Sf9细胞存在Dm0-like核纤层蛋白的证据
Sf9细胞存在Dm0-like核纤层蛋白的证据
卫文强†, 季少平†*, 张银燕
(河南大学医学院,河南 开封475004)
摘要为了确定Sf9细胞是否存在核纤层(lamina)及其性状,该文首先用已知的昆虫的核纤层蛋白(Lamin)的基因序列在Spodobase数据库搜索Sf9细胞的同源序列,并将推导的氨基酸序列与其他物种的同源蛋白进行比对。再利用抗果蝇Lamin Dm0抗体ADL67通过免疫印迹法(Western blotting)对Sf9细胞的蛋白裂解物进行检测,并通过免疫荧光技术(immunofluore-scence)对Sf9细胞进行染色。在Spodobase数据库搜索到1条Sf9细胞的Dm0-like lamin EST序列,同源比对显示它与其他物种的Lamin存在一定的同源性,尤其与家蚕、果蝇的同源性相对较高。免疫印迹结果表明Sf9细胞裂解物中存在大小约为70 ku的蛋白,免疫荧光检测表明Sf9细胞核周呈现阳性反应,这些特征与已知的其他物种的核纤层的性状相似。结果表明,Sf9细胞可能存在Dm0-like核纤层蛋白,可作为探讨杆状病毒核衣壳穿过核纤层的机制之依据.
关键词Sf9细胞; 核纤层; 免疫荧光; 免疫印迹
中图分类号Q 96文献标志码A

Evidence for the existence of Dm0-like Lamin in Sf9 cells. Journal of ZhejiangUniversity(Agric. & LifeSci.), 2015,41(3):245-251
Wei Wenqiang†, Ji Shaoping†*, Zhang Yinyan (MedicalCollege,HenanUniversity,Kaifeng475004,Henan,China)
SummaryThe nuclear membrane of mammalian cells was composed of inner nuclear membrane, outer nuclear membrane and perinuclear space. The lamina was localized under the nucleoplasm face of inner nuclear membrane. It has been known that the lamina was distributed in the nucleus of mammalian cells, insect cells and plant cells. Lamina plays important roles in the celluar life cycle,including DNA replication, transcription, chromatin organization as well as nuclear assembly. Moreover, lamina is an obstacle for the egress of some viruses, like herpes simplex virus (HSV). Sf9 cells were mostly used for the infection ofAutographacalifornicamultiple nucleopolyhedrovirus (AcMNPV). However, there was no comprehensive study to observe whether Sf9 cells also have the lamina. So, it is difficult to know how AcMNPV pass through the lamina of Sf9 cells and then arrive at the inner nuclear membrane for nuclear egress of capsids.
To determine the nucleotide sequence oflamingene in Sf9 cells, we searched the Spodobase database with the known insectlamingenes. The identity of the nucleotide and amino acid sequences of the homologouslaminswas respectively analyzed. To analyze the molecular mass of Lamin of Sf9 cells, the monolayer cells were harvested and the whole-cell protein extracts were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and the protein size of Lamin was detected usingDrosophilaDm0 monoclonal antibody ADL67 by Western blotting. To observe the shape and distribution of lamina of Sf9 cells, the living cells were immunostained with ADL67 by immunofluorescence assay.
From the Spodobase database, aDm0-likelaminEST of Sf9 cells was obtained. The nucleotide and amino acid homology comparison indicated that this sequence showed some identities to thelaminsof other species, especiallyBombyxmori. Western blotting assay showed that the protein size of Dm0-like Lamin of Sf9 cells was approximately 70 ku. Immunofluorescence staining indicated that the observed lamina of Sf9 cells was localized evenly along with the nuclear membrane.
