Phenolic and flavonoid contents, antioxidant and antimicrobial activities of leaf extracts from ten Algerian Ficus carica L. varieties
Phenolic and flavonoid contents, antioxidant and antimicrobial activities of leaf extracts from ten Algerian Ficus carica L. varieties
Souhila Mahmoudi1*, Mustapha Khali1, Abderahim Benkhaled2, Karima Benamirouche3, Imen Baiti21Department of Food Sciences, University“Saad Dahleb”of Blida, Blida, Algeria
2Department of Microbiology and Biochemistry, University of M'sila, M'sila, Algeria
3Scientific and Technical Research Center in Physicochemical Analyses, Boumail, Algeria
Floral research http://dx.doi.org/10.1016/j.apjtb.2015.12.010
Tel: +213 561357916
E-mail: mahmoudisouhila@yahoo.fr
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 9 Nov 2015
Received in revised form 1 Dec 2015 Accepted 10 Dec 2015
Available online 31 Dec 2015
Keywords:
Fig leaves
Phenolics
Flavonoids
Antioxidant activity
Antimicrobial activity
ABSTRACT
Objective: To determine the total phenolic and flavonoid contents, antioxidant and antimicrobial activities of methanolic leaf extracts of ten Algerian fig (Ficus carica L.) varieties (uniferous, biferous and caprifig tree).
Methods: Phenolics were extracted by Soxhlet method and analyzed by the Folin-Ciocalteu colorimetric method. Flavonoids were determined by aluminum trichloride assay and the antioxidant capacity was determined by the 2,2-diphenyl-1-picrylhydrazyl radical scavenging assay. The antimicrobial activity was studied with the disc diffusion method and a macrodilution broth method was used to determine the minimal inhibitory concentrations and minimal lethal concentrations.
Results: The mean extract yield was 14.10%±0.66% (n = 10). Leaf extract of biferous followed by uniferous varieties had the highest total phenolic contents [(52.296±5.232) and (48.973±2.015) mg gallic acid equivalent/g of dry plant extract respectively],flavonoids [(14.388±0.333) and (14.136±1.082) mg quercetin equivalent/g of dry plant extract] and antioxidant capacity [IC50(798.754±108.590) and (825.004±110.835) mg/ mL]. Antioxidant capacity of fig leaves was significantly correlated with phenolic contents (r = 0.748). These extracts showed bactericidal activity and moderate antifungal activity, and the minimal inhibitory concentrations and minimal lethal concentrations were determined on Bacillus cereus and Staphylococcus aureus.
Conclusions: All tested extracts contain phenolic compounds and exhibited an antioxidant activity and an antimicrobial effect against Gram-positive and Gram-negative bacteria. Further researches on identification and purification of phenolic compounds are required.
1. Introduction
Phenolic compounds are common plant secondary metabolites which have not only physiological functions in plants but also positive effects for human health because they can act as antioxidants[1]. Antioxidants play important roles in preventing pathogenic processes related to cancer, cardiovascular disease, macular degeneration, cataracts and asthma, and can enhance immune function. Antioxidant defenses protect the body from the detrimental effects of free radicals generated as byproducts of normal metabolism [2].
In addition to antioxidative roles, phenolic compounds from different plants had been reported to have antimicrobial activity against different pathogenic microorganisms [3-5]. There is an increasing interest in medicinal plants as an alternative to synthetic drugs, particularly against microbial agents because of the growth of antibiotic resistance [6]. The search for new antimicrobial agents like phenolic compounds has therefore become indispensable.
Thousands of plants are well known in traditional medicine system for their medicinal and therapeutic potentials worldwidealike fig [Ficus carica (F. carica)] which is a deciduous tree belonging to the Moraceae family. It is one of the earliest cultivated fruit trees and an important crop worldwide for both dry and fresh consumption [1,7,8]. Its fruit, root and leaves are used in the native system of medicine in different disorders such as gastrointestinal (colic, ulcers, indigestion, loss of appetite and diarrhea), respiratory (sore throats, coughs and bronchial problems), inflammatory, furuncles, cancer and cardiovascular disorders [9,10].
Infusions or decoctions of fig tree leaves have been traditionally employed in the treatment of tumors and diseases associated with inflammation, in the prevention of nutritional anemia and as anthelmintic[10,11]. Some biological activities of different parts from F. carica, namely, antioxidant, antimicrobial, acetyl cholinesterase inhibition, anticarcinogenic, anti-inflammatory, inhibition of low density lipoprotein oxidation in humans and antidiabetic have been reported [12-25].
Some phenolic compounds, with reported pharmacological properties have already been isolated from fig leaves, namely, furanocoumarins like psoralen and bergapten,flavonoids like quercetin 3-O-rutinoside and phenolic acids like ferulic acid, 3-O-caffeoylquinic acid and 5-O-caffeoylquinic acid[11].
The aim of the present study was to determine the total phenolic and flavonoid contents of leaf extracts obtained from ten Algerian F. carica varieties and to evaluate their biological activity, especially as antioxidant and antimicrobial agent. To our knowledge, this is the first report comparing phenolic composition and bioactivity of the Algerian fig leaves varieties.
2. Materials and methods
2.1. Standards and reagents
Folin-Ciocalteu, gallic acid, quercetin, butylhydroxytoluene (BHT), 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich (USA). Methanol, acetic and hydrochloric acids, isoamylic alcohol, ammonium, benzene, sodium carbonate, ferric trichloride, aluminum trichloride, dimethyl sulfoxide (DMSO) were obtained from Merck (Germany), Rectapur, Cheminova (France) and Fluka. Mueller-Hinton agar and broth and Sabouraud dextrose agar were obtained from Pasteur Institute (Algeria).
