In vitro antihistamine-releasing activity of a peptide derived from wasp venom of Vespa orientalis
In vitro antihistamine-releasing activity of a peptide derived from wasp venom of Vespa orientalis
Jafar Jalaei1, Mehdi Fazeli1, Hamid Rajaian1, Somayeh Layeghi Ghalehsoukhteh1*, Alireza Dehghani2, Dominic Winter21Department of Pharmacology and Toxicology, School of Veterinary Medicine, Shiraz University, Shiraz, Iran
2Institute of Biochemistry and Molecular Biology, University of Bonn, Bonn, Germany
Entomological research http://dx.doi.org/10.1016/j.apjtb.2015.12.001
Tel: +98 7136138634
E-mail: s.layeghi@shirazu.ac.ir
All experimental procedures involving animals were conducted in accordance to Guiding Principles for the Care and Use of Research Animals of Shiraz University and approved by Animal Care Committee for the Shiraz University of Veterinary Medicine.
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 29 Apr 2015
Received in revised form 27 Jun, 2nd revised form 13 Nov 2015
Accepted 20 Nov 2015
Available online 2 Jan 2016
Keywords:
Vespa orientalis
Antihistamine release
Lactate dehydrogenase assay Peptide
ABSTRACT
Objective: To investigate the antihistamine-releasing effect of a peptide isolated from wasp venom of Vespa orientalis.
Methods: This peptide was separated from crude venom by chromatography methods and mass spectrometry. Then various concentrations (2, 4, 8, 16, 32, 64, 128 and 256 mmol/L) of the peptide were incubated with mast cells and lactate dehydrogenase assay was performed.
Results: No significant effect was observed in lactate dehydrogenase absorbance under 128 mmol/L concentration. This implied that the peptide did not cause cell death in mast cells and consequently, histamine release did not happen. Moreover, the results showed the IC50of mast cells degranulation at 126 mmol/L, which was approximately high implying that this peptide had high selectivity for normal cells and did not cause histamine release from these cells.
Conclusions: This would be a great aim in new drug development, in which an agent acts potentially on its target tissue without activating the immune system.
1. Introduction
Mast cells are the most important effector cells in inflammatory and allergic diseases [1]. Thus, researchers concerning mast cells should focus on their detrimental pathologic effects. However, mast cells play important roles in homeostasis and may have a significant function in defense against parasitic and bacterial infections, the healing of wounds, and participating in innate and adaptive immunity [2]. Mast cells are the initial storage location of histamine in mammalian tissues and placed in secretory granules [3]. These granules in rats contain serotonin and a highly charged matrix of heparin and protein which is released along with histamine [4]. Preventing mast cell degranulation is critically important in drug administration since immune system activation would have destructive effect not only on exogenous particles, but also may affect all body cells. Both inflammatory and antiinflammatory responses against several venoms, especially bee venom, have been shown in many studies[5,6]. In this study, the attempt was aimed to elucidate the antihistamine-releasing activity of a specific peptide derived from wasp venom of Vespa orientalis (V. orientalis).
2. Materials and methods
2.1. Venom extraction
V. orientalis specimens were collected from Abarkooh located in central part of Iran. The dissected wasp's venom glands were paralyzed at 4°C and then immersed in liquid nitrogen. By using pestle and liquid nitrogen, the glands were crushed into a clean mortar. About 20 mL of 0.1 mol/L phosphate buffer (pH = 7.4) was added to the powdered samples following liquid nitrogen evaporation. After centrifugation at 1500 r/min at 4°C for 15 min, the supernatant was siphoned to another clean tube and further homogenized. Subsequently, the tube content was lyophilized and kept at−20°C till it was analyzed for further assay.
2.2. Purification of venom
The methodology described above produced a partially purified extract of the venom. The extract was subjected to other purification steps, the most frequently of which are chromatography procedures and mass spectrometry. The selection of one or other (or both) depends on the knowledge of the peptide characteristics.