These results indicate that the Dm0-like Lamin may exist in Sf9 cells. How the capsids of budded virus of AcMNPV pass through the lamina will depend on the structure and cellular distribution of lamina. If the distribution of lamina in the inner nuclear membrane is discontinuous, the viral capsids will cross the blind spots to arrive at the inner membrane directly. Also, We cannot exclude the possibility that the lattice of lamina of Sf9 cell is enough wide and the layers of lamina are thin, and the capsids will pass through the lattice without difficulties. On the contrary, if the distribution of lamina is compact and the layers of lamina are thick, the baculovirus may disrupt the lamina for capsids egress. This study may lay the foundation for the exploration of the mechanism of baculovirus capsids transport across the lamina.
Key wordsSf9 cells; lamina; immunofluorescence; Western blotting
核纤层(lamina)是真核细胞核膜的组成部分,位于内核膜的核质面。核纤层由核纤层蛋白(Lamin)组成,在维持核膜的形态、保持染色质的有序性、基因表达、细胞分裂、核膜的解体和重建等过程中发挥重要作用[1-4]。人类lamin的突变与多种疾病有关,例如,早衰综合征、核纤层蛋白病、右心室心律失常型心肌症、新疆维吾尔族特发性扩张性心肌病等[5-10].lamin突变在乳腺癌、结肠癌的发生和发展中也可能起一定的作用[11-13]。Lamin性状受蛋白激酶的调控。细胞或病毒编码的蛋白激酶可磷酸化Lamin导致其溶解[14-15];JIL-1激酶与果蝇Dm0蛋白相互作用,影响细胞核的形态与完整性[16]。对各种生物Lamin结构与功能的研究,丰富了人们对核纤层的认识,为人类lamin突变所致疾病的治疗奠定了理论基础[17-18]。目前已知Lamin在人、小鼠、果蝇等真核生物的细胞内均存在。
核纤层附着在内核膜上,核纤层纤维排列通常比较致密,病毒等大分子很难通过它。而病毒则通过某种机制打破核纤层,以促进子代病毒的出核。对单纯疱疹病毒(herpes simplex virus,HSV)、巨细胞病毒(human cytomegalovirus,HCMV)、鼻咽癌病毒(epstein-barr virus,EBV)等动物细胞的DNA病毒研究表明,子代病毒粒子出核必须打破核纤层[19-23]。昆虫细胞Sf9是来自草地贪夜蛾(Spodopterafrugiperda)蛹期卵巢组织的商业细胞系,是研究杆状病毒模式株AcMNPV感染机制的常用细胞。研究表明,杆状病毒感染后在细胞核内装配后的核衣壳沿着内核膜排列,通过出芽的方式进入核周间隙[24-25]。通过麦胚凝集素-胶体金(WGA-gold)标记核膜技术发现,杆状病毒感染致核膜向核质内伸出形成突起,核衣壳则移向此突起,沿着突起上形成的小孔,移向细胞质[26]。这使我们想到Sf9细胞是否存在核纤层,以及杆状病毒如何穿越核纤层这个有趣的问题。但对Sf9细胞核纤层的研究目前还比较少,限制了对该问题的理解。
本研究利用抗果蝇Lamin Dm0单抗ADL67通过免疫印迹法对Sf9细胞裂解物进行检测;再利用抗体ADL67通过免疫荧光技术染色Sf9细胞并在荧光共聚焦显微镜下观察。同时,用其他昆虫的已知lamin序列在Spodobase[27]数据库(专门存放来自草地贪夜蛾的EST序列数据库)搜索Sf9细胞的lamin同源序列,并进行核苷酸和氨基酸序列比对。结果初步表明,Sf9细胞存在Dm0-like核纤层蛋白。
1材料与方法
1.1材料和试剂
1.1.1昆虫细胞Sf9细胞.用含10%胎牛血清的Grace培养基置于28 ℃培养.胎牛血清购自Gibco公司.Grace培养基购自Invitrogen公司.