2.2. Plant material
Ten Algerian varieties of F. carica (uniferous:“Bidha”, “Hamra”,“Onk Elhamam”,“Zarrouk”,“Chatwi”,“Boughandjo”and“Safra”; biferous:“Bakkor”and“Bither”and caprifig tree:“Dhokkar”) leaves were collected in Lakhdaria, Province of Bouira (northeast of Algeria). The leaves were airdried at room temperature for 20 days and were powdered and stored for later analysis.
2.3. Extracts preparation
Thirty gram of powdered leaves samples were extracted with 300 mL pure methanol for 8 h using the Soxhlet apparatus. Afterwards, the resulting extracts were filtered and solvent was evaporated under reduced pressure at 35°C using rotary vacuum evaporator (B¨UCHI). At last, the residues were kept in small sterile bottles under refrigerated conditions until used. The yield (%) of evaporated dried extracts was calculated as 100 DWext/DWsamp, where DWextwas dry weight of extract after evaporation of solvent and DWsampwas the dry weight of sample.
2.4. Microbial strains
F. carica leaf extracts were tested against two strains of fungi: Aspergillus brasiliensis (ATCC 16404) (A. brasiliensis) and Candida albicans (ATCC 10231) (C. albicans). Of the nine tested bacteria,five were Gram-positive [Bacillus cereus (ATCC 10876) (B. cereus), Bacillus subtilis (ATCC 9372) (B. subtilis), Staphylococcus aureus (ATCC 6538) (S. aureus), Enterococcus faecalis (ATCC 29200) (E. faecalis) and Micrococcus luteus (ATCC 4698)] and four were Gram-negative [Klebsiella pneumoniae (ATCC 4352), Pseudomonas aeruginosa (ATCC 27853), Escherichia coli (ATCC 25922) (E. coli) and Salmonella sp.]. These microorganisms were obtained from culture collection of Pasteur Institute (Algiers), Laboratory of Microbiology of SAIDAL (Bridge of Constantine, Algiers) and Algerian Drugs Laboratory (Tipaza, Algeria).
2.5. Phytochemical analysis
Phytochemical tests of the aqueous leaf extracts of fig (maceration of 5 g of leaf powder in 50 mL of distilled water for 30 min) were carried out qualitatively for the presence of anthraquinones, coumarins, alkaloids,flavonoids, saponins, anthocyanin and tannins according to the standard methods[26].
2.6. Total phenolic contents
Total phenolic contents of each sample were measured by the Folin-Ciocalteu's method [27]. Total phenolic content was expressed as milligrams gallic acid equivalents per gram of dry plant extract (mg GAE/g DE) through the calibration curve of gallic acid that its linearity range was from 10 to 100 mg/mL (R2>0.99).
2.7. Total flavonoid contents
Total flavonoid content was determined using aluminum trichloride assay [28]. Total flavonoid content was expressed as milligrams quercetin equivalents per gram of dry plant extract (mg QE/g DE) through the calibration curve of quercetin that its linearity range was from 0.5 to 8 mg/mL (R2>0.99).
2.8. Antioxidant activity
Briefly, all extracts were dissolved in pure methanol at eight different concentrations (50-2800 mg/mL). A total of 0.3 mL of extract was mixed with 2.7 mL of methanol solution containing DPPH radical (6×10−5mol/L). The mixture was shaken for 20 s and the absorbance was measured at 517 nm (Schimadzu-UV-2401 PC) after 60 min incubation at room temperature and dark area. Pure methanol was used as blank solution and DPPH solution was used as a control. The inhibition percentage of the absorbance was calculated using the equation:
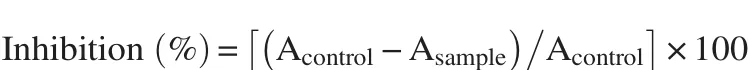
where, Acontrolwas the absorbance of the solution without extract and Asamplewas the absorbance of solution with extract in different concentrations [29]. The sample concentration providing IC50was calculated by plotting inhibition percentages against concentrations of the sample. BHT and gallic acid were used as standards.
2.9. Antimicrobial activity
2.9.1. Disc diffusion assay
F. carica leaf extracts were dissolved in DMSO and were sterilized by filtration on 0.45 mm Millipore filters. Disc diffusion method was employed for the determination of antimicrobial activity of the extracts. A total of 100 mL of suspensions containing 107CFU/mL of bacteria, in exponential growth phase, and 106CFU/mL of yeast were spread on Mueller-Hinton agar medium and Sabouraud dextrose agar respectively [30]. Filter paper disks (9 mm of diameter) were impregnated with 50 mL of each extract (7.5 mg/disc) and placed on the inoculated Petri dishes. Negative control was performed using DMSO solvent employed to dissolve the different extracts. Ciprofloxacin (100 mg/disc), oxacillin (500 mg/disc) and lamidaz (100 mg/disc) were individually used as positive controls for bacteria and fungi. Petri dishes were then incubated during 24 h at 37°C for bacterial strains and 48 h at 30°C for fungi. Antimicrobial activity was evaluated by measuring the inhibition zone (mm) against the studied microorganisms, including disc diameter.
2.9.2. Macrodilution assay
A macrodilution broth method was used to determine the minimal inhibitory concentrations (MIC) and minimal lethal concentrations (MLC) for S. aureus and B. cereus which were determined as highly sensitive to F. carica leaf extracts (inhibition diameter: 15 mm) in disc diffusion assay. Serial doubling dilution of each extract was prepared in DMSO with final concentrations ranging from 1.09 to 35.00 mg/mL. A total of 950 mL of Mueller-Hinton broth was mixed with 50 mL of bacterial suspension (107CFU/mL) and 1 000 mL of each extract dilution. Mixture was incubated for 24 h at 37°C [30].
To evaluate MLC, aliquots (10 mL) of broth were taken from each negative tube, after MIC determination and cultured in Mueller-Hinton agar plates. Plates were then incubated for 24 h at 37°C.