2.3. Ion exchange chromatography
Venom (20 mg/mL) sample was dissolved in distilled water after lyophilization, then it was loaded onto a fast protein liquid chromatography system on a HiTrap™SPHP (Sweden) cation exchange column equilibrated with 30 mmol/L sodium bicarbonate buffer (pH 7.2). Venom was eluted with a linear gradient of elution buffer 0.01 mol/L NaCl in the same buffer and the flow rate was 2 mL/min[7]. At a rate of 4 mL/min, fractions were collected and monitored at 220 nm. The antihistamine-releasing activities of the pooled fractions were investigated. In addition, the active fractions were lyophilized and subjected to further analysis.
2.4. Gel filtration chromatography
After cation exchange chromatography, the collected active fraction of peptide was dissolved in distilled water and loaded onto a column (1 cm×25 cm) of Sephadex G-25 gel filtration which was initially balanced with distilled water[8]. The column was eluted with distilled water at a flow rate of 60 mL/h followed bycollectingeachfractionatavolumeof2mLanditsabsorbance wasmonitoredat220nmbyspectrophotometry,andeachfraction illustrating antihistamine-releasing activities was pooled, lyophilized and kept for next steps.
2.5. Reverse phase high performance liquid chromatography (RP-HPLC)
Peptide detection was done by using a Knauer HPLC system. Peptide aliquots (20 mL) were detected by a Vertex plus C18 column (250 mm×4.6 mm) balanced with acidified water [0.05% trifluoroacetic acid (TFA)]. The acidified water was used for column washing for 5 min. Elution was then proceeded with a linear gradient of 20%-80% acetonitrile in acidified water (0.05% TFA) over 60 min at a flow rate of 1 mL/min. Fractions were collected into 3 mL volumes. The purification of the final fraction was proceeded using the Smart HPLC (Amersham Pharmacia Biotech) system equipped with a Sephasil peptide C18 column (4.6 mm×250 mm, Amersham Pharmacia Biotech). Fraction elution was done with acidified water (0.05% TFA in water) for 7 min, a linear gradient of 0%-60% solvent D (0.05% TFA in acrylonitrile) for 20 min and finally with 60%-100% solvent D for 1 min completed the elution process. The detection wave length was set at 220 nm in which the flow rate was 0.1 mL/min [9].
2.6. Mass spectrometry
Electrospray ionization spectra were recorded using a Quattro II triple quadrupole mass spectrometer (Micromass)fixed with an electrospray source. On-line liquid chromatography-mass spectrometry1 was implemented using the 2nd step separation procedure (C2/C18 column). A post-column split of nearly 10:1 (fraction collector and electrospray ionization mass spectrometer) was used to enable similar fractionation and mass determination. Two alternating scan functions were employed as follows: one at a sampling cone potential of 35 V to give the molecular weight of the peptides and a second scan at a sampling cone potential of 100 V to produce low mass fragment ions to enable the determination of any post-translational variation, such as glycosylation. The primary UV wave length of the Smart system was also noted by the data system of the mass spectrometer so that the UV and total ion chromatogram outputs were aligned [10].
2.7. Mast cell preparation
Mast cells were isolated from peritoneal area of adult female Wistar rats weighing 300-350 g by using Kurosawa and Parker modified method [11]. Firstly, 10 mL of cold Hank's balanced salt solution was injected intraperitoneally and then the abdomen was softly massaged for 2 min. The suspension was aspirated and centrifuged at 2500 r/min at 4°C for 5 min. Based on Hachisuka et al. method, mast cells were purified by percoll density centrifugation [12]. Twice washing with Hank's balanced salt solution containing 1 mg/mL bovine serum albumin was done on purified mast cells.
2.8. Mast cell degranulation
The release of b-D-glucosaminidase was measured to determine the mast cell degranulation co-localized with histamine [13]. Different concentrations (2, 4, 8, 16, 32, 64, 128 and 256 mmol/L) of peptide were incubated with mast cells at 37°C for 15 min. The tubes were centrifuged and then supernatants were sampled for D-glucosaminidase assay. Briefly, 50 mL of each sample was incubated in 50 mL of the substrate (3 mg of p-nitrophenyl-N-acetyl-b-D-glucosaminidine dissolved in 10 mL of 0.2 mol/L sodium citrate solution at pH 4.5) overnight at 37°C. The absorbance of the colored product was assessed at 405 nm and the values were expressed as the percentage of total b-D-glucosaminidase activity from rat mast cell suspensions, characteristic in lysed mast cells in the presence of 0.1% (v/v) Triton X-100 [14].