1.1.2抗体ADL67抗体由美国石溪大学Paul A。Fisher教授馈赠.四甲基异硫氰酸罗丹明(tetraethyl rhodamine isothiocyanate,TRITC)标记的羊抗鼠IgG抗体购自北京中衫金桥生物技术公司。辣根过氧化物酶(horseradish peroxidase,HRP)标记的羊抗鼠抗体购自北京博奥森生物技术公司。
1.2方法
1.2.1序列比对用已知的lamin基因搜索Spodobase数据库,通过Multalian在线软件(http://multalin.toulouse.inra.fr/multalin/)将搜到的Sf9细胞Dm0-likelaminEST序列及推导出的氨基酸序列与其他物种的lamin进行比对,并用ClustalW2在线软件(http://www.ebi.ac.uk/tools/msa/clustalw2/)分析它们的核苷酸和氨基酸的同源性.
1.2.2免疫印迹Sf9细胞(1×106/35-mm小皿)收获后加入5×蛋白上样缓冲液,煮样10 min后上样进行十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(SDS-polyacrylamide gel electrophoresis,SDS-PAGE),将蛋白条带经湿转法转到PVDF膜上。5%脱脂奶粉室温孵育1 h。以单抗ADL67(1∶100)4 ℃过夜孵育,用带吐温-20的Tris缓冲盐溶液(tris-buffered saline with tween-20,TBST)洗3次,每次10 min。加入辣根过氧化物酶标记的羊抗鼠二抗(1∶2 500)室温孵育1 h,TBST洗3次,每次10 min。同时,取表达Dm0的细菌裂解物(由Nico Stuurman教授馈赠的质粒pETDmLFL在BL21细胞中诱导表达果蝇Dm0蛋白)作为阳性对照。最后以化学发光法检测Lamin.
1.2.3免疫荧光检测Sf9细胞(1×106/35-mm小皿)以4%多聚甲醛室温固定10 min,再用磷酸缓冲盐溶液(phosphate buffer saline,PBS)洗3遍.然后,分别以0.15% Triton X-100处理10 min,100%纯甲醇-20 ℃处理20 min,PBS洗涤3次,每次5 min.以1%牛血清白蛋白(albumin from bovine serum,BSA)37 ℃封闭1 h.加ADL67(1∶10)室温过夜孵育,用带吐温-20的磷酸缓冲盐溶液(phosphate buffer saline with tween-20,PBST)洗3次,每次5 min.加四甲基异硫氰酸罗丹明标记的羊抗鼠二抗(1∶100),室温孵育1 h,PBST洗3次,每次5 min.加入Hoechst 33258,室温孵育20 min.用PBST洗3次.将细胞置于荧光共聚焦显微镜下,观察细胞核内核纤层的分布、形态特征.
2结果
2.1Sf9细胞Dm0-like Lamin的大小
为了确定Dm0-like Lamin的分子质量,取Sf9细胞总蛋白,经SDS-PAGE后转移到PVDF膜上,然后用抗体ADL67通过免疫印迹进行检测.结果显示,在大约70 ku处有1条明显的阳性条带,其大小与果蝇的Dm0蛋白接近(图1).

Dm:果蝇的Dm0蛋白;Sf9:Sf9细胞的Dm0-like Lamin;M:蛋白标志物. Dm: Dm0 protein of D. melanogaster; Sf9: Dm0-like Lamin of Sf9 cells; M: Protein marker. 图1 免疫印迹检测Sf9细胞Dm0-like Lamin Fig.1 Detection of Dm0-like Lamin of Sf9 cells by Western blotting
2.2Sf9细胞Dm0-like Lamin与其他物种同源蛋白序列比对
为了确定Sf9细胞是否存在lamin序列,我们用已知的lamin序列搜索Spodobase数据库.结果搜到1条来自Sf9细胞的Dm0-likelamin同源序列(Sf9LR450003-5-1-C1112),再由其核苷酸序列推导出氨基酸序列.核苷酸序列比对结果显示,Sf9Dm0-likelamin与其他物种的lamin核苷酸序列同源性达56%~80%,其中,与家蚕的同源性达80%,与果蝇的同源性达64%(图2A),但与其他物种lamin同源性相对较低.氨基酸序列比对结果显示,Sf9 Dm0-like Lamin与其他物种的同源蛋白同源性达16%~96%.该序列与家蚕的Lamin同源性最高(96%),其次是果蝇(59%),而与其他物种的同源性较低(图2B).