2.9.3. Statistical analysis
All measurements were performed in triplicate and the results were represented as mean±SEM. Statistical analyses were realized with the GraphPad Prism 6 statistics program. Data statistical analyses were achieved by using One-way ANOVA and Tukey-test. The level of significance was set at P<0.05.
3. Results
3.1. Phytochemical analysis
The results of our preliminary phytochemical analysis revealed that the aqueous extract of dried powdered leaves tested contained flavonoids, alkaloids, coumarins and saponins.
3.2. Yieldofextract,totalphenolicandflavonoidcontents
Yield of extract shown in Table 1 ranged between 12.52% for “Bakkor”variety and 19.80%for“Safra”variety. The methanolic extracts of“Bither”,“Bidha”and“Chatwi”fig leaves presented the highest quantities of phenolic compounds [(58.704±0.455), (53.519±0.417) and (52.370±0.353) mg GAE/g DE respectively] (Table 1). Indeed, the total phenolic content was significantly different among the ten varieties (P<0.05) and the biferous followed by uniferous varieties had the highest total phenolic contents [means: (52.296±5.232) and (48.973±2.015) mg GAE/g DErespectively].Whereascaprifigtreehadthelowest total phenolics [(46.074±0.134) mg GAE/g DE at mean].
In our study, the highest amounts of flavonoids were noted in “Chatwi”and“Safra”varieties with (16.211±0.156) and (16.093±0.166) mg QE/g DE correspondingly (Table 1). The lowest and similar values were recorded in“Dhokkar”and “Zarrouk”varieties. It seemed that flavonoid content was significantly different among the ten leaf extracts studied (P<0.05) and biferous followed by uniferous varieties had the highest flavonoid amount [means: (14.388±0.333) and (14.136±1.082) mg QE/g DE].

Table 1 Yield, total phenolic contents and total flavonoids of fig leaf extracts.
3.3. Antioxidant capacities
Leaf extracts of the ten Algerian fig varieties were investigated and control samples of gallic acid and BHT exhibited DPPH scavenging capacity, in a concentration-dependent way (Figures 1 and 2).
The results of antioxidant capacity were shown in Table 2. The lowest IC50values indicated the highest free radical scavenging activity of the extract. In general, the amount of antioxidant capacity (IC50) of fig leaf extracts ranged between 659.97 and 1119.59 mg/mL with an average of 849.21 mg/mL “Chatwi”,“Onk Elhamam”,“Bither”,“Bidha”and“Zarrouk”were the varieties with stronger ability to scavenge free radical DPPH, which was related with the highest phenolic contents comparing to the other varieties. Antioxidant capacity of fig leaves was significantly correlated with phenolic contents (r = 0.748) but not with flavonoid values (r = 0.007).
In comparison, it seemed that the radical scavenging activities of the positive controls, gallic acid and BHT [IC50= (15.48±0.13) and (82.77±0.43) mg/mL, respectively] were higher than that of the F. carica leaf extracts.
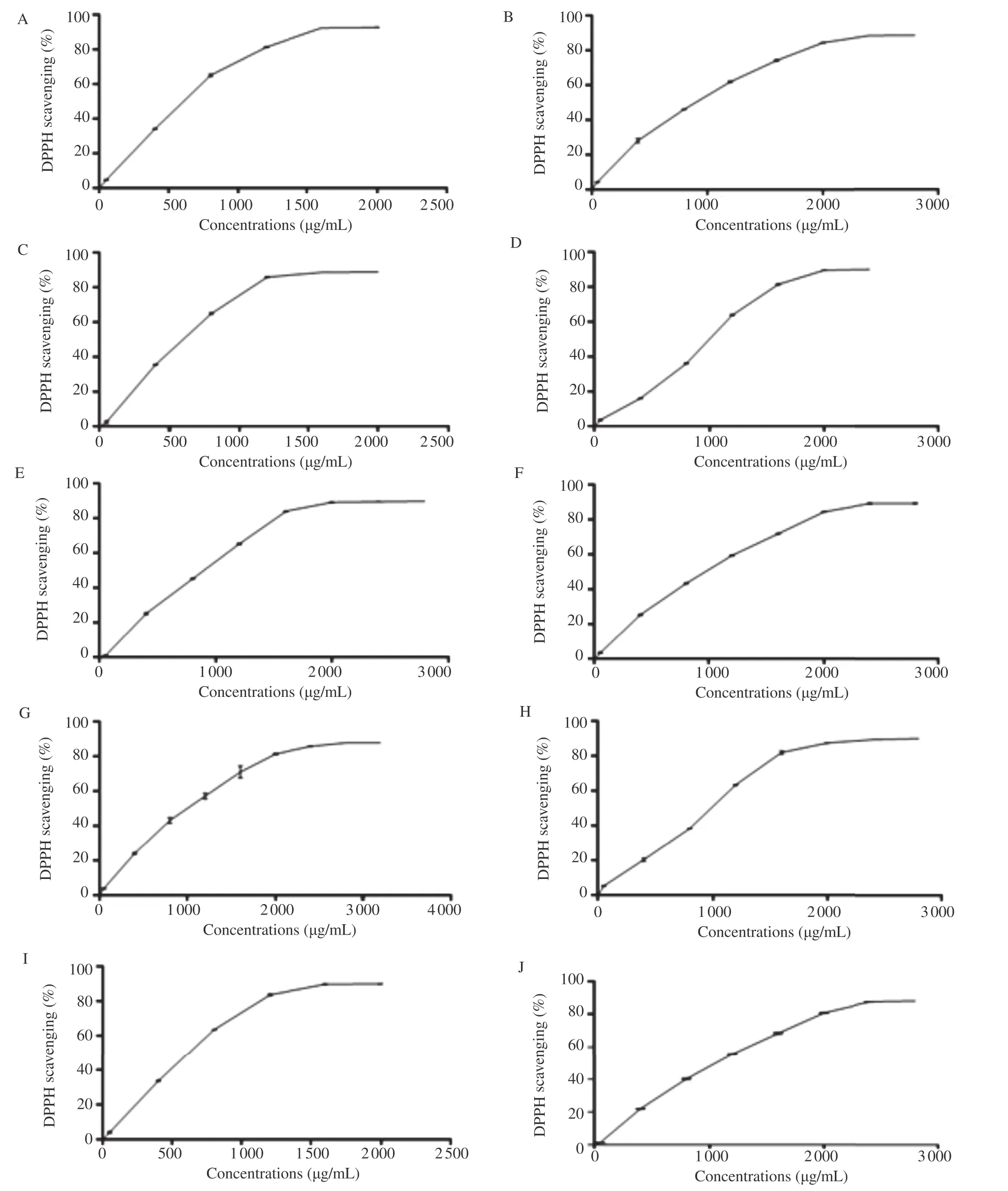
Figure 1. The DPPH free radical scavenging activity (%) of F. carica leaf extracts at different concentrations.A: Onk Elhamam; B: Safra; C: Chatwi; D: Zarrouk; E: Dhokkar; F: Boughandjo; G: Hamra; H: Bither; I: Bidha; J: Bakkor; Each value was represented as mean±SEM of three measurements.
