Quantitative determination of vitexin in Passiflora foetida Linn. leaves using HPTLC
Quantitative determination of vitexin in Passiflora foetida Linn. leaves using HPTLC
Aussavashai Shuayprom1, Donruedee Sanguansermsri2,3, Phanchana Sanguansermsri1, Ian Hamilton Fraser4, Nalin Wongkattiya5*1Department of Biochemistry, Faculty of Medical Science, Naresuan University, Phitsanulok 65000, Thailand
2Department of Microbiology and Parasitology, Faculty of Medical Science, Naresuan University, Phitsanulok 65000, Thailand
3Centre of Excellence in Medical Biotechnology, Faculty of Medical Science, Naresuan University, Phitsanulok 65000, Thailand
4School of Chemistry, Monash University, Clayton 3800, Victoria, Australia
5Program in Biotechnology, Faculty of Science, Maejo University, Chiang Mai 50290, Thailand
Floral research http://dx.doi.org/10.1016/j.apjtb.2015.11.006
Tel: +66 877880432
E-mail: nalin.wongkattiya@gmail.com
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 25 Sep 2015
Received in revised form 14 Oct, 2nd revised form 3 Nov, 3rd revised form 10 Nov 2015
Accepted 25 Nov 2015
Available online 2 Jan 2016
Keywords:
HPTLC
Thin layer chromatography Vitexin
Passiflora foetida Linn.
ABSTRACT
Objective: To establish a simple, rapid, precise and accurate high performance thin layer chromatography (HPTLC) method with densitometric detection for the determination of vitexin in Passiflora foetida Linn. (P. foetida).
Methods: Ethanolic extract of the plant leaf powder was used for the experimental work. Separation was performed on silica gel 60 F254HPTLC plates with ethyl acetate: methanol: distilled water: formic acid in the proportion of 50:2:3:6 (v/v), as the mobile phase. The determination was carried out using the densitometric absorbance mode at 340 nm.
Results: Vitexin response was linear over the range of 2.5-17.5 mg/mL with a correlation coefficient of 0.996. Intraday and interday precision studies showed the relative SD was <3%. Accuracy of the method was determined and the average recovery was 100.3%. The limit of quantitation and limit of detection were 0.879 and 0.290 mg/mL, respectively. The contents of vitexin in P. foetida leaf extracts were within the range of 0.030%-0.310%.
Conclusions: The method was evaluated for sensitivity, accuracy, precision and reproducibility. Each analysis by HPTLC is less expensive than current methods. This method is suitable for routine quality control of raw material of the leaves of P. foetida extract and its products.
1. Introduction
Passiflora foetida Linn. (P. foetida) (family Passifloraceae) is native to South America, which has spread to tropical regions around the world, including Thailand. The leaves of this plant are also utilized as traditional medicine for the treatment of hysteria, fever, ear infections, emmenagogue, asthma, insomnia and skin disease [1-6]. The ethanolic extracts of the leaves of P. foetida display remarkable activity against Pseudomonas putida, Vibrio cholerae, Shigella flexneri and Streptococcus pyogenes. A further study conducted by Mohanasundari et al. did not show the active compound present in the crude extract of P. foetida [7]. The major phytochemical constituents of P. foetida have several active constituents like hydrocyanic acid, groups of flavonoids, harman alkaloids, passifloricins, polyketides, a-pyrones and vitexin [2,8,9]. Vitexin has been reported to have antioxidant, anti-inflammatory, anti-thyroid, anti-arteriosclerotic, antihypertensive and antihepatotoxic properties [10-23]. Levels of vitexin in different plants extracts have been determined by numerous techniques, including spectroscopic and chromatographic methods. High performance thin layer chromatography (HPTLC), coupled with densitometricdetection, is among the various methods reported for determination of vitexin [24-30]. HPTLC has advantages because of high sample throughput at low operating cost, easy sample preparation, short analysis time and use of a small quantity of mobile phase unlike high performance liquid chromatography. The major advantage of HPTLC is that it is both a qualitative and a quantitative technique [31-37]. The objective of this work was to develop a quick, economical HPTLC method for vitexin determination in the ethanolic extract of leaves of P. foetida. The proposed method was validated in compliance with International Conference on Harmonization guidelines [38].