2.9. Lactate dehydrogenase (LDH) assay
Various concentrations (2, 4, 8, 16, 32, 64, 128 and 256 mmol/L) of peptide were incubated with mast cells for 24 h. The medium was removed without damaging cells for LDH assay. Briefly, 100 mL of each well medium was transferred to a new plate and 100 mL of LDH catalyst and reagent (45:1) (Roche cytotoxicity detection, Germany) was added to the supernatant and incubated for 30 min in dark at room temperature. Diaphorase and aldehyde dehydrogenase as a blue compound, and iodo tetrazolium chloride plus sodium lactate as a red one were urgent need to make LDH media (11/125 mmol/L of red and 0/25 mmol/L of blue). The absorbance was recorded at 490 nm using an ELISA reader. Aliquot of 100 mL of 2% Triton X-100 was added to the untreated cells considered as a high control and media with cells as a low control. Cytotoxicity was correlated with the percentage of LDH release [15].
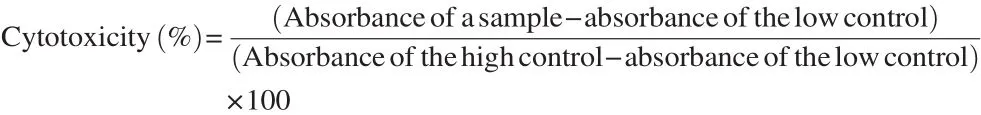
3. Results
3.1. Ion exchange chromatography
Fast protein liquid chromatography peptide detection results indicated three clear fractions at 280 nm (Figure 1). The third fraction showed antihistamine-releasing activity (Figure 2). This fraction was located in a 100 salt gradient region reflecting its net positive charge. Its tight junction with column required more buffer salt for separation. Additionally, the concentration of third fraction was increased after filtration with Millipore tubes and peptides with molecular weight of 5000 Da and more were passed.

