Screening and antibacterial efficacy of selected Indian medicinal plants
Screening and antibacterial efficacy of selected Indian medicinal plants
Suresh Mickymaray1,2*, Mohammad Saleh Al Aboody1, Pradipta Kumar Rath2, Panneerselvam Annamalai3, Thajuddin Nooruddin41Department of Medical Laboratories, College of Science Al-Zulfi, Majmaah University, Al-Zulfi, Kingdom of Saudi Arabia
2Department of Diagnostic Microbiology and Molecular Biology, Doctors' Diagnostic Centre, Tiruchirappalli, 620 003, Tamil Nadu, India
3Post Graduate & Research Department of Botany & Microbiology, A. Veeriya Vandayar Memorial Sri Pushpam College (Autonomous), Poondi, 613 503, Thanjavur District, Tamil Nadu, India
4Department of Microbiology, School of Life Sciences, Bharathidasan University, Tiruchirappalli, 620 024, Tamil Nadu, India
Floral research http://dx.doi.org/10.1016/j.apjtb.2015.12.005
Tel: +966 595592263
E-mail: druresh.maray@gmail.com
Foundation project: Supported by Department of Biotechnology Govt. of India (BT/PR4815/AAQ/3/587/2012).
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 8 Oct 2015
Received in revised form 23 Oct, 2nd revised form 29 Oct 2015
Accepted 16 Nov 2015
Available online 4 Jan 2016
Keywords:
Antibacterial efficacy Medicinal plants
Bacterial pathogens Phytoconstituents
ABSTRACT
Objective: To evaluate the antibacterial efficacy of five Indian medicinal plants such as Acalypha indica L. (A. indica), Aerva lanata (L.) Juss. ex Schult. (A. lanata), Clerodendrum inerme (L.) Gaertn., Pergularia daemia (Forsk.) Chiov. and Solanum surattense Burm. f. against opportunistic bacterial pathogens isolated from HIV infected patients for the potential phytoconstituents in plant extracts.
Methods: The opportunistic bacterial pathogens such as Escherichia coli (E. coli), Pseudomonas aeruginosa, Salmonella typhi and Serratia marcescens from Gramnegative group and Staphylococcus aureus from Gram-positive group were isolated from HIV infected patients. The antibacterial efficacy of ethanolic extracts of selected medicinal plants was carried out by disc diffusion method. The potential phytoconstituents of medicinal plant extracts were identified by gas chromatography and mass spectrometry (GC-MS) analysis.
Results: Among the five medicinal plants tested, A. indica and A. lanata showed the significant antibacterial activity. A. indica showed potential activity against Staphylococcus aureus and E. coli. A. lanata significantly exhibited antibacterial activity against E. coli, Salmonella typhi and Pseudomonas aeruginosa. A total of 19 phytoconstituents were identified in the ethanolic extract of A. indica and A. lanata by GC-MS analysis respectively.
Conclusions: The results of the present investigation revealed that A. indica and A. lanata, possessed significant antibacterial activity when compared with the other plant extracts tested. The presence of 3-O-methyl-D-glucose by GC-MS analysis in both A. indica and A. lanata extracts has not been reported elsewhere in the literature and the findings in this study could be the first one to report.
1. Introduction
Medicinal plants have been identified as a part of the evolution of human healthcare for thousands of years. Medicinal components from plants play an important role in traditional as well as modern medicine. Antimicrobial resistance is an increasingly serious threat to global public health. According to World Health Organization (WHO) report on antimicrobial resistance in 2014, overcoming the antibiotic resistance is the major issue to the WHO for the next millennium. Screening of plants for antimicrobial agents has gained much importance because WHO is encouraging and promoting in the development and utilization of medicinal plant resources in the traditional system of medicine. Accordingly, the last decade witnessed an increase in the investigation of plants as a source of human infectious disease management.
Acalypha indica L. (A. indica) (family: Euphorbiaceae) is an annual herbaceous weed and widely distributed throughout the tropical region of India. According to ethno-medicinal uses, it is used for treating pneumonia, jaundice, piles, asthma, rheumatism, bedsores, wounds and skin disorders. It has been reported to have wound healing activity, snake venom neutralizing properties, antibacterial activity and antiurolithiatic activity [1-4].
Aerva lanata (L.) Juss. ex Schult. (A. lanata) (family: Amaranthaceae) is a herbaceous perennial weed. It is commonly distributed throughout India. It is used as diuretic and demulcent. Its diuretic action is very effective in the treatment of urethral discharges and gonorrhea. It is also used for treating headache, cough, liver congestion, jaundice, biliousness, dyspepsia, pneumonia, typhoid, urinary and gall stones, skin diseases, scorpion stings and snake bites [5]. It is extensively studied for various pharmacological activities such as antimicrobial, anti-diabetic, antitumor, nephronprotective, hepatoprotective, immunomodulatory, antiurolithiatic, antifertility, anti-metastatic and anti-HIV activity [6-19].
Clerodendrum inerme (L.) Gaertn. (C. inerme) (family: Verbenaceae) is a perennial shrub. It is used to treat fever, cough, scrofulous infection, venereal infection and skin diseases. It is also used to treat umbilical cord infection and for cleaning the uterus [20,21]. It has been accounted for antimalarial, anticancer, relieve hyperlocomotion and antimicrobial activities [22-25].
Pergularia daemia (Forsk.) Chiov. (P. daemia) (family: Asclepiadaceae) is a perennial climber throughout hot parts of India. It is used as stomachic, laxative and diuretic. It is also used to treat diarrhea, fever, cough, asthma, biliousness and sore eyes. The methanolic extract of P. daemia plant was evaluated for its antiurolithiatic and antidiabetic activities[26,27].