3.4. Antimicrobial activity
3.4.1. Disc diffusion assay
Most extracts showed bactericidal activity against different species of Gram-positive and Gram-negative bacteria and a moderate antifungal activity (Figure 3). S. aureus and B. cereus bacteria were more sensitive to F. carica extracts.
No inhibition was observed with the solvent control (DMSO) which was used as solvent to solubilize the dry extracts. Bacterial and fungal growth was inhibited by the antibiotics andused as control. Ciprofloxacin inhibition zones varied from (30.67±0.67) mm for E. faecalis to (48.00±0.58) mm for Salmonella sp., oxacillin inhibition zones ranged between (17.67±0.67) mm for B. cereus and (58.67±0.33) mm for B. subtilis and lamidaz inhibition zones were (20.67±0.67) mm for C. albicans and (32.33±1.45) mm for A. brasiliensis.
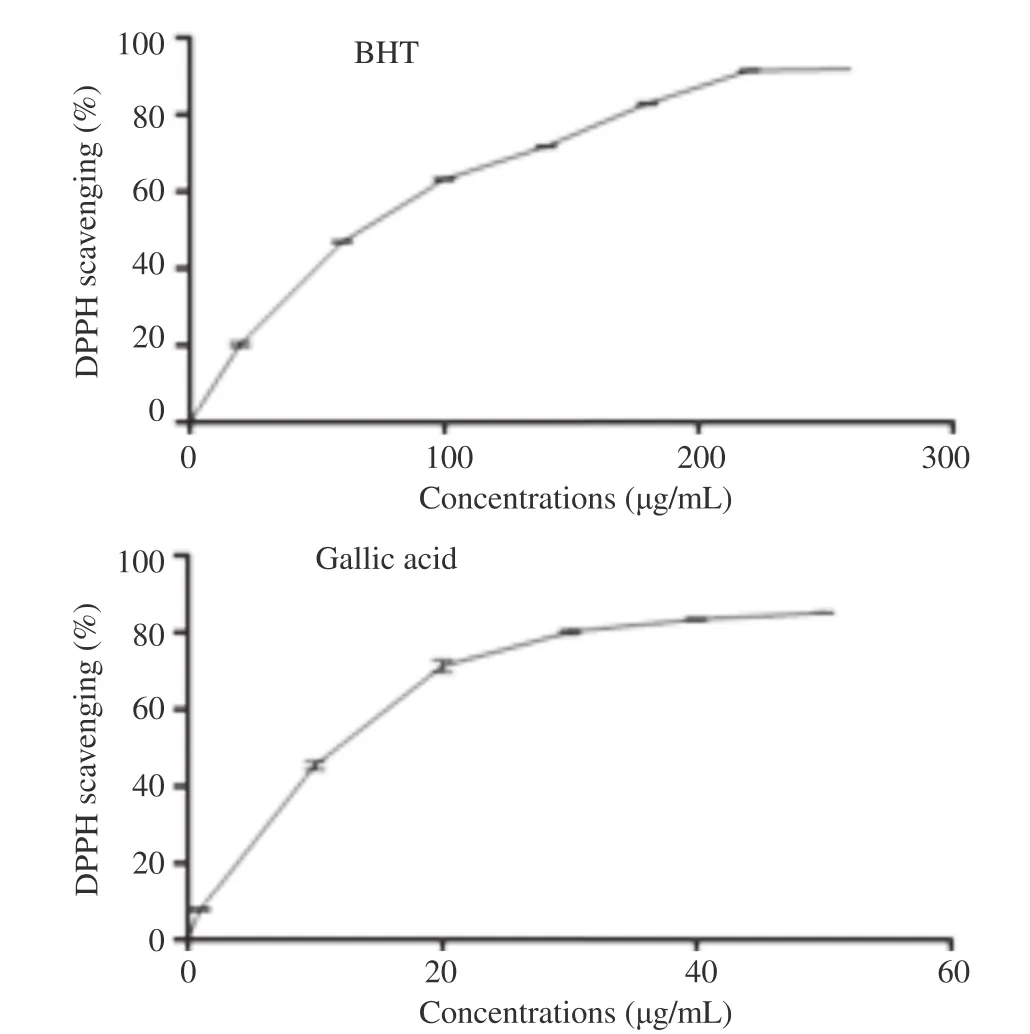
Figure 2. The DPPH free radical scavenging activity of gallic acid and BHT at different concentrations (mg/mL).Each value was represented as mean±SEM of three measurements.