Figure 1. V. orientalis venom detection result on exchange cationic column HiTrap™in equivalent with 300 mmol/L bicarbonate and salt gradient.
3.2. Gel filtration chromatography
Resin G-25 Sephadex was used in this assay that diameter was 8-20 mm and separated salt and peptide. Results of gel filtration chromatography were shown in Figure 3.
3.3. RP-HPLC
The chromatogram of V. orientalis venom illustrated more than 100 different agents and 20 high picks were shown in Figure 4. RP-HPLC analysis eluted two picks, named formin homology-1 (FH-1) and FH-2.
Following further analytical RP-HPLC analysis of samples from each of these fractions, a histamine-releasing peptide of novel primary structure was found in the fraction FH-1, therefore, this was further purified (Figure 5).
The primary structure of this novel peptide was established by using tandem mass spectrometry fragmentation (Table 1).
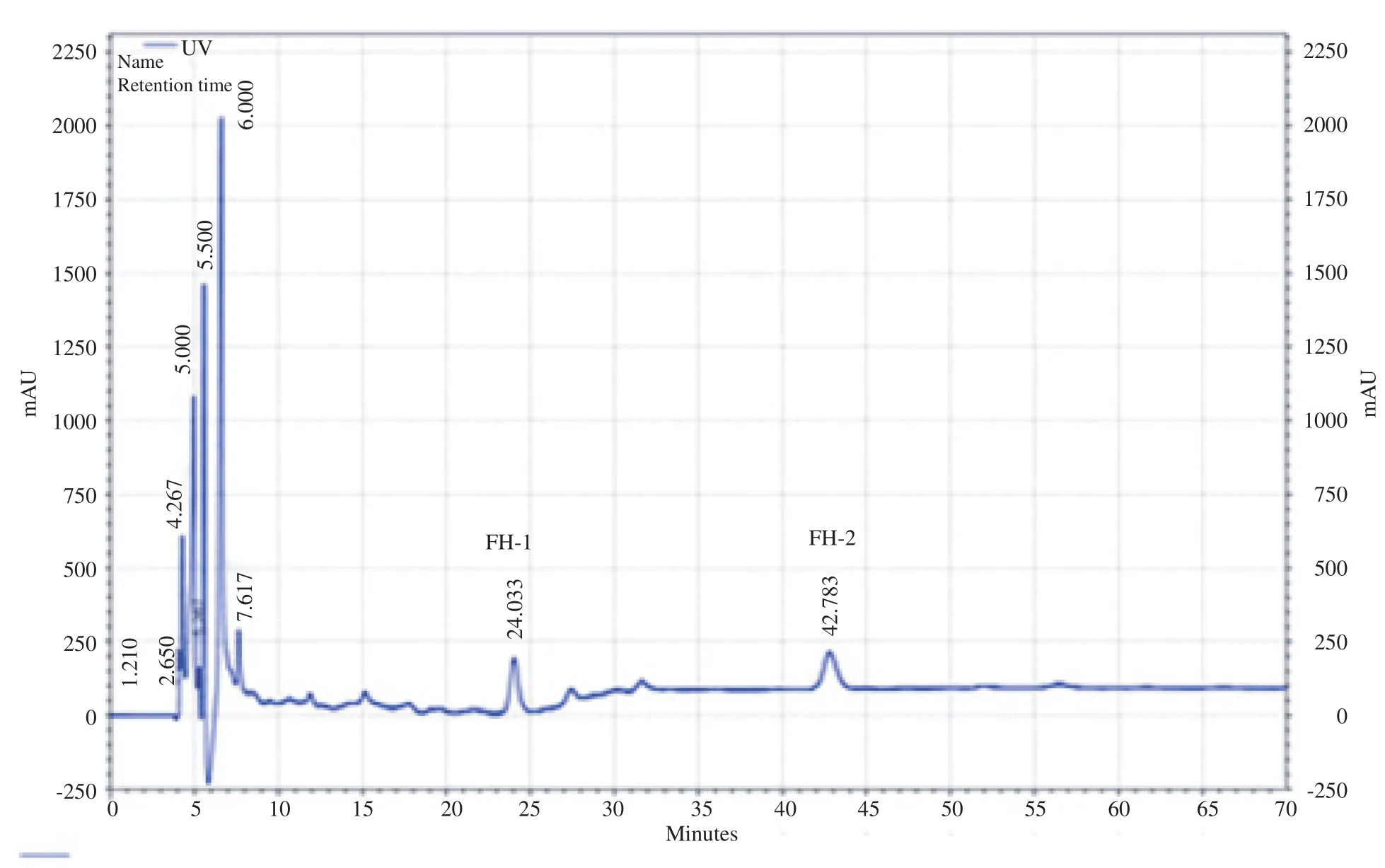
Figure 2. Fast protein liquid chromatography and gel filtration chromatography of antihistamine-releasing peptide.
3.4. Cytotoxic activity
The cytotoxic activity of the peptide was examined on mast cells by LDH assay. LDH was an enzyme present in all cells and was released in culture medium after cell death. In this assay, NADH/H+ reacted with the peptide in the presence of the catalyst to form a red color compound, i.e. formazan, which wasmeasured at 490 nm [16]. The peptide did not show marked cytotoxic effect on mast cells, caused concentration dependent increase in LDH and had an IC50of 80 mmol/L on mast cells (Figure 6).
