heinz E.13C-NMR analysis of the positional distribution of fatty acids in plant glycolipids. Chem Phys Lipids 1995; 77(2): 147-53.
[21] Yamaguchi M, Kasamo K. Modulation in the activity of purified tonoplast H+-ATPase by tonoplast glycolipids prepared from cultured rice (Oryza sativa L. var. Boro) cells. Plant Cell Physiol 2001; 42(5): 516-23.
[22] Whitaker BD. Fatty-acid composition of polar lipids in fruit and leaf chloroplasts of“16:3”- and“18:3”-plant species. Planta 1986; 169(3): 313-9.
[23] Walker GC. Determination of flour glycolipids as their benzoyl derivatives by high-performance liquid chromatography with ultraviolet detection. Cereal Chem 1988; 65(5): 433-5.
[24] Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65(1-2): 55-63.
[25] Schinazi RF, Chou TC, Scott RT, Yao XJ, Nahmias AJ. Delayed treatment with combinations of antiviral drugs in mice infected with herpes simplex virus and application of the median effect method of analysis. Antimicrob Agents Chemother 1986; 30(3): 491-8.
[26] Elion GB. Mechanism of action and selectivity of acyclovir. Am J Med 1982; 73: 7-13.
[27] Thormar H, Isaacs CE, Kim KS, Brown HR. Inactivation of visna virus and other enveloped viruses by free fatty acids and monoglycerides. Ann N Y Acad Sci 1994; 724: 465-71.
[28] Yoosook C, Panpisutchai Y, Chaichana S, Santisuk T, Reutrakul V. Evaluation of anti-HSV-2 activities of Barleria lupulina and Clinacanthus nutans. J Ethnopharmacol 1999; 67(2): 179-87.
[29] Yoosook C, Bunyapraphatsara N, Boonyakiat Y, Kantasuk C. Anti-herpes simplex virus activities of crude water extracts of Thai medicinal plants. Phytomedicine 2000; 6(6): 411-9.
*Corresponding author:Donruedee Sanguansermsri, Faculty of Medical Science, Naresuan University, Phitsanulok 65000, Thailand.
 Asian Pacific Journal of Tropical Biomedicine2016年3期
Asian Pacific Journal of Tropical Biomedicine2016年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Cyclical mastalgia: Prevalence and associated determinants in Hamadan City, Iran
- Natural antibacterial remedy for respiratory tract infections
- Bee pollen extract of Malaysian stingless bee enhances the effect of cisplatin on breast cancer cell lines
- In vitro antihistamine-releasing activity of a peptide derived from wasp venom of Vespa orientalis
- Changes in energetic profile of pregnant ewes in relation with the composition of the fetal fluids
- The inhibition of Typhonium flagelliforme Lodd. Blume leaf extract on COX-2 expression of WiDr colon cancer cells