Solanum surattense Burm. f. (S. surattense) (family: Solanaceae) is a perennial herbaceous weed. It is distributed throughout the tropical regions of India. It is used for treating asthma, cough, leprosy, dropsy, sore throat and constipation. It has been reported for antimalarial and antioxidant activities[28-31].
As per the currently available literature, only few studies were evaluated the antibacterial properties of the selected medicinal plants. Consequently, the present research was planned to evaluate the antibacterial activity of ethanolic extracts of the selected medicinal plants against the opportunistic bacterial pathogens isolated from HIV infected patients and its potential phytoconstituents.
2. Materials and methods
2.1. Collection of plant leaves
The fresh aerial parts of medicinal plants including A. indica, A. lanata, C. inerme, P. daemia and S. surattense were collected from Tiruchirappalli District. The taxonomic identification of the plants was done and the voucher herbarium specimens were deposited in the Post Graduate & Research Department of Botany & Microbiology, A. Veeriya Vandayar Memorial Sri Pushpam College (Autonomous), Poondi, Thanjavur District, Tamil Nadu, India. All the plant leaves were cleaned and shade dried (Figure 1).
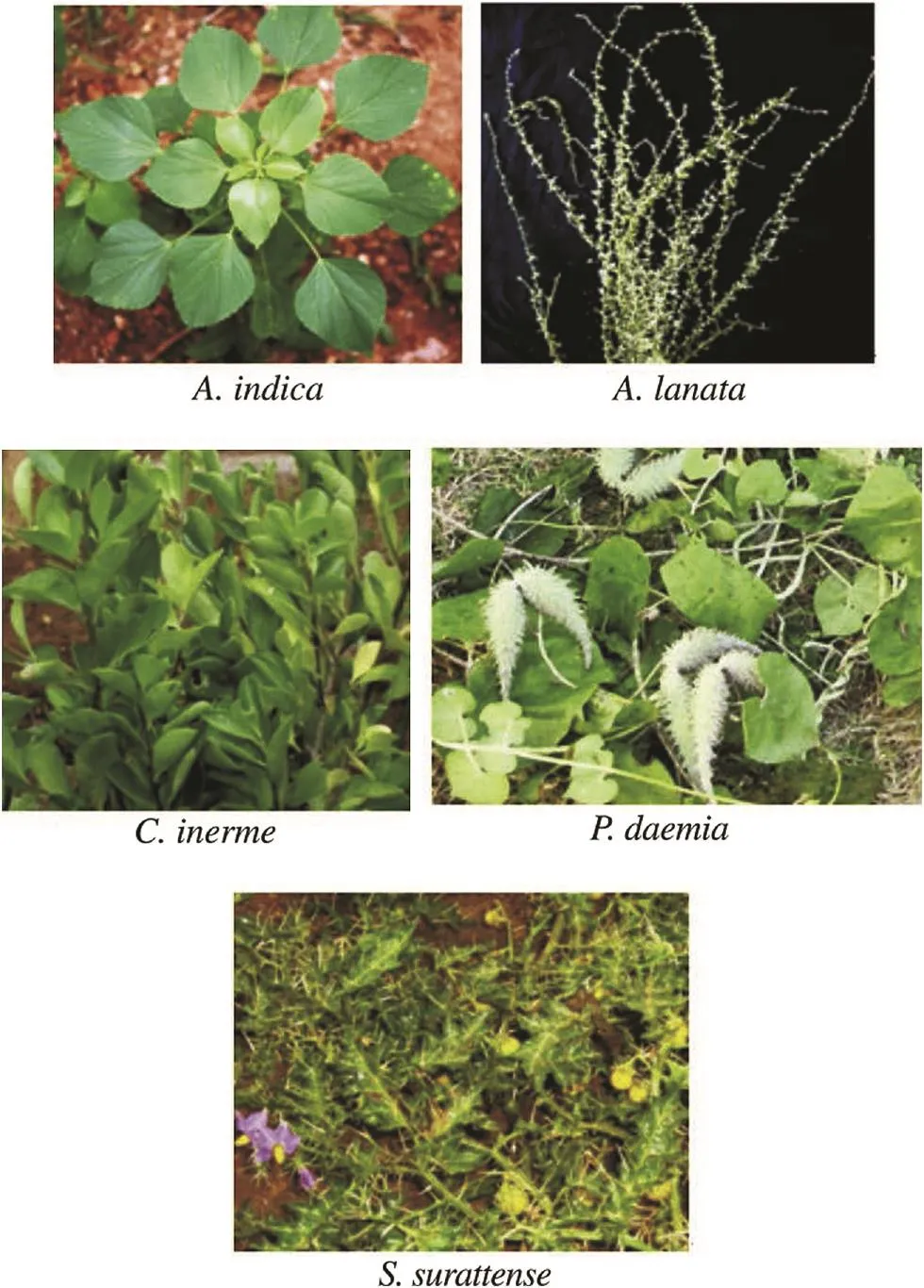
Figure 1. Selected Indian medicinal plants.
2.2. Preparation of ethanolic extracts
The shade dried leaves of selected plants were pulverized. About 50 g of powdered sample was extracted with the 100 mL of ethanol by using a Soxhlet extractor for 24 h. The obtained extracts were concentrated by using a rotary flash evaporator. The extracts were well preserved in airtight containers for further analysis.
2.3. Preparation of disc
The sterile discs (6 mm, Himedia) were impregnated with different concentrations of 5, 10, 20 and 30 mg of the plant extracts. The discs were identified with labeling and stored in airtight containers with silica gel desiccant.