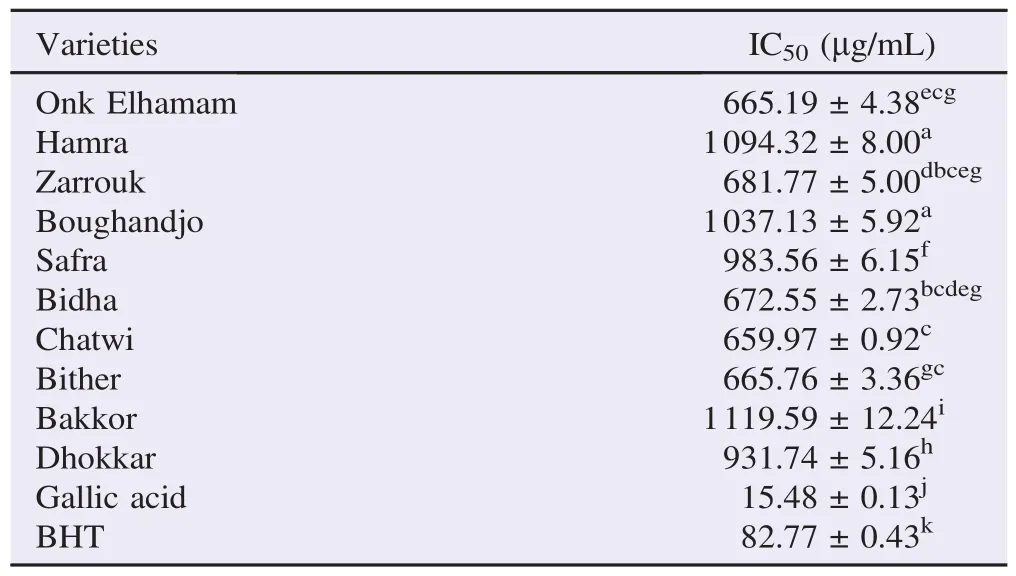
Table 2 Free radical scavenging capacities of fig leaf extracts, gallic acid and BHT.
3.4.2. Macrodilution assay
Evaluation of MIC and MLC of the ten F. carica leaf extracts showed a variability of inhibition among the bacterial strains tested (Table 3). B. cereus showed more sensibility to these extracts when compared with S. aureus. The leaf extracts of “Dhokkar”variety were proved to be more active with MIC and MLC values ranging from 2.19 to 8.75 mg/mL and 4.38-17.50 mg/mL, respectively (Table 3).
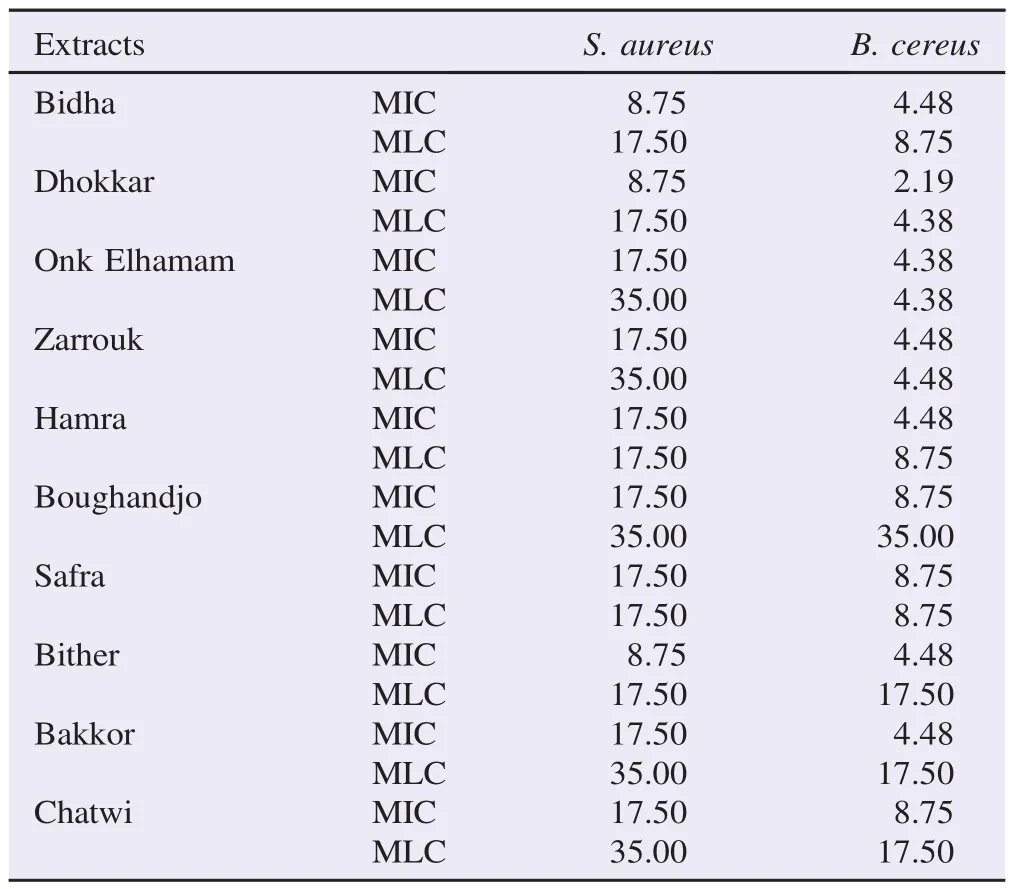
Table 3 Antibacterial activity (MIC and MLC) of F. carica leaf extracts for S. aureus and B. cereus. mg/mL.