2. Materials and methods
2.1. Apparatus
A Camag HPTLC system equipped with an automatic thin layer chromatography (TLC) sampler 4 (ATS 4, connected to a nitrogen tank), TLC scanner 3 (winCATS version 1.3.4), and UV cabinet (Reprostar 3) and automatic developing chamber was used for the analysis.
2.2. Reagents and materials
Ten samples of P. foetida were collected from areas where it has colonized in Thailand during January 2013. The samples were identified by comparison with the plant specimens in the Herbarium Section, Forestry Department, Bangkok, Thailand. Solvents were obtained from Merck (Darmstadt, Germany) and were of analytical grades. Standard vitexin (95% pure) was obtained from Sigma-Aldrich Chemie (Steinheim, Germany). Precoated silica gel 60 F254HPTLC plates were procured from Merck (Darmstadt, Germany).
2.3. Preparation of standard stock solution
A vitexin (100 mg/mL) stock solution was prepared with methanol to give a series of working standard solutions with concentrations of 2.5, 5.0, 10.0, 12.5 and 17.5 mg/mL.
2.4. Sample preparation
The leaves of P. foetida were separated from the stems, washed thoroughly and dried in an oven at 50°C. The dried samples were ground to powder. The powder was extracted with ethanol (3×10 mL),filtered and concentrated under vacuum to obtain the crude extract. A total of 200 mg of the accurately weighed extract was transferred to a 10 mL volumetric flask. Methanol was added to the volumetric flask (final concentration of 20000 mg/mL).
2.5. Mobile phase
The mobile phase was comprised of ethyl acetate: methanol: distilled water: formic acid in the proportion of 50:2:3:6 (v/v).
2.6. Method validation
The analytical method was validated for linearity, precision, accuracy, limit of detection (LOD) and limit of quantitation (LOQ) according to the International Conference on Harmonization guidelines.
2.7. Linearity
Standard vitexin solutions from above were used for the determination experiment using silica gel 60 F254HPTLC plates with a mobile phase of ethyl acetate: methanol: distilled water: formic acid (50:2:3:6, v/v). A total of 10 mL of each standard solution was applied to the plate as per the method of Kumar et al. [37]. A wavelength of 340 nm (λmax) was used for quantitation. This was repeated in triplicate and the mean was used for calculation.
2.8. Precision
The precision of the method was studied by analyzing standard solutions of vitexin (5 and 10 mg/mL) after application on TLC plate (n = 6) on the same day for intraday precision and on 3 different days for interday precision by proposed method. The results were expressed as percentage of relative SD (%RSD).
2.9. Accuracy
Accuracy of the method was studied by performing recovery studies at 3 levels of vitexin reference standard added to the samples. Three different volumes (500, 750 and 1000 mL) of standard solution (containing 5 mg/mL vitexin in methanol) were added to the sample solution (20000 mg/mL) and analyzed by the densitometric HPTLC method.
2.10. LOD and LOQ
A plot of experimental concentration (mg/mL) against SD was performed to reflect a linear correlation and the value of the SD on the y-axis intercept was obtained. This intercept value was then multiplied by a factor of 3 to give the LOD or by 10 to give the LOQ.
2.11. Quantification of vitexin content in the leaves of P. foetida extract
Ten microliters of the plant leaf extract was applied to the HPTLC plate in triplicate and run as per the determination experiment above. The amount of vitexin in the extracted samples was then determined using linearity equation.
3. Results
The method developed was a normal phase HPTLC method adapted from Kumar using silica gel 60 F254stationary phase precoated on aluminum sheet for the analysis[37]. Mobile phase used was ethyl acetate: methanol: distilled water: formic acid in the proportion of 50:2:3:6 (v/v), which gave good separation of vitexin (Rf= 0.70) from the other phytochemicals of P. foetida. Concentrations of vitexin in leaf extracts from various sites were in the range of 0.030%-0.310% (Table 1).