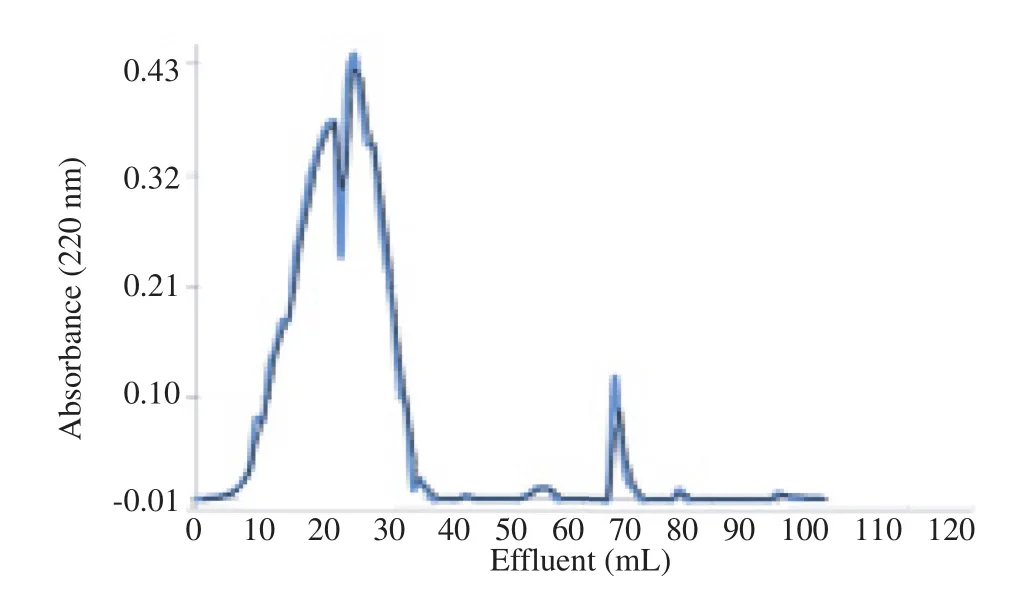
Figure 3. Gel filtration chromatography of V. orientalis venom on G-25 Sephadex.
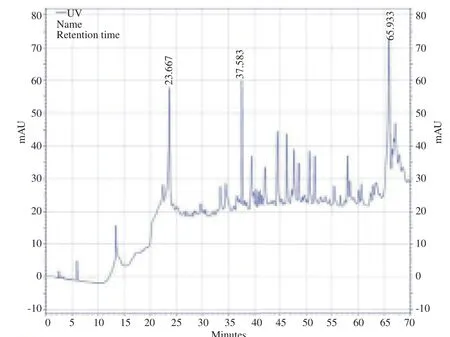
Figure 4. RP-HPLC chromatogram of V. orientalis venom.

Figure 5. RP-HPLC chromatogram of antihistamine-releasing peptide.
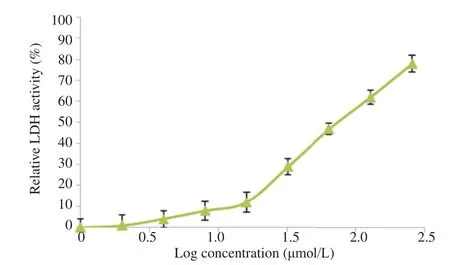
Figure 6. LDH activity on mast cells in presence of the peptide and 0.1% Triton.
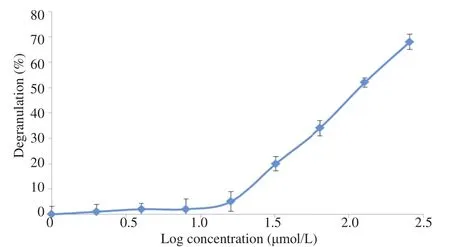
Figure 7. The peptide degranulation activity on peritoneal mast cells.