2.4. Inoculum preparation
The opportunistic bacterial pathogens such as Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa), Salmonella typhi (S. typhi), Serratia marcescens (S. marcescens) and Staphylococcus aureus (S. aureus) were isolated from HIV infected patients, who were referred to Doctors' Diagnostic Center, Tiruchirappalli. All these pathogens were suspended in 0.85% saline corresponding to No. 0.5 McFarland turbidity standard. All cultures were incubated on a shaker at 37°C for 18 h and then diluted to 1/10 concentration to yield a culture density of approximately 1.5×108CFU/mL.
2.5. Antibacterial assay
The antibacterial activity of ethanolic extracts was assessed by disc diffusion method [32]. Mueller-Hinton agar (0.2 g beef extract, 1.75 g peptone, 0.15 g starch, 2.0 g agar, 100 mL distilled water, pH 7.5) was prepared with lawn culture using desired test organisms. The inoculated plates were kept aside for a few minutes. The plant extract impregnated discs were placed over the medium. After diffusion, the plates were incubated at 37°C for 24 h. After incubation, the diameter (mm) of the zone of inhibition was measured and compared with the standard antibiotics such as ofloxacin (5 mg/disc) for Gram-negative bacteria and vancomycin (30 mg/disc) for the Gram-positive bacteria (S. aureus). All the tests were performed in three replicates and the activity was expressed as the mean of inhibition diameters (mm) produced by the plant extracts.
2.6. Gas chromatography-mass spectrometry (GC–MS) analysis
About 20 g of powdered plant material was soaked in 50 mL of absolute alcohol overnight and then filtered through Whatman Grade No. 41 quantitative filter paper along with 2 g sodium sulfate to remove the sediments and traces of water in the filtrate. Before filtering, the filter paper along with sodium sulfate was wetted with absolute alcohol. The filtrate was concentrated by bubbling nitrogen gas into the solution and reduced the volume to 1 mL. The extract contained both polar and non-polar phytocomponents of the plant material used.
The GC-MS analysis was carried out using a Clarus 500 PerkinElmer (AutoSystem XL) gas chromatograph equipped and coupled to a mass detector, turbo mass gold-PerkinElmer turbo mass 5.1 spectrometer with an elite-1 (100% dimethyl poly siloxane), 30 m×0.25 mm (inner diameter)×1 mm of capillary column. The instrument was set to an initial temperature of 110°C and maintained at this temperature for 2 min. At the end of the period, the oven temperature was rose up to 280°C at the rate of an increase of 5°C/min and maintained for 9 min. Injection port temperature was ensured as 250°C and helium flow rate was ensured as 1 mL/min. The ionization voltage was 70 eV. The samples were injected in split mode as 10:1. Mass spectral scan range was set at 45-450 (m/z). Using computer searches on a National Institute of Standards and Technology ver.2.1 MS data library and comparing the spectrum obtained through GC-MS compounds present in the plants sample were identified.
3. Results
3.1. Antibacterial activity of selected Indian medicinal plants
The antibacterial activity of ethanolic plant extracts was evaluated against the five opportunistic bacterial pathogens isolated from HIV infected patients. The results of antibacterial activity of different concentrations of A. indica extracts were summarized in Table 1. The results revealed that A. indica ethanolic extracts showed the maximum activity against S. aureus and E. coli and the minimum activity against S. typhi, P. aeruginosa and S. marcescens at 30 mg concentration. The antibacterial activity was distinctly varied against the bacterial test pathogens.
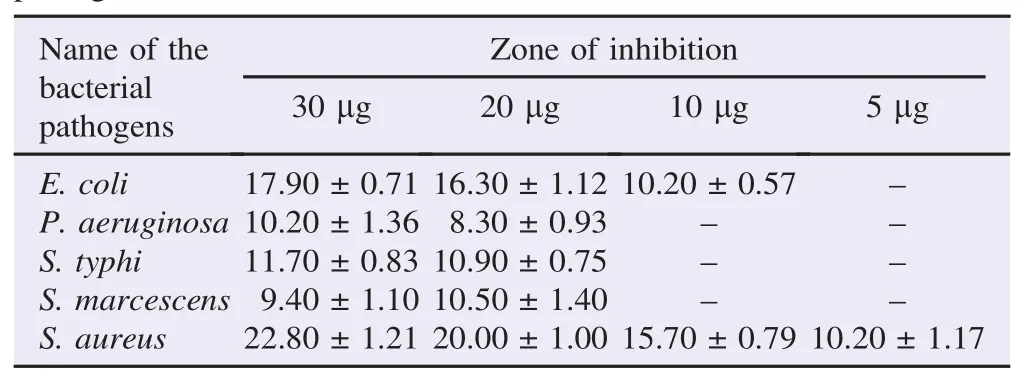
Table 1 Antibacterial activity of ethanolic extract of A. indica against the clinical pathogens. mm.
Table 2 demonstrates the results of antibacterial activity of A. lanata ethanolic extracts. The maximum antibacterial activity of A. lanata plant extract was exhibited against E. coli, P. aeruginosa and S. aureus. A minimal antibacterial activity was identified against S. typhi and S. marcescens at 30 mg concentration. The activity was concentration-dependent.