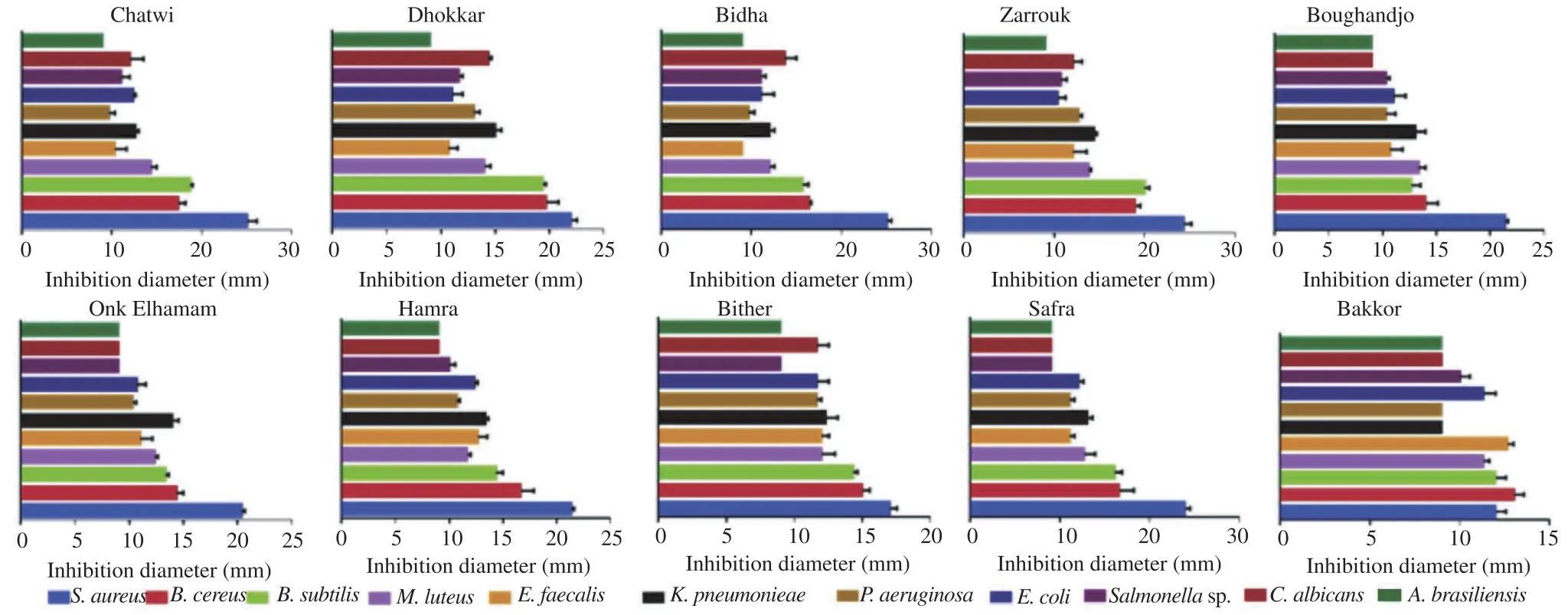
Figure 3. Inhibition zones of growth of Gram-positive and Gram-negative bacteria and fungi, including disc diameter.Data were represented as mean±SEM of three measurements. Significant differences (P<0.05) were observed between tested microorganisms among the same variety. M. luteus: Micrococcus luteus; K. pneumoniae: Klebsiella pneumoniae; P. aeruginosa: Pseudomonas aeruginosa.
4. Discussion
F. carica leaves may constitute an excellent source of bioactive compounds, specifically, phenolic compounds. Phenolic contents in our study were highest than the sum of the determined phenolic compounds registered by Oliveira et al.[18] on“Branca Tradicional”and“Pingo de Mel”fig leaves and by Konyalio glu et al.[31]. On the other hand, stem was the rich fig part on phenolic compounds [(133.00±3.50) mg GAE/g DM] [32]. In fact, the total phenolic content is significantly different among the three vegetal materials, following the order: leaves>peels>pulps [18,33]. This fact is not surprising since these compounds, especially flavonoids, act as UV filters, protecting some cell structures, like chloroplasts, from harmful effects of UV radiation [34]. In the review by Saoudi and El Feki,fig stem was shown to have a high amount of flavonoids [(43.25±2.00) mg QE/g DE][32].
The qualitative composition of fig leaves extracts revealed three hydroxycinnamic acids (3- and 5-O-caffeoylquinic acids and ferulic acid), one flavonoid glycoside (quercetin 3-O-rutinoside) and two furanocoumarins (psoralen and bergapten) [11,18]. In addition, Teixeira et al. identified chlorogenic acid in fig leaves [35].
Antioxidants have recently become a topic of increasing interest to health and food science researchers and medical experts [36]. The antioxidant potential of F. carica pulps, peels and leaves was checked [13,18]. All materials exhibited activity against DPPH and nitric oxide radicals. However, only the leaves presented capacity to scavenge superoxide radical. Leaves were always the most effective part, which seems to be related with phenolic compounds [18]. Similar to our results, a strong correlation between the phenolic content and the antioxidant capacity of figs has been previously reported by different authors [1,18,31,37].
The effect of phenolic compounds on preventing radical scavenging was studied and it is generally assumed the ability of these compounds to act as hydrogen donors[12,38]. Antioxidant capacities of our studied varieties were lower than those of Oliveira et al. on“Branca Tradicional”and“Pingo de Mel”fig varieties [18]. Flavonoids, carotenoids and triterpenes have antioxidant activity by scavenging reactive oxygen species which prevent potential damage to cellular components such as DNA, proteins and lipids [39].
Fig extracts and latex showed antimicrobial activity against a wide range of bacteria including antibiotic-resistant species and fungal species[17,30]. Our results showed that the Gram-positive bacteria were more sensitive to inhibition by fig leaf extracts [(15.4±0.6) mm at mean, n = 50] than Gram-negative bacteria [(11.3±0.2) mm at mean, n = 40]. This phenomenon was previously reported [40,41]. It is not known exactly why Gramnegative bacteria should be less susceptible, but it may be related to the outer membrane which contains peptidoglycan and lipopolysaccharide, endows the bacterial surface with strong hydrophilicity and acts as strong permeability barrier [42]. Hydro-alcoholic F. carica leaf extract and its derived fractions display moderate antimicrobial potential against S. aureus, E. coli and Pseudomonas, in the range of 0%-13%[13].