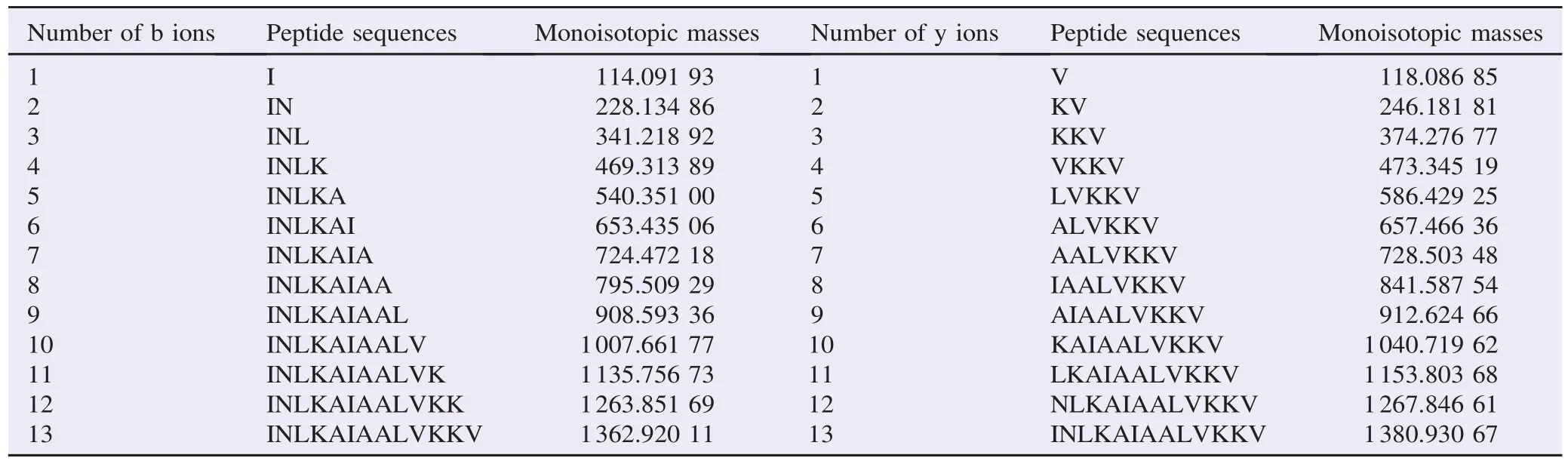
Table 1 Molecular masses, primary structures and identities of histamine releasing peptide present in semi-preparative RP-HPLC fraction.
3.5. Mast cell degranulation
The absorbance of different concentrations of the peptide with adherent cells in microplate after overnight incubating was read. The results showed the IC50of mast cells degranulation 126 mmol/L, which was approximately high, implying that this peptide had high selectivity for normal cells and had immunomodulation property which led the prevention of immune system activation (Figure 7).
4. Discussion
Avoiding mast cell degranulation and consequent histamine release, immune system activation would be the goal of new drug development. A constituent of bee venom named mast cell degranulating (MCD) peptide has both anti-inflammatory and inflammatory activities. Mizuno et al. showed that mastoparan (5-30 mmol/L), a tetradecapeptide isolated from wasp venom, caused histamine release from RBL-2H3 cells in a concentration and time-dependent manner [17], whereas based on our results, wasp venom peptide exhibited dissimilar effect, which has previously been shown in bee venom [18]. On the other hand, different kinds of mastoparan have shown histamine releasing activity [19], which is in contrast with our findings explaining the presence of different peptides with diverse activity in wasp venom. Besides, more investigations showed that bee venom contains several peptides with biological activity, and the major known peptides are melittin and apamin with hemolytic and neurotoxic activities respectively and a number of minor peptides. The researchers showed anti-inflammatory property just for mast cell degranulating peptide in bee venom [20]. Apamin, a small conductance Ca2+-activated K+channel blocker, significantly inhibited both ovalbumins which induced tracheal contraction and histamine release from lung tissues [21]. The results of some investigations suggest that apamin could reduce allergic airway inflammation by means of mast cell stabilizing effect [22]. Apamin has also an anti-allergic activity by inhibiting mast cells histamine releasing [22]. It is claimed that the main reason of antihistamine-releasing effect of this peptide is disulfide exchange bindings between its receptors and immunoglobulin E (IgE) on mast cell surface in a dose-response manner which partially inhibits mast cell degranulation [23]. Moreover, it has been found that mast cell degranulating inhibition by this peptide could take place at concentrations higher than those that would cause the release of histamine and it might be because of its interaction with the IgE molecule, which is associated with allergic reactions [24]. It was assumed that disulfide exchange between IgE and the MCD peptide on the mast cell surface at high doses might prevent the release of histamine, which would allow the MCD peptide to act as an anti-allergic agent [22]. By contrast, our peptide had no histamine releasing effects even on low doses. The venom of the European honey bee (Apis mellifera) has long been considered to be a rich source of anti-inflammatory activities. To date, apitherapy has continued to be a common practice in China, Eastern Europe and South America in different types of inflammation and infection [20,24,25]. Cytotoxicity data from a LDH release assay, which measure damage to the plasma membrane showed no significant increase in percentage of LDH release and its IC50was nearly 128 mL, so we confirmed that LDH absorbance was not affected by this peptide. Taken together, the data strongly suggest that 128 mL or lower peptide concentrations have no cytotoxic action after incubation period. In another study, the anti-inflammatory activities of peptide 401, contained 22 residues and occurred in the venom of the common European honey bee, and compound 48/80 completely suppressed skin reactions to the mast cell-derived amines. Pretreatment of rats with compound 48/80 also suppressed the apparent anti-inflammatory actions of peptide 401 and compound 48/80 [26]. These findings would potentially confirm the present study results which claimed V. orientalis wasp venom contained both inflammatory and anti-inflammatory peptide, and detection of such peptide would be great goal in immune system disorder treatment. Moreover, LDH assay strongly suggests that cell death is not a mechanism by which isolated peptide from V. orientalis inhibits mast cell degranulation.