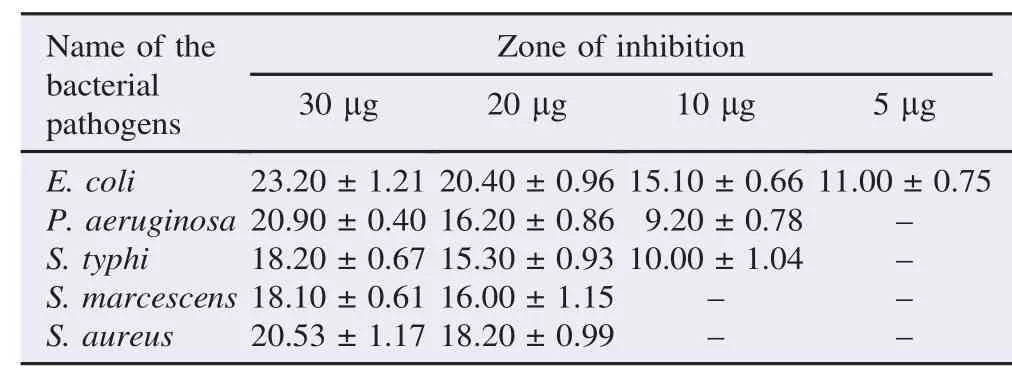
Table 2 Antibacterial activity of ethanolic extract of A. lanata against the clinical pathogens. mm.
The antibacterial activity of C. inerme plant extract showed the activity against only S. typhi with the zone of inhibition (10.60±0.85) mm at 30 mg concentration and didn't show any inhibitory activity against the other test pathogens such as E. coli, S. aureus, P. aeruginosa and S. marcescens. The results of antibacterial activity of P. daemia plant extract revealed the activity against only E. coli [(14.10±0.75) mm and (12.10±0.80) mm] at 30 mg and 20 mg concentrations and didn't show any inhibitory activity against the other tested pathogens. The antibacterial activity of S. surattense plant extract showed the activity against only E. coli [(10.10±0.91) mm] at 30 mg concentration and did not show any inhibitory activity against the other test pathogens such as S. aureus, S. typhi, P. aeruginosa and S. marcescens.
The results of standard antibiotics sensitivity test on clinical pathogens were given in Table 3. Among the five plant extracts tested, the ethanolic extracts of A. lanata exhibited significant antibacterial activity when compared with the standard antibiotics tested.
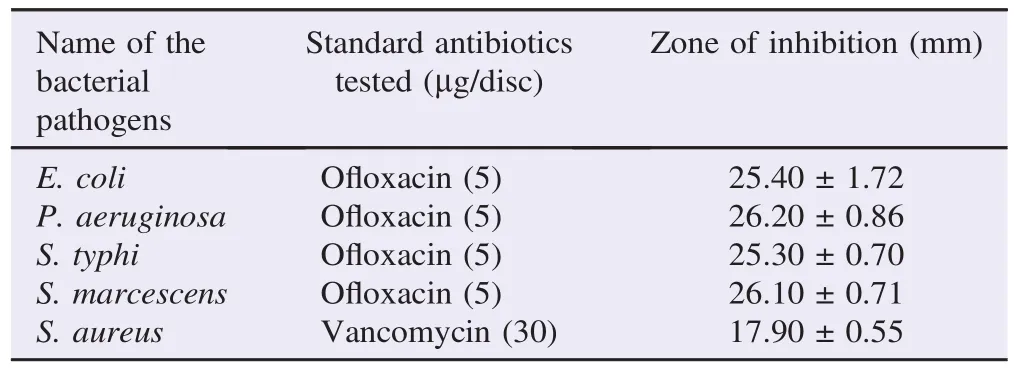
Table 3 Standard antibiotics sensitivity test on clinical pathogens.
3.2. Assessment of phytocomponents in selected medicinal plants
Among the five plants, A. indica and A. lanata showed the significant antibacterial activity. Consequently, it was subjected to GC-MS analysis. The mass spectrum of ethanol extracts of A. indica showed 10 prominent peaks (Figure 2). The major phytoconstituents were 3-O-methyl-D-glucose with 50.18% peak area and9,12,15-octadecatrienoicacid,(Z,Z,Z)-with17.50%peakarea. The identified phytoconstituents of A. indica extracts and its biologicalactivityweregiveninTable4.Thebiologicalactivitieslisted were based on Dr. Duke's phytochemical and ethnobotanical databases created by Dr. Jim Duke of the Agricultural Research Service/United States Department of Agriculture.
The mass spectrum of ethanolic extracts of A. lanata showed 9 prominent peaks (Figure 3) and the identified phytoconstituents were given in Table 5. The major phytoconstituents were 3-O-methyl-D-glucose with 25.56% peak area, 9,12-octadecadienoic acid (Z,Z)- with 18.90% peak area, n-hexadecanoic acid with 16.02% peak area and squalene with 14.04% peak area.

Figure 2. GC-MS chromatogram formed by ethanolic extract of A. indica.