Our results of antibacterial activity of fig leaf extracts against S. aureus were lower than those obtained by Lee and Cha (MIC: 2.5-20 mg/mL and MLC: 5-20 mg/mL), with the same part of plant against clinical isolates of methicillin-resistant S. aureus [17]. Whereas, Olufemi and Olusegun registered a higher MIC (25 mg/mL) with F. carica leaf aqueous extracts and a lower MIC (6.25 mg/mL) with ethanolic extracts against S. aureus[41].
At last of this work,fig leaves of different tested varieties appeared as a good source of health-promoting polyphenols and flavonoids and had beneficial effects like antioxidant and antimicrobial activities against Gram-positive and Gram-negative bacteria. To increase the antioxidant and the antimicrobial effects of leaf extracts from fig tree, it seems important to identify and purify their phenolic compounds in further studies.
Conflict of interest statement
We declare that we have no conflict of interest.
References
[1]Çalis¸kan O, Polat AA. Phytochemical and antioxidant properties of selected fig (Ficus carica L.) accessions from the eastern Mediterranean region of Turkey. Sci Hortic 2011; 128: 473-8.
[2] Nakilcio glu E, Hıs¸ıl Y. Research on the phenolic compounds in sarilop (Ficus carica L.)fig variety. GIDA 2013; 38(5): 267-74.
[3] Megdiche-Ksouri W, Trabelsi N, Mkadmini K, Bourgou S, Noumi A, Snoussi M, et al. Artemisia campestris phenolic compounds have antioxidant and antimicrobial activity. Ind Crops Prod 2015; 63: 104-13.
[4] Stefanovi c OD, Teˇsi c JD,ˇComi c LR. Melilotus albus and Dorycnium herbaceum extracts as source of phenolic compounds and their antimicrobial, antibiofilm, and antioxidant potentials. J Food Drug Anal 2015; 23: 417-24.
[5] T¨urkyılmaz M, Ta gı S¸, Dereli U,¨Ozkan M. Effects of various pressing programs and yields on the antioxidant activity, antimicrobial activity, phenolic content and colour of pomegranate juices. Food Chem 2013; 138: 1810-8.
[6] Tavares AC, Gonçalves MJ, Cavaleiro C, Cruz MT, Lopes MC, Canhoto J, et al. Essential oil of Daucus carota subsp. halophilus: composition, antifungal activity and cytotoxicity. J Ethnopharmacol 2008; 119: 129-34.
[7] Dueñas M, P´erez-Alonso JJ, Santos-Buelga C, Escribano-Bail´on T. Anthocyanin composition in fig (Ficus carica L.). J Food Compost Anal 2008; 21: 107-15.
[8] Barolo MI, Ruiz Mostacero N, L´opez SN. Ficus carica L. (Moraceae): an ancient source of food and health. Food Chem 2014; 164: 119-27.
[9] Patil VV, Patil VR. Evaluation of anti-inflammatory activity of Ficus carica Linn. Indian J Nat Prod Resour 2011; 2(2): 151-5.
[10] Lansky EP, Paavilainen HM, Pawlus AD, Newman RA. Ficus spp. (fig): ethnobotany and potential as anticancer and antiinflammatory agents. J Ethnopharmacol 2008; 119: 195-213.
[11] Oliveira AP, Baptista P, Andrade PB, Martins F, Pereira JA, Silva BM, et al. Characterization of Ficus carica L. cultivars by DNA and secondary metabolite analysis: is genetic diversity reflected in the chemical composition? Food Res Int 2012; 49: 710-9.
[12] Soltana H, Tekaya M, Amri Z, El-Gharbi S, Nakbi A, Harzallah A, et al. Characterization of fig achenes' oil of Ficus carica grown in Tunisia. Food Chem 2016; 196: 1125-30.
[13] Weli AM, Al-Blushi AAM, Hossain MA. Evaluation of antioxidant and antimicrobial potential of different leaves crude extracts of Omani Ficus carica against food borne pathogenic bacteria. Asian Pac J Trop Dis 2015; 5(1): 13-6.
[14] Viuda-Martos M, Barber X, P´erez-´Alvarez JA, Fern´andez-L´opez J. Assessment of chemical, physico-chemical, techno-functional and antioxidant properties of fig (Ficus carica L.) powder co-products. Ind Crops Prod 2015; 69: 472-9.
[15] Lazreg-Aref H, Mars M, Fekih A, Aouni M, Said K. Chemical composition and antibacterial activity of a hexane extract of Tunisian caprifig latex from the unripe fruit of Ficus carica. Pharm Biol 2012; 50: 407-12.
[16] Oliveira AP, Silva LR, Ferreres F, Guedes de Pinho P, Valentão P, Silva BM, et al. Chemical assessment and in vitro antioxidantcapacity of Ficus carica latex. J Agric Food Chem 2010; 58: 3393-8.
[17] Lee YS, Cha JD. Synergistic antibacterial activity of fig (Ficus carica) leaves extract against clinical isolates of methicillinresistant Staphylococcus aureus. Korean J Microbiol Biotechnol 2010; 38(4): 405-13.
[18] Oliveira AP, Valentão P, Pereira JA, Silva BM, Tavares F, Andrade PB. Ficus carica L.: metabolic and biological screening. Food Chem Toxicol 2009; 47: 2841-6.
[19] Jasmine R, Manikandan K, Karthikeyan K. Evaluating the antioxidant and anticancer property of Ficus carica fruits. Afr J Biotechnol 2015; 14(7): 634-41.
[20] Hashemi SA, Abediankenari S, Ghasemi M, Azadbakht M, Yousefzadeh Y, Dehpour AA. The effect of fig tree latex (Ficus carica) on stomach cancer line. Iran Red Crescent Med J 2011; 13(4): 272-5.