Identity of vitexin was confirmed by overlay of spectrum chromatograms obtained with the Camag TLC scanner. The vitexin from the plant extract was compared with the vitexin standard. The detector response of vitexin showed linearity overthe range of 2.5-17.5 mg/mL with a correlation coefficient of 0.996 (Table 2). The overlay of UV spectrum of standard vitexin and vitexin in plant extract was shown in Figure 1. HPTLC chromatograms of standard vitexin and vitexin in the plant extract were shown in Figure 2.
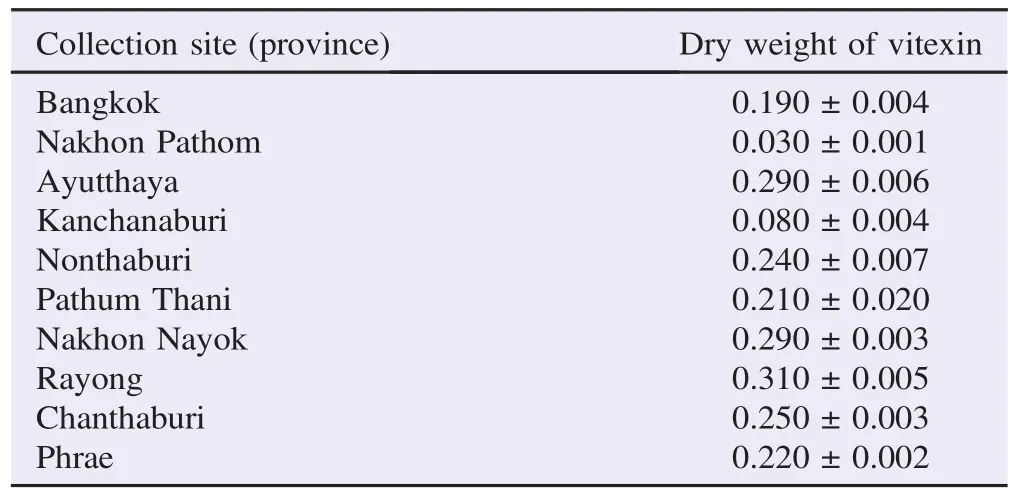
Table 1 Vitexin contents in P. foetida. %.
Instrument precision and interday and intraday precision were measured to determine the overall precision of the method. Percentage of RSD was found to be<3% (Table 3).
Vitexin at various concentrations was determined by the TLC-densitometric method. The percent recovery at 3 different levels of vitexin was found to be 98.3%, 103.0% and 99.7%, with a mean value of 100.3% (Table 4).

Table 2 Method validation parameters for the quantification of vitexin by the proposed TLC-densitometric method.
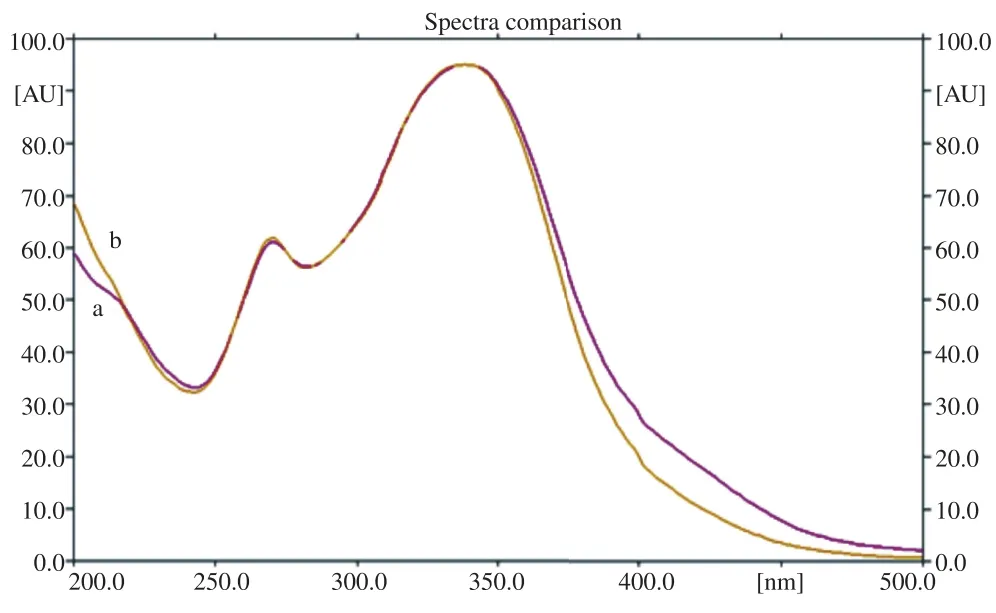
Figure 1. Overlay UV absorption spectra.a: Vitexin standard; b: Vitexin in the sample extract of the leaves of P. foetida.