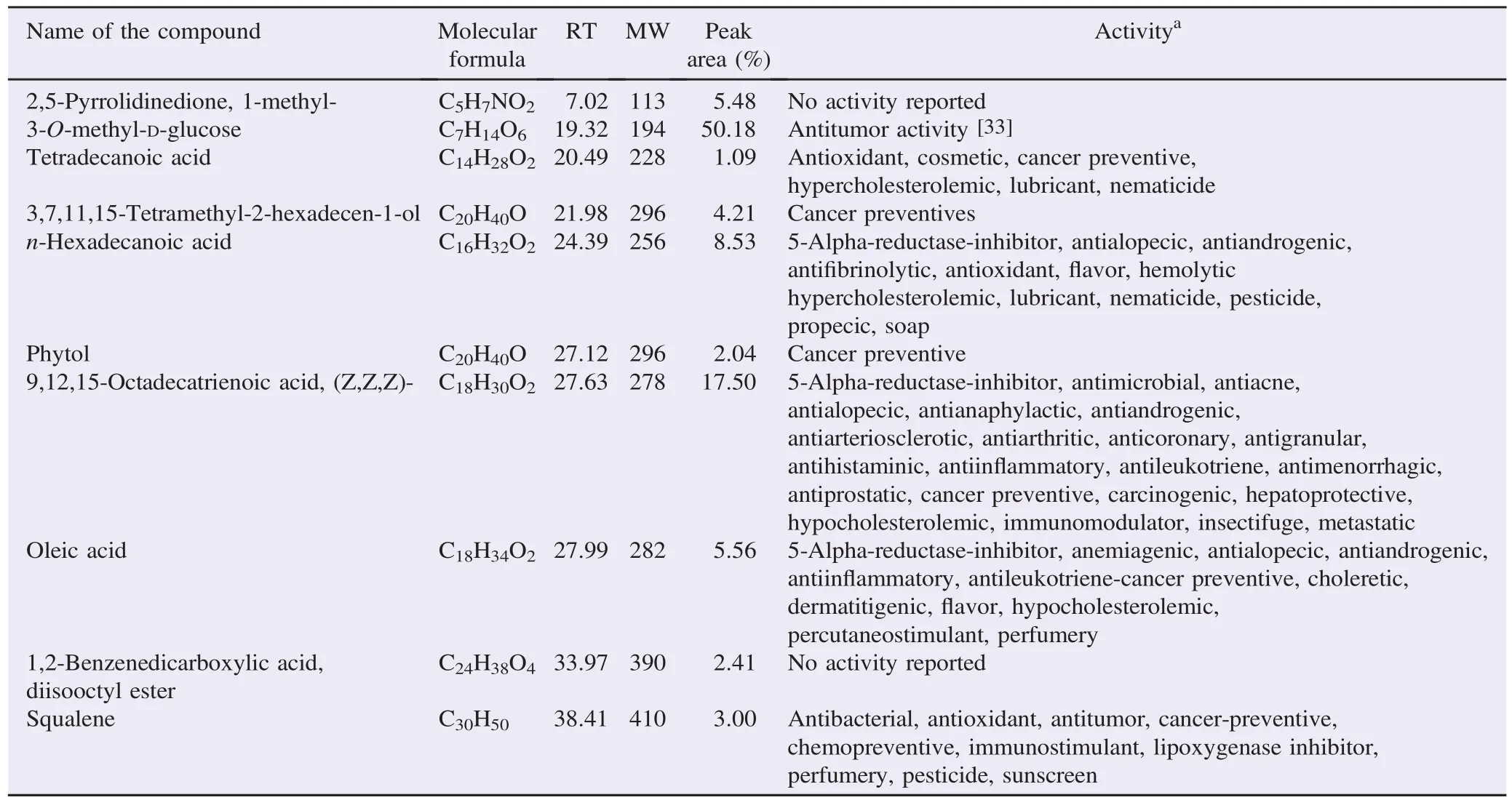
Table 4 Phytochemicals identified in the ethanolic extract of A. indica by GC-MS.

Figure 3. GC-MS chromatogram formed by ethanolic extract of A. lanata.
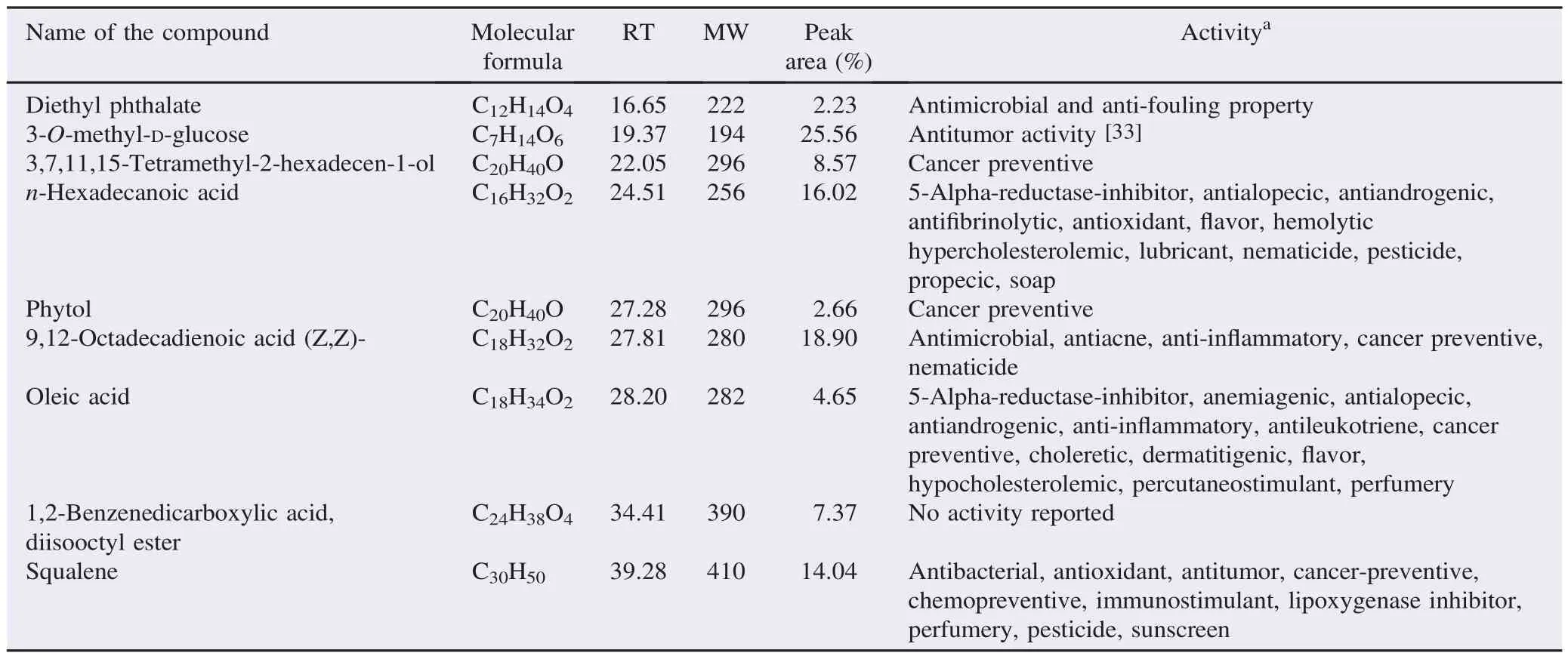
Table 5 Phytochemicals identified in the ethanolic extract of A. lanata by GC-MS.