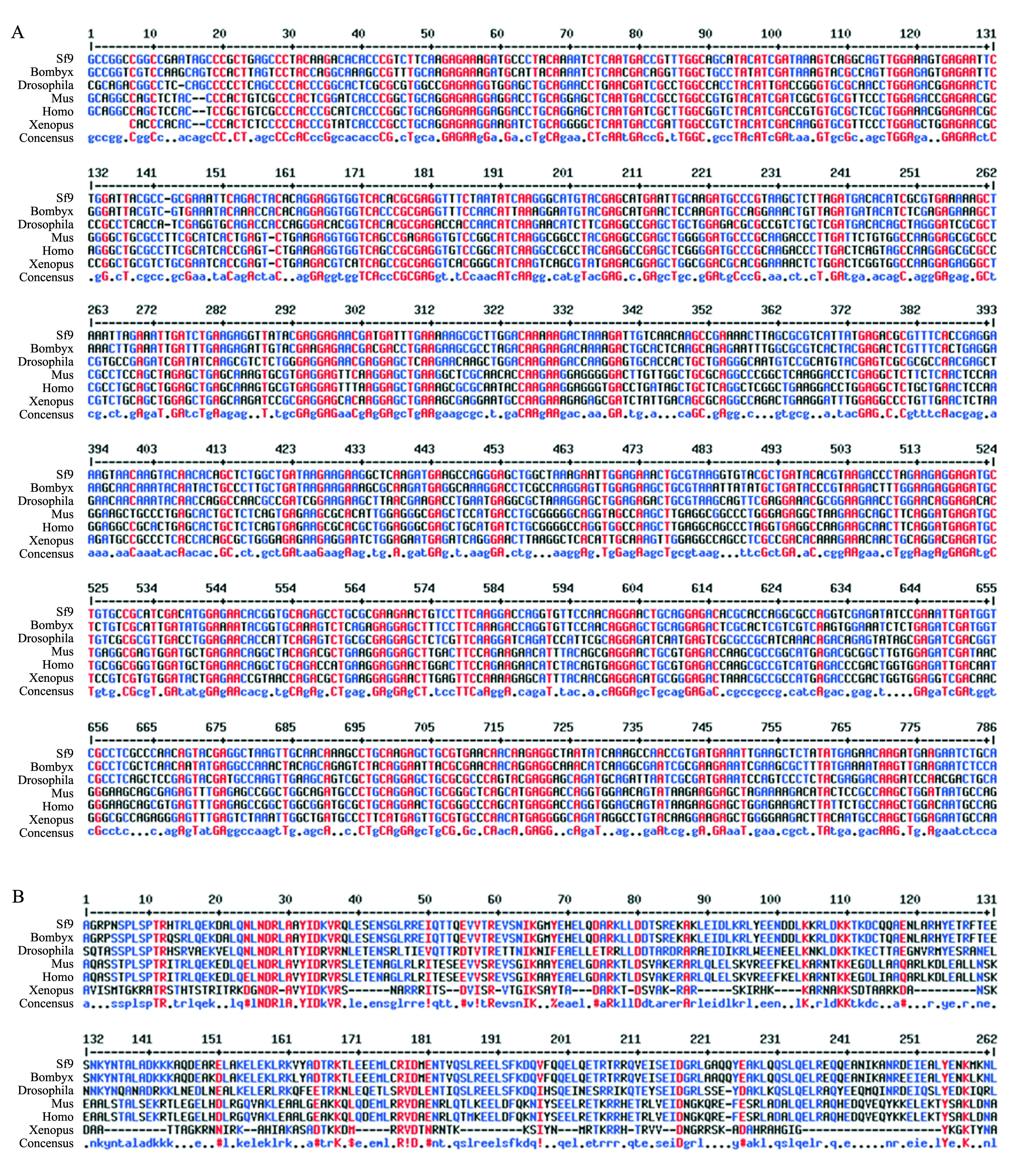
其他物种包括:Bombyx:家蚕(GI:512922266);Drosophila:果蝇(GI:667674288);Mus:小鼠(GI:15929760);Homo:人(GI:224901);Xenopus:非洲爪蟾(GI:156119432). Other species include Bombyx (Bombyx mori, GI: 512922266), Drosophila (Drosophila melanogaster, GI: 667674288), Mus (Mus musculus, GI: 15929760), Homo (Homo sapiens, GI: 224901), Xenopus (Xenopus laevis, GI: 156119432). 图2 Sf9和其他物种Lamin的核苷酸(A)与推导的氨基酸(B)序列比对 Fig.2 Comparison of nucleotide (A) and deduced amino acid (B) sequences of Lamin among Sf9 cells and other species