Figure 2. HPTLC chromatograms.a: Standard vitexin (2.5 mg/mL); b: Standard vitexin (5 mg/mL); c: Vitexin in the ethanolic extract of leaves of P. foetida.
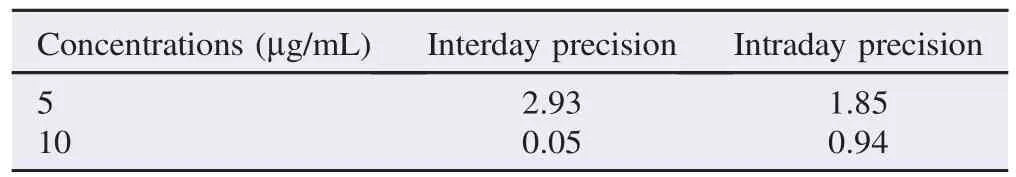
Table 3 Interday and intraday precision for quantification of vitexin determination by proposed TLC-densitometric methoda. %.
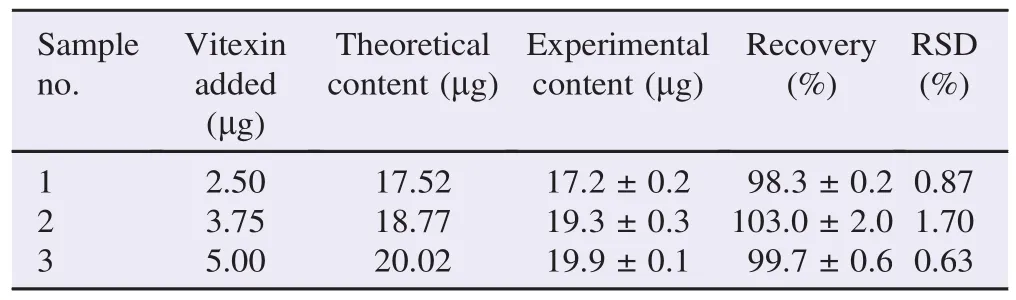
Table 4 Accuracy determined for the TLC-densitometric method.
4. Discussion
Quantitative determination of vitexin in P. foetida was performed by HPTLC with densitometric detection. Tools used such as sample applicator, densitometer and chromatogram evaluation with electronic image acquisition, make HPTLC to be an important analytical technique. Various steps of HPTLC were run by fully automated machines but method development requires primary knowledge of TLC. Generally, there are two ways of sample application on HPTLC plate: contact application and spray-on technique. Contact application of sample causes irregular distribution of the sample and spots may be broad and asymmetric after development [39]. In this study, sample application using the spray-on technique (automatic TLCsampler 4) produced narrow bands to ensure the best resolution of the samples.
The mobile phase for HPTLC was selected on the basis of the analyst's experience. In this study, vitexin was successfully separated with a mobile phase of ethyl acetate: methanol: distilled water: formic acid (50:2:3:6) which provided vitexin Rfof 0.70. Previous works on HPTLC determination of flavonoids and phenolic acid reported similar mobile phase systems of ethyl acetate: acetic acid: formic acid: distilled water (100:11:11:26) [40,41].
The linearity and sensitivity shown by this method was shown to be superior to that of reverse phase high performance liquid chromatography with detection limits, an order of magnitude lower using HPTLC. The response for vitexin in this study was shown to be linear over the range of 2.5-17.5 mg/mL compared to reverse phase high performance liquid chromatography that gave the range of 25-500 mg/mL [9].
Percentage of RSD of interday and intraday precision for quantification of vitexin determination by TLC-densitometric method in this study was found to be<3%. This suggests that the proposed method is highly precise and reproducible for vitexin[38].