4. Discussion
The discovery of novel antimicrobial metabolites from medicinal plants is an important alternative to overcome the increasing levels of drug resistance by human pathogens. Due to the world's urgent need for new antibiotics and chemotherapeutic agents, growing interest is taken into the research on the chemistry of medicinal plants. Antimicrobial activity of medicinal plants and their phytoconstituents have been deliberated in the late 19th century [34]. In the present study,five ethnobotanical herbs including A. indica, A. lanata, C. inerme, P. daemia and S. surattense were evaluated for antibacterial activity against opportunistic bacterial pathogens isolated from HIV infected patients.
Among the five plants, the significant antibacterial property was observed in A. indica. The present results were in concurrence with the findings of Mohan et al.[35]. Their results revealed that various extracts of A. indica showed considerable antibacterial activity against S. aureus and E. coli. The antibacterial potential of A. lanata plant extract showed significant activity against E. coli in all concentrations and potential activity against S. aureus, S. typhi, P. aeruginosa and S. marcescens. Results of antibacterial activity were comparable with the findings of Vidhya and Udayakumar, who found that A. lanata possessed significant antibacterial activity[36].
The antibacterial property of C. inerme, P. daemia and S. surattense plant extracts showed very minimal antibacterial activity. The plant extract of these plants could have lost their antibacterial potentials in drying due to heat sensitive nature of the phytochemical constituents in the plants.
GC-MS analysis plays a key role in the analysis of components of plant origin. Generally, the plant materials are highly complexes, which make GC-MS well suited for their analysis because of its high sensitivity and selectivity. It is considered to be the gold standard in scientific analysis [37,38]. Several phytochemical screening studies have been carried out in different parts of the world by using GC-MS [39,40]. Among the five plants, only two plants were justifiably chosen for bioactive compounds investigation using the GC-MS analysis.
In this study, we reported for the first time a high resolution GC-MS method for the evaluation of the chemical constituents of A. indica and A. lanata plant extracts. This accurate and sensitive analysis of A. indica and A. lanata revealed the various phytoconstituents present in the extracts.
The major bioactive compounds in A. indica and A. lanata are 3-O-methyl-D-glucose. The presence of 3-O-methyl-D-glucose in A. indica and A. lanata extracts has not been reported in any previous research work and the findings in this study could be the first one to report. 3-O-methyl-D-glucose is a nontoxic nonmetabo lizable derivative of glucose and it has been reported for antitumor activity [33]. The antibacterial activity of A. indica and A. lanata may be due to presence of 3-O-methyl-D-glucose.
9,12,15-Octadecatrienoic acid, (Z,Z,Z)-, 9,12-octadecadienoic acid (Z,Z)- and n-hexadecanoic acid are another major compounds in the studied plant extracts and have been reported to have antimicrobial, antioxidant and anti-inflammatory activities [41-44]. The antibacterial activity of A. indica and A. lanata could be attributed to the presence of these phytoconstituents.
The n-hexadecanoic acid (synonym: palmitic acid) and 9,12-octadecadienoic acid (linoleic acid) were also reported in Benincasa hispida and Carissa congesta plant extracts [40]. Similarly, these phytocompounds were identified in various plants such as Allium nigrum, Kielmeyera coriacea, Cyrtocarpa procera, Labisia pumila and Rosa indica [42-46]. The identified squalene (14.04%) in A. lanata extract has been reported for antioxidant, antibacterial and anticancer activities[47].
The results of the present investigation also complement the ethnobotanical usage of the studied plants which possess several phytoconstituents with biological activity. Based on the present investigation, it is concluded that A. indica and A. lanata have potentialsourceofbioactivecompoundswithgreatpharmaceutical value.Thestudycanbeextendedforseparationandinvitro,invivo evaluation of bioactive compounds in novel drug discovery.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
We gratefully acknowledge the Department of Biotechnology Govt. of India (BT/PR4815/AAQ/3/587/2012) for their financial assistance.
References
[1] Reddy JS, Rao PR, Reddy MS. Wound healing effects of Heliotropium indicum, Plumbago zeylanicum and Acalypha indica in rats. J Ethnopharmacol 2002; 79(2): 249-51.
[2] Shirwaikar A, Rajendran K, Bodla R, Kumar CD. Neutralization potential of Viper russelli russelli (Russell's viper) venom by ethanol leaf extract of Acalypha indica. J Ethnopharmacol 2004; 94(2-3): 267-73.
[3] Govindarajan M, Jebanesan A, Reetha D, Amsath R, Pushpanathan T, Samidurai K. Antibacterial activity of Acalypha indica L. Eur Rev Med Pharmacol Sci 2008; 12: 299-302.