2.3Sf9细胞Dm0-like Lamin的定位
为了观察Sf9细胞是否存在核纤层及核纤层蛋白的细胞定位,使用抗体ADL67对Sf9细胞进行免疫荧光染色.同时,用Hoechst 33258对细胞核进行染色.荧光共聚焦显微镜观察显示在Sf9细胞的核外周分布一层环状的蛋白,其形状不规则,有的呈规则的圆形,有的则呈椭圆形,这与其他物种的核纤层形态特征相似(图3).
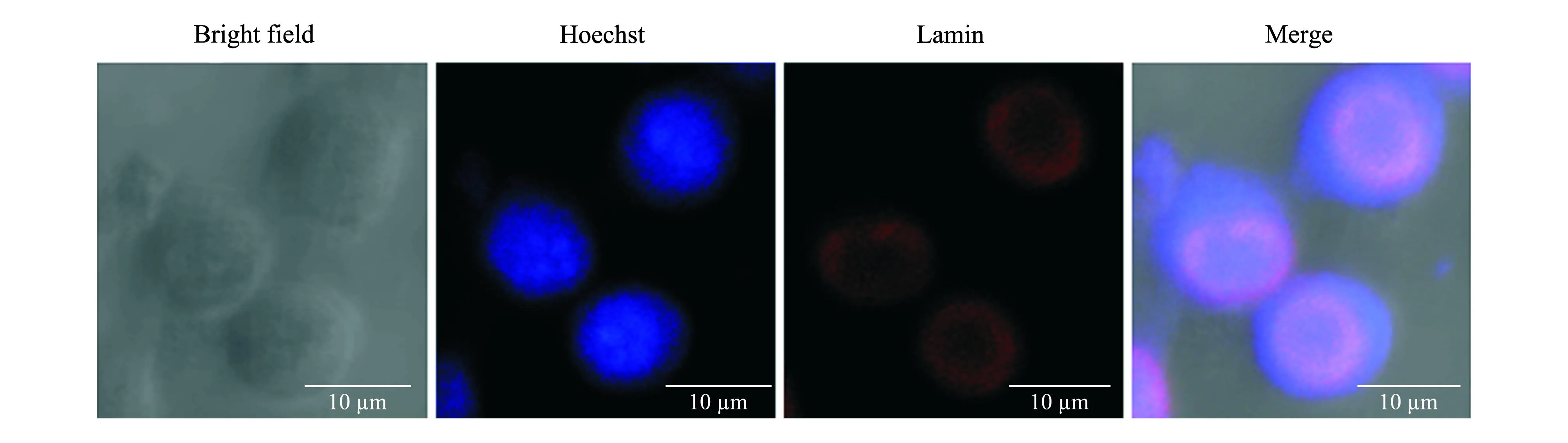
图3 免疫荧光检测Dm0-like Lamin在Sf9细胞的定位 Fig.3 Localization of Dm0-like Lamin of Sf9 cells observed by immunofluorescence
3讨论
本研究通过生物信息学分析、免疫印迹、免疫荧光等方法初步证明Sf9细胞存在Dm0-likelamin.这为下一步研究杆状病毒核衣壳穿过核纤层的机制提供了依据.