The present method provides a lower LOD than current methods. It is practical for routine analysis and could be used for pharmaceutical quality control of raw materials for regulatory purposes. The percent recovery for the method proposed here is comparable with previous methods [9]. The percentage of RSD and correlation coefficient values indicate a high reproducibility of the method and the proposed method is specific for determination of vitexin from a single plant species. The pattern produced is characteristic of P. foetida and can be readily profiled.
A TLC densitometric method was validated in terms of linearity, accuracy, precision, LOD and LOQ. This method offers high degree of sensitivity, economic and rapid analysis combined with single-step sample preparation. Simultaneously, a large number of samples along with the standard can be analyzed in one TLC plate and solvent requirement is negligible, thus making it far less expensive when compared to high performance liquid chromatography. In addition, it requires very small amount of sample and can detect active principle concentration at the nanogram level. The proposed method is simple, precise, accurate and sensitive and can be used for routine quality control of P. foetida, and standardizing the vitexin.
Conflict of interest statement
We declare that we have no conflict of interest.
References
[1] Puricelli L, Dell'Aica I, Sartor L, Garbisa S, Caniato R. Preliminary evaluation of inhibition of matrix-metalloprotease MMP-2 and MMP-9 by Passiflora edulis and P. foetida aqueous extracts. Fitoterapia 2003; 74(3): 302-4.
[2] Dhawan K, Dhawan S, Sharma A. Passiflora: a review update. J Ethnopharmacol 2004; 94(1): 1-23.
[3] Arung ET, Kusuma IW, Christy EO, Shimizu K, Kondo R. Evaluation of medicinal plants from Central Kalimantan for antimelanogenesis. J Nat Med 2009; 63(4): 473-80.
[4] Chivapat S, Bunjob M, Shuaoprom A, Bansidhi J, Chavalittumrong P, Rangsripipat A, et al. Chronic toxicity of Passiflora foetida L. extract. Int J Appl Res Nat Prod 2011; 4(2): 24-31.
[5] Sasikala V, Saravanan S, Parimelazhagan T. Analgesic and antiinflammatory activities of Passiflora foetida L. Asian Pac J Trop Med 2011; 4(8): 600-3.
[6] Joseph Asir P,Priyanga S,Hemmalakshmi S,Devaki K.Invitrofree radical scavenging activity and secondary metabolites in Passiflora foetida L. Asian J Pharm Res Health Care 2014; 6(2): 3-11.
[7] Mohanasundari C, Natarajan D, Srinivasan K, Umamaheswari S, Ramachandran A. Antibacterial properties of Passiflora foetida L. - a common exotic medicinal plant. Afr J Biotechnol 2007; 6(23): 2650-3.
[8] Echeverri F, Arango V, Quiñones W, Torres F, Escobar G, Rosero Y, et al. Passifloricins, polyketides alpha-pyrones from Passiflora foetida resin. Phytochemistry 2001; 56(8): 881-5.
[9] Pongpan N, Luanratana O, Suntornsuk L. Rapid reversed-phase high performance liquid chromatography for vitexin analysis and fingerprint of Passiflora foetida. Curr Sci 2007; 93(3): 378-82.
[10] Choi HJ, Eun JS, Kim BG, Kim SY, Jeon H, Soh Y. Vitexin, an HIF-1alpha inhibitor, has anti-metastatic potential in PC12 cells. Mol Cells 2006; 22(3): 291-9.
[11] Picerno P, Mencherini T, Della Loggia R, Meloni M, Sanogo R, Aquino RP. An extract of Lannea microcarpa: composition, activity and evaluation of cutaneous irritation in cell cultures and reconstituted human epidermis. J Pharm Pharmacol 2006; 58(7): 981-8.
[12] Kim J, Lee I, Seo J, Jung M, Kim Y, Yim N, et al. Vitexin, orientin and other flavonoids from Spirodela polyrhiza inhibit adipogenesis in 3T3-L1 cells. Phytother Res 2010; 24(10): 1543-8.