[4] Sathya M, Kokilavani R, Teepa KS, Balakrishnan A. Biopotency of Acalypha indica Linn on membrane bound ATPases and marker enzymes urolithic rats. Anc Sci Life 2011; 31(1): 3-9.
[5] Upadhyay OP, Kumar K, Tiwari RK. Ethnobotanical study of skin treatment uses of medicinal plants of Bihar. Pharm Biol 1998; 36: 167-72.
[6] Chowdhury D, Sayeed A, Islam A, Shah Alam Bhuiyan M, Astaq Mohal Khan GR. Antimicrobial activity and cytotoxicity of Aerva lanata. Fitoterapia 2002; 73: 92-4.
[7] Vetrichelvan T, Jegadeesan M. Anti-diabetic activity of alcoholic extract of Aerva lanata (L.) Juss. ex Schultes in rats. J Ethnopharmacol 2002; 80: 103-7.
[8] Agrawal R, Sethiya NK, Mishra SH. Antidiabetic activity of alkaloids of Aerva lanata roots on streptozotocin-nicotinamide induced type-II diabetes in rats. Pharm Biol 2013; 51(5): 635-42.
[9] Riya MP, Antu KA, Pal S, Chandrakanth KC, Anilkumar KS, Tamrakar AK, et al. Antidiabetic property of Aerva lanata (L.) Juss. ex Schult. is mediated by inhibition of alpha glucosidase, protein glycation and stimulation of adipogenesis. J Diabetes 2015; 7(4): 548-61.
[10] Nevin KG, Vijayammal PL. Effect of Aerva lanata on solid tumor induced by DLA cells in mice. Fitoterapia 2003; 74: 578-82.
[11] Shirwaikar A, Issac D, Malini S. Effect of Aerva lanata on cisplatin and gentamicin models of acute renal failure. J Ethnopharmacol 2004; 90: 81-6.
[12] Nevin KG, Vijayammal PL. Effect of Aerva lanata against hepatotoxicity of carbon tetrachloride in rats. Environ Toxicol Pharmacol 2005; 20: 471-7.
[13] Nevin KG, Vijayammal PL. Pharmacological and immunomodulatory effects of Aerva lanata in Daltons lymphoma ascites-bearing mice. Pharm Biol 2005; 43: 640-6.
[14] Soundararajan P, Mahesh R, Ramesh T, Begum VH. Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Indian J Exp Biol 2006; 44: 981-6.
[15] Dinnimath BM, Jalalpure SS. In silico antiurolithiatic screening of Aerva lanata (L) isolated constituents. Indian J Pharm Educ Res 2015; 49(2): 126-33.
[16] Gunatilake M, Lokuhetty MD, Bartholameuz NA, Edirisuriye DT, Kularatne MU, Date A. Aerva lanata (Polpala): its effects on the structure and function of the urinary tract. Pharmacogn Res 2012; 4(4): 181-8.
[17] Savadi RV, Alagawadi KR. Antifertility activity of ethanolic extracts of Plumbago indica and Aerva lanata on albino rats. Int J Green Pharm 2009; 3: 230-3.
[18] Siveen KS, Kuttan G. Inhibition of B16F-10 melanoma-induced lung metastasis in C57BL/6 mice by Aerva lanata via induction of apoptosis. Integr Cancer Ther 2013; 12(1): 81-92.
[19] Gujjeti RP, Namthabad S, Mamidala E. HIV-1 reverse transcriptase inhibitory activity of Aerva lanata plant extracts. BMC Infect Dis 2014; 14(Suppl 3): P12.
[20] Ur-Rehman A, Begum S, Saied S, Choudhary MI, Akhtar F. A steroidal glycoside from Clerodendron inerme. Phytochemistry 1997; 45: 1721-2.
[21] Kanchanapoom T, Kasai R, Chumsri P, Hiraga Y, Yamasaki K. Megastigmane and iridoid glucosides from Clerodendrum inerme. Phytochemistry 2001; 58: 333-6.
[22] Kalyanasundaram M, Das PK. Larvicidal and synergistic activity of plant extracts for mosquito control. Indian J Med Res 1985; 82: 19-23.
[23] Manoharan S, Kavitha K, Balakrishnan S, Rajalingam K. Clerodendron inerme protects cellular integrity during 7,12-dimethylbenz[A]-anthracene induced hamster buccal pouch carcinogenesis. Afr J Tradit Complement Altern Med 2008; 5: 213-22.
[24] Chen HL, Lee HJ, Huang WJ, Chou JF, Fan PC, Du JC, et al. Clerodendrum inerme leaf extract alleviates animal behaviors, hyperlocomotion, and prepulse inhibition disruptions, mimicking tourette syndrome and schizophrenia. Evid Based Complement Altern Med 2012; 2012: 284301.
[25] Rajasekar A, Hemalatha S. Anti bacterial activity and phytochemical analysis of Clerodendrum inerme. Int Res J Pharm 2015; 6(2): 169-72.
[26] Vyas B, Vyas R, Joshi S, Santani D. Antiurolithiatic activity of whole-plant hydroalcoholic extract of Pergularia daemia in rats. J Young Pharm 2011; 3(1): 36-40.
[27] Sijuade AO, Omotayo OO, Oseni OA. Hypoglyceamic effect of methanolic extract of Pergularia daemia in alloxan-induced diabetic mice. Br J Pharm Res 2014; 4(22): 2614-21.
[28] Ramazani A, Zakeri S, Sardari S, Khodakarim N, Djadidt ND. In vitro and in vivo anti-malarial activity of Boerhavia elegans and Solanum surattense. Malar J 2010; 9: 124.