Antihistamine-releasing activity of a peptide isolated from V. orientalis wasp venom was shown in this study. This peptide had no significant effect on LDH release from mast cells, which indicates that it would not cause cellular death on these cells. This is an advantage for a peptide with potential medical use as immune system of the host will not be activated.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This research was financially supported by Natural Antimicrobial Centre of Excellence of Shiraz University. Authors are also thankful to Mrs. Maryam Tavana and Hossein Ali Shamsaei for their technical expertise.
References
[1] Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med 2012; 18(5): 693-704.
[2] Tsai M, Starkl P, Marichal T, Galli SJ. Testing the‘toxin hypothesis of allergy’: mast cells, IgE, and innate and acquired immune responses to venoms. Curr Opin Immunol 2015; 36: 80-7.
[3] Mecheri S. Contribution of allergic inflammatory response to the pathogenesis of malaria disease. Biochim Biophys Acta 2012; 1822(1): 49-56.
[4] Beaven MA. Factors regulating availability of histamine at tissue receptors. In: Ganellin CR, Parsons ME, editors. Pharmacology of histamine receptors. Amsterdam: Elsevier Ltd.; 1982.
[5] Moreno M, Giralt E. Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: melittin, apamin and mastoparan. Toxins 2015; 7(4): 1126-50.
[6] Park D, Jung JW, Lee MO, Lee SY, Kim B, Jin HJ, et al. Functional characterization of naturally occurring melittin peptide isoforms in two honey bee species, Apis mellifera and Apis cerana. Peptides 2014; 53: 185-93.
[7] Qian ZJ, Jung WK, Kim SK. Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin, Rana catesbeiana Shaw. Bioresour Technol 2008; 99(6): 1690-8.
[8] Qian ZJ, Jung WK, Byun HG, Kim SK. Protective effect of an antioxidative peptide purified from gastrointestinal digests of oyster, Crassostrea gigas against free radical induced DNA damage. Bioresour Technol 2008; 99(9): 3365-71.
[9] Dang XL, Tian JH, Yi HY, Wang WX, Zheng M, Li YF, et al. Inducing and isolation of antibacterial peptides from oriental fruit fly, Bactrocera dorsalis Hendel. Insect Sci 2006; 13(4): 257-62.
[10] Charpentier S, Amiche M, Mester J, Vouille V, Le Caer JP, Nicolas P, et al. Structure, synthesis, and molecular cloning of dermaseptins B, a family of skin peptide antibiotics. J Biol Chem 1998; 273(24): 14690-7.
[11] Kurosawa M, Parker CW. Protein phosphorylation in rat mast cell granules. Cyclic AMP dependent phosphorylation of a 44K protein associated with broken granules. Biochem Pharmacol 1987; 36(1): 131-40.
[12] Hachisuka H, Kusuhara M, Higuchi M, Okubo K, Sasai Y. Purification of rat cutaneous mast cells with Percoll density centrifugation. Arch Dermatol Res 1988; 280(6): 358-62.
[13] Souza BM, Cabrera MP, Gomes PC, Dias NB, Stabeli RG, Leite NB, et al. Structure-activity relationship of mastoparananalogs: effects of the number and positioning of Lys residues on secondary structure, interaction with membrane-mimetic systems and biological activity. Peptides 2015; 72: 164-74.
[14] Ribeiro SP, Mendes MA, Dos Santos LD, de Souza BM, Marques MR, de Azevedo WF Jr, et al. Structural and functional characterization of N-terminally blocked peptides isolated from the venom of the social wasp Polybia paulista. Peptides 2004; 25(12): 2069-78.