[13] Sahreen S, Khan MR, Khan RA. Hepatoprotective effects of methanol extract of Carissa opaca leaves on CCl4-induced damage in rat. BMC Complement Altern Med 2011; 11: 48.
[14] Lee CY, Chien YS, Chiu TH, Huang WW, Lu CC, Chiang JH, et al. Apoptosis triggered by vitexin in U937 human leukemia cells via a mitochondrial signaling pathway. Oncol Rep 2012; 28(5): 1883-8.
[15] Yao Y, Cheng XZ, Wang LX, Wang SH, Ren G. Major phenolic compounds, antioxidant capacity and antidiabetic potential of rice bean (Vigna umbellata L.) in China. Int J Mol Sci 2012; 13(3): 2707-16.
[16] Abbasi E, Nassiri-Asl M, Sheikhi M, Shafiee M. Effects of vitexin on scopolamine-induced memory impairment in rats. Chin J Physiol 2013; 56(3): 184-9.
[17] Demir¨Ozkay U, Can OD. Anti-nociceptive effect of vitexin mediated by the opioid system in mice. Pharmacol Biochem Behav 2013; 109: 23-30.
[18] Lu CC, Xu YQ, Wu JC, Hang PZ, Wang Y, Wang C, et al. Vitexin protects against cardiac hypertrophy via inhibiting calcineurin and CaMKII signaling pathways. Naunyn Schmiedebergs Arch Pharmacol 2013; 386(8): 747-55.
[19] Simpson MJ, Hjelmqvist D, L´opez-Alarc´on C, Karamehmedovic N, Minehan TG, Yepremyan A, et al. Anti-peroxyl radical quality and antibacterial properties of rooibos infusions and their pure glycosylated polyphenolic constituents. Molecules 2013; 18(9): 11264-80.
[20] Yang SH, Liao PH, Pan YF, Chen SL, Chou SS, Chou MY. The novel p53-dependent metastatic and apoptotic pathway induced by vitexin in human oral cancer OC2 cells. Phytother Res 2013; 27(8): 1154-61.
[21] Sahreen S, Khan MR, Khan RA. Comprehensive assessment of phenolics and antiradical potential of Rumex hastatus D. Don. roots. BMC Complement Altern Med 2014; 14: 47.
[22] Yang L, Yang ZM, Zhang N, Tian Z, Liu SB, Zhao MG. Neuroprotective effects of vitexin by inhibition of NMDA receptors in primary cultures of mouse cerebral cortical neurons. Mol Cell Biochem 2014; 386(1-2): 251-8.
[23] Yang ZB, Tan B, Li TB, Lou Z, Jiang JL, Zhou YJ, et al. Protective effect of vitexin compound B-1 against hypoxia/reoxygenationinduced injury in differentiated PC12 cells via NADPH oxidase inhibition. Naunyn Schmiedebergs Arch Pharmacol 2014; 387(9): 861-71.
[24] Pereira CA, Yariwake JH, Lanças FM, Wauters JN, Tits M, Angenot L. A HPTLC densitometric determination of flavonoids from Passiflora alata, P. edulis, P. incarnata and P. caerulea and comparison with HPLC method. Phytochem Anal 2004; 15(4): 241-8.
[25] Ringl A, Prinz S, Huefner A, Kurzmann M, Kopp B. Chemosystematic value of flavonoids from Crataegus x macrocarpa (Rosaceae) with special emphasis on (R)- and (S)-eriodictyol-7-O-glucuronide and luteolin-7-O-glucuronide. Chem Biodivers 2007; 4(2): 154-62.
[26] Zhang Y, Tie X, Bao B, Wu X, Zhang Y. Metabolism of flavone C-glucosides and p-coumaric acid from antioxidant of bamboo leaves (AOB) in rats. Br J Nutr 2007; 97(3): 484-94.
[27] Wang J, de Yue Y, Jiang H, Tang F. Rapid screening for flavone C-glycosides in the leaves of different species of bamboo and simultaneous quantitation of four marker compounds by HPLCUV/DAD. Int J Anal Chem 2012; 2012: 205101.