[21] Khodarahmi GA, Ghasemi N, Hassanzadeh F, Safaie M. Cytotoxic effects of different extracts and latex of Ficus carica L. on Hela cell line. Iran J Pharm Res 2011; 10(2): 273-7.
[22] Park S, Han J, Im K, Whang WK, Min H. Antioxidative and antiinflammatory activities of an ethanol extract from fig (Ficus carica) branches. Food Sci Biotechnol 2013; 22(4): 1071-5.
[23] Ali B, Mujeeb M, Aeri V, Mir SR, Faiyazuddin M, Shakeel F. Anti-inflammatory and antioxidant activity of Ficus carica Linn. leaves. Nat Prod Res 2012; 26(5): 460-5.
[24] Mawa S, Husain K, Jantan I. Ficus carica L. (Moraceae): phytochemistry, traditional uses and biological activities. Evid Based Complement Altern Med 2013; http://dx.doi.org/10.1155/2013/ 974256.
[25] Ahmad MZ, Ali M, Mir SR. Anti-diabetic activity of Ficus carica L. stem barks and isolation of two new flavonol esters from the plant by using spectroscopical techniques. Asian J Biomed Pharm Sci 2013; 3(18): 22-8.
[26] Makanjuola OY, Dada OE, Akharaiyi FC. Antibacterial potentials of Parquetina nigrescens extracts on some selected pathogenic bacteria. J Nat Prod 2010; 3: 124-9.
[27] Fu L, Xu BT, Xu XR, Gan RY, Zhang Y, Xia EQ, et al. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem 2011; 129: 345-50.
[28] Koolen HHF, da Silva FMA, Gozzo FC, de Souza AQL, de Souza ADL. Antioxidant, antimicrobial activities and characterization of phenolic compounds from buriti (Mauritia flexuosa L. f.) by UPLC-SI-MS/MS. Food Res Int 2013; 51: 467-73.
[29] Koh PH, Mokhtar RA, Iqbal M. Antioxidant potential of Cymbopogon citratus extract: alleviation of carbon tetrachloride-induced hepatic oxidative stress and toxicity. Hum Exp Toxicol 2012; 31(1): 81-91.
[30] Lazreg-Aref H, Salah KBH, Fekih A, Chemli R, Mars M, Aouni M, et al. Variability in antimicrobial activity of latex from two varieties of Ficus carica. Afr J Microbiol Res 2011; 5(12): 1361-7.
[31] Konyalio glu S, Sa glam H, Kivçak B. a-Tocopherol,flavonoid, and phenol contents and antioxidant activity of Ficus carica leaves. Pharm Biol 2005; 43(8): 683-6.
[32] Saoudi M, El Feki A. Protective role of Ficus carica stem extract against hepatic oxidative damage induced by methanol in male Wistar rats. Evid Based Complement Altern Med 2012; http:// dx.doi.org/10.1155/2012/150458.
[33] Vallejo F, Marín JG, Tom´as-Barber´an FA. Phenolic compound content of fresh and dried figs (Ficus carica L.). Food Chem 2012; 130(3): 485-92.
[34] Treutter D. Significance of flavonoids in plant resistance: a review. Environ Chem Lett 2006; 4: 147-57.
[35] Teixeira DM, Patão RF, Coelho AV, da Costa CT. Comparison between sample disruption methods and solid-liquid extraction (SLE) to extract phenolic compounds from Ficus carica leaves. J Chromatogr A 2006; 1103: 22-8.
[36] Ben Mansour A, Porter EA, Kite GC, Simmonds MS, Abdelhedi R, Bouaziz M. Phenolic profile characterization of Chemlali olive stones by liquid chromatography-ion trap mass spectrometry. J Agric Food Chem 2015; 63(7): 1990-5.
[37] Veberic R, Colaric M, Stampar F. Phenolic acids and flavonoids of fig fruit (Ficus carica L.) in the northern Mediterranean region. Food Chem 2008; 106: 153-7.
[38] Obied HK, Bedgood DR Jr, Prenzler PD, Robards K. Chemical screening of olive biophenol extracts by hyphenated liquid chromatography. Anal Chim Acta 2007; 603: 176-89.
[39] Ksouri WM, Medini F, Mkadmini K, Legault J, Magn´e C, Abdelly C, et al. LC-ESI-TOF-MS identification of bioactive secondary metabolites involved in the antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Zygophyllum album Desf. Food Chem 2013; 139: 1073-80.
[40] Smith-Palmer A, Stewart J, Fyfe L. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett Appl Microbiol 1998; 26: 118-22.
[41] Olufemi BE, Olusegun OV. Antibacterial properties of ethanolic extract of Ficus carica on microorganisms isolated from pepper Capsicum frutescens. WebPub J Sci Res 2013; 1(1): 7-15.
[42] Mann A, Abalaka ME, Garba SA. The antimicrobial activity of the leaf extracts of Calotropis procera. Biomed Lett 1997; 55: 205-10.
*Corresponding author:Souhila Mahmoudi, Department of Food Sciences, University“Saad Dahleb”of Blida, Blida, Algeria.
 Asian Pacific Journal of Tropical Biomedicine2016年3期
Asian Pacific Journal of Tropical Biomedicine2016年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Cyclical mastalgia: Prevalence and associated determinants in Hamadan City, Iran
- Quantitative determination of vitexin in Passiflora foetida Linn. leaves using HPTLC
- Screening and antibacterial efficacy of selected Indian medicinal plants
- Anti-herpes simplex virus activities of monogalactosyl diglyceride and digalactosyl diglyceride from Clinacanthus nutans, a traditional Thai herbal medicine
- Analgesic and anti-inflammatory potential of aerial parts of the Daphne mucronata Royle extract in mice: Opioid-independent action
- Sub-chronic effects of a Phthirusa pyrifolia aqueous extract on reproductive function and comparative hormone levels in male rats