[29] Joseph JM, Sowndhararajan K, Rajendrakumaran D, Manian S. In vitro antioxidant potential of different parts of Solanum surattense Burm. f. Food Sci Biotechnol 2011; 20(2): 477-83.
[30] Thirumalai T, David E, Viviyan TS, Elumalai EK. Effect of Solanum surattense seed on the oxidative potential of cauda epididymal spermatozoa. Asian Pac J Trop Biomed 2012; 2(1): 21-3.
[31] Muruhan S, Selvaraj S, Viswanathan PK. In vitro antioxidant activities of Solanum surattense leaf extract. Asian Pac J Trop Biomed 2013; 3(1): 28-34.
[32] Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 1966; 45: 493-6.
[33] Wick MM, Rossini A, Glynn D. Reduction of streptozotocin toxicity by 3-O-methyl-D-glucose with enhancement of antitumor activity in murine L1210 leukemia. Cancer Res 1977; 37: 3901-3.
[34] Zaika LL. Spices and herbs: their antimicrobial activity and its determination. J Food Saf 1988; 9: 97-118.
[35] Mohan SC, Dinakar S, Anand T, Elayaraja R, SathiyaPriya B. Phytochemical, GC-MS analysis and antibacterial activity of a medicinal plant Acalypha indica. Int J Pharm Tech Res 2012; 4(3): 1050-4.
[36] Vidhya R, Udayakumar R. Antibacterial potential of different parts of Aerva lanata (L.) against some selected clinical isolates from urinary tract infections. Br Microbiol Res J 2015; 7(1): 35-47.
[37] Sajewicz M, Rzepa J, Hajnos M, Wojtal Ł, Staszek D, Kowalska T, et al. GC-MS study of the performance of different techniques for isolating the volatile fraction from sage (Salvia L.) species, and comparison of seasonal difference in the composition of this fraction. Acta Chromatogr 2009; 21(3): 453-71.
[38] Balamurugan K, Nishanthini A, Mohan VR. GC-MS analysis of Polycarpaea corymbosa (L.) Lam whole plant. Asian Pac J Trop Biomed 2012; 2(Suppl 3): S1289-92.
[39] Dubey D, Patnaik R, Ghosh G, Padhy RN. In vitro antibacterial activity, gas chromatography-mass spectrometry analysis of Woodfordia fruticosa Kurz. leaf extract and host toxicity testing with in vitro cultured lymphocytes from human umbilical cord blood. Osong Public Health Res Perspect 2014; 5(5): 298-312.
[40] Doshi GM, Nalawade VV, Mukadam AS, Chaskar PK, Zine SP, Somani RR, et al. Structural elucidation of chemical constituents from Benincasa hispida seeds and Carissa congesta roots by gas chromatography: mass spectroscopy. Pharmacogn Res 2015; 7(3): 282-93.
[41] Wei LS, Wee W, Siong JY, Syamsumir DF. Characterization of anticancer, antimicrobial, antioxidant properties and chemical compositions of Peperomia pellucida leaf extract. Acta Med Iran 2011; 49(10): 670-4.
[42] Rouis-Soussi LS, Ayeb-Zakhama AE, Mahjoub A, Flamini G, Jannet HB, Harzallah-Skhiri F. Chemical composition and antibacterial activity of essential oils from the Tunisian Allium nigrum L. EXCLI J 2014; 13: 526-35.
[43] Martins Cde M, do Nascimento EA, de Morais SAL, de Oliveira A, Chang R, Cunha LCS, et al. Chemical constituents and evaluation of antimicrobial and cytotoxic activities of Kielmeyera coriacea Mart. & Zucc. essential oils. Evid Based Complement Altern Med 2015; 2015: 842047.
[44] Martinez-Elizalde KS, Jimenez-Estrada M, Flores CM, Hernandez LB, Rosas-Lopez R, Duran-Diaz A, et al. Evaluation of the medicinal properties of Cyrtocarpa procera Kunth fruit extracts. BMC Complement Altern Med 2015; 15: 74.
[45] Karimi E, Jaafar HZ, Ghasemzadeh A, Ebrahimi M. Fatty acid composition, antioxidant and antibacterial properties of the microwave aqueous extract of three varieties of Labisia pumila Benth. Biol Res 2015; 48: 9.
[46] Rasheed HM, Khan T, Wahid F, Khan R, Shah AJ. Chemical composition and vasorelaxant and antispasmodic effects of essential oil from Rosa indica L. petals. Evid Based Complement Altern Med 2015; 2015: 279247.
[47] Popa O, B abeanu NE, Popa I, Nit¸ a S, Dinu-Pˆarvu CE. Methods for obtaining and determination of squalene from natural sources. Biomed Res Int 2015; 2015: 367202.
*Correspondingauthor:Suresh Mickymaray,Departmentof Medical Laboratories, College of Science Al-Zulfi, Majmaah University, Al-Zulfi, Kingdom of Saudi Arabia.
 Asian Pacific Journal of Tropical Biomedicine2016年3期
Asian Pacific Journal of Tropical Biomedicine2016年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Cyclical mastalgia: Prevalence and associated determinants in Hamadan City, Iran
- Natural antibacterial remedy for respiratory tract infections
- Bee pollen extract of Malaysian stingless bee enhances the effect of cisplatin on breast cancer cell lines
- In vitro antihistamine-releasing activity of a peptide derived from wasp venom of Vespa orientalis
- Changes in energetic profile of pregnant ewes in relation with the composition of the fetal fluids
- The inhibition of Typhonium flagelliforme Lodd. Blume leaf extract on COX-2 expression of WiDr colon cancer cells