[28] Zucolotto SM, Fagundes C, Reginatto FH, Ramos FA, Castellanos L, Duque C, et al. Analysis of C-glycosyl flavonoids from South American Passiflora species by HPLC-DAD and HPLC-MS. Phytochem Anal 2012; 23(3): 232-9.
[29] da Silva Morrone M, de Assis AM, da Rocha RF, Gasparotto J, Gazola AC, Costa GM, et al. Passiflora manicata (Juss.) aqueous leaf extract protects against reactive oxygen species and protein glycation in vitro and ex vivo models. Food Chem Toxicol 2013; 60: 45-51.
[30] Yan C, Liu H, Lin L. Simultaneous determination of vitexin and isovitexin in rat plasma after oral administration of Santalum album L. leaves extract by liquid chromatography tandem mass spectrometry. Biomed Chromatogr 2013; 27(2): 228-32.
[31] Reich E, Widmer V. Plant analysis 2008-planar chromatography. Planta Med 2009; 75(7): 711-8.
[32] Sherma J. Review of HPTLC in drug analysis: 1996-2009. J AOAC Int 2010; 93(3): 754-64.
[33] Attimarad M, Ahmed KK, Aldhubaib BE, Harsha S. High-performance thin layer chromatography: a powerful analytical technique in pharmaceutical drug discovery. Pharm Methods 2011; 2(2): 71-5.
[34] Kaale E, Risha P, Layloff T. TLC for pharmaceutical analysis in resource limited countries. J Chromatogr A 2011; 1218(19): 2732-6.
[35] Shewiyo DH, Kaale E, Risha PG, Dejaegher B, Smeyers-Verbeke J, Vander Heyden Y. HPTLC methods to assay active ingredients in pharmaceutical formulations: a review of the method development and validation steps. J Pharm Biomed Anal 2012; 66: 11-23.
[36] Kaale E, Manyanga V, Makori N, Jenkins D, Michael Hope S, Layloff T. High-performance thin layer chromatography to assess pharmaceutical product quality. Trop Med Int Health 2014; 19(6): 747-51.
[37] Kumar KA, Shetty SR, Narasu L. HPTLC method development and validation for determination of rutin in flavanoidal fraction of Hibiscus micranthus Linn. E J Chem 2011; 8(3): 1444-50.
[38] Renger B, V´egh Z, Ferenczi-Fodor K. Validation of thin layer and high performance thin layer chromatographic methods. J Chromatogr A 2011; 1218(19): 2712-21.
[39] CAMAG. Instruments, tools and concepts for HPTLC. Switzerland: CAMAG; 2015. [Online] Available from: http://www. camag.com [Accessed on 25th September, 2015]
[40] Vunda c VB, Maleˇs Z, Plazibat M, Golja P, Cetina-ˇCiˇzmek B. HPTLC determination of flavonoids and phenolic acids in some Croatian Stachys taxa. J Planar Chromatogr Mod TLC 2005; 18: 269-73.
[41] Amanzadeh Y, Khanavi M, Khatamsaz M, Rajabi A, Ebrahimi SES. High-performance thin-layer chromatographic fingerprints of flavonoids and phenol carboxylic acids for standardization of Iranian species of the genus Crataegus L. Iran J Pharm Sci 2007; 3(3): 143-52.
*Corresponding author:Nalin Wongkattiya, Program in Biotechnology, Faculty of Science, Maejo University, Chiang Mai 50290, Thailand.
 Asian Pacific Journal of Tropical Biomedicine2016年3期
Asian Pacific Journal of Tropical Biomedicine2016年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Cyclical mastalgia: Prevalence and associated determinants in Hamadan City, Iran
- Comparative repellency effect of three plant extracts on Paederus beetles (Coleoptera: Staphylinidae), the cause of linear dermatitis in Iran
- Screening and antibacterial efficacy of selected Indian medicinal plants
- Anti-herpes simplex virus activities of monogalactosyl diglyceride and digalactosyl diglyceride from Clinacanthus nutans, a traditional Thai herbal medicine
- Analgesic and anti-inflammatory potential of aerial parts of the Daphne mucronata Royle extract in mice: Opioid-independent action
- Sub-chronic effects of a Phthirusa pyrifolia aqueous extract on reproductive function and comparative hormone levels in male rats