[15] Schmitt A, Schmitt J, M¨unch G, Gasic-Milencovic J. Characterization of advanced glycation end products for biochemical studies: side chain modifications and fluorescence characteristics. Anal Biochem 2005; 338(2): 201-15.
[16] Gholivand MB, Mohammadi M, Khodadadian M, Rofouei MK. Novel platinum(II) selective membrane electrode based on 1,3-bis(2-cyanobenzene)triazene. Talanta 2009; 78(3): 922-8.
[17] Mizuno K, Nakahata N, Ohizumi Y. Characterization of mastoparan-induced histamine release from RBL-2H3 cells. Toxicon 1998; 36(3): 447-56.
[18] Dotimas EM, Hamid KR, Hider RC, Ragnarsson U. Isolation and structure analysis of bee venom mast cell degranulating peptide. Biochim Biophys Acta 1987; 911(3): 285-93.
[19] Mukai H, Kikuchi M, Suzuki Y, Munekata E. A mastoparan analog without lytic effects and its stimulatory mechanisms in mast cells. Biochem Biophys Res Commun 2007; 362(1): 51-5.
[20] Li R, Zhang L, Fang Y, Han B, Lu X, Zhou T, et al. Proteome and phosphoproteome analysis of honeybee (Apis mellifera) venom collected from electrical stimulation and manual extraction of the venom gland. BMC Genomics 2013; 14: 766.
[21] Karimzadeh L, Nabiuni M, Sheikholeslami A, Irian S. Bee venom treatment reduced C-reactive protein and improved follicle quality in a rat model of estradiol valerate-induced polycystic ovarian syndrome. J Venom Anim Toxins Incl Trop Dis 2012; 18(4): 384-92.
[22] Shin SH, Kim YH, Kim JK, Park KK. Anti-allergic effect of bee venom in an allergic rhinitis mouse model. Biol Pharm Bull 2014; 37(8): 1295-300.
[23] Oschatz CT. Mechanisms and functions of the mast cell-activated contact system in inflammatory reactions. W¨urzburg: Oschatz CT; 2012. [Online] Available from: https://opus.bibliothek. uni-wuerzburg.de/opus4-wuerzburg/frontdoor/deliver/index/docId/ 5933/file/DrArbeit_Chris_Oschatz.pdf [Accessed on 8th April, 2015]
[24] Koh PS, Seo BK, Cho NS, Park HS, Park DS, Baek YH. Clinical effectiveness of bee venom acupuncture and physiotherapy in the treatment of adhesive capsulitis: a randomized controlled trial. J Shoulder Elb Surg 2013; 22(8): 1053-62.
[25] Ali MAA. Studies on bee venom and its medical uses. Int J Adv Res Technol 2012; 1(2): 69-83.
[26] Banks BE, Dempsey CE, Vernon CA, Warner JA, Yamey J. Antiinflammatory activity of bee venom peptide 401 (mast cell degranulating peptide) and compound 48/80 results from mast cell degranulation in vivo. Br J Pharmacol 1990; 99(2): 350-4.
*Corresponding author:Somayeh Layeghi Ghalehsoukhteh, School of Veterinary Medicine, Department of Pharmacology and Toxicology, Shiraz University, Shiraz, Iran.
 Asian Pacific Journal of Tropical Biomedicine2016年3期
Asian Pacific Journal of Tropical Biomedicine2016年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Cyclical mastalgia: Prevalence and associated determinants in Hamadan City, Iran
- Quantitative determination of vitexin in Passiflora foetida Linn. leaves using HPTLC
- Screening and antibacterial efficacy of selected Indian medicinal plants
- Anti-herpes simplex virus activities of monogalactosyl diglyceride and digalactosyl diglyceride from Clinacanthus nutans, a traditional Thai herbal medicine
- Analgesic and anti-inflammatory potential of aerial parts of the Daphne mucronata Royle extract in mice: Opioid-independent action
- Sub-chronic effects of a Phthirusa pyrifolia aqueous extract on reproductive function and comparative hormone levels in male rats