利用已知的昆虫lamin序列,在Spodobase[27]数据库进行序列同源性搜索,结果找到1条Sf9细胞的Dm0-likelaminEST序列.生物信息学分析表明,该序列及其翻译后的氨基酸序列与其他物种的Lamin具有一定的同源性,尤其与家蚕的Lamin同源性最高.Nico Stuurman通过对Lamin内含子的进化分析认为,目前在昆虫中仅知道果蝇存在2种Lamin蛋白,即Dm0和Lamin C,Lamin C为果蝇所独有.而家蚕仅有1种Lamin蛋白.Sf9细胞也很可能只存在1种Lamin蛋白[28-29].所以,抗体ADL67检测到的细胞蛋白可能是由搜到的这个Dm0-likelamin基因表达。随后,根据这段EST序列设计特异性引物,利用从Sf9细胞提取的RNA进行反转录PCR(reverse transcription PCR,RT-PCR),扩增到预期大小的条带(未展示),测序结果也符合预期.该段序列的发现为下一步克隆Sf9Dm0-likelamin基因全长并深入研究其蛋白结构与功能奠定了基础.
虽然在Spodobase数据库搜到1个Sf9Dm0-likelaminEST序列,但其完整的开放阅读框(open reading frame,ORF)尚未获得,故其蛋白大小也无法预测.尽管Sf9 Dm0-like Lamin与家蚕Lamin的同源性最高,但家蚕Lamin的抗体市面上尚没有.而针对果蝇Dm0的单抗ADL67目前已经存在,而且Sf9 Dm0-like Lamin与果蝇的Dm0 Lamin的同源性也达到59%.所以,我们尝试用该抗体对Sf9细胞的Dm0-like Lamin的性状进行分析.利用ADL67对Sf9全细胞裂解物进行免疫印迹检测,结果检测到大小约为70 ku的蛋白条带.果蝇的Dm0蛋白(NM_001258963)大小为71 ku.这说明Lamin蛋白大小在不同昆虫间可能是相对保守的.根据氨基酸序列比对的结果可知,获得的Sf9细胞的Dm0-likelaminEST翻译后的氨基酸序列是Lamin N端的一部分,而ADL67的抗原识别表位对应果蝇的Dm0的C端的548~620位氨基酸,故目前无法对果蝇ADL67抗原表位与Sf9细胞Dm0-like Lamin的序列同源性进行比对.但ADL67能识别Sf9细胞的Dm0-like Lamin,说明ADL67识别表位在不同昆虫Lamin间可能具有较高的保守性.
核纤层蛋白一般分布于真核细胞核膜的核质面,荧光显微镜下观察呈环形.为了确定抗体ADL67检测到的70 ku左右的蛋白在Sf9细胞内是否也定位于核膜,再次利用该抗体进行了免疫荧光实验.结果显示,在细胞核的周围观察到环状的阳性荧光信号,与其他物种的细胞核纤层形态、分布相似.免疫荧光实验直观地说明Sf9细胞存在核纤层蛋白.
基于上述实验结果,初步认为在Sf9细胞存在Dm0-like核纤层蛋白.这表明杆状病毒BV的核衣壳出核应先通过核纤层再靠近内核膜.至于杆状病毒是如何穿越核纤层则取决于核纤层的结构,推测有3种可能性:一是核纤层排列致密且层数较多,核衣壳必须打破核纤层方可出核;二是核纤层在核膜上不连续分布,核衣壳直接穿过分布盲区到达核膜;三是核纤层层数较少,杆状的核衣壳可穿过核纤层纤维之间的网格而到达核膜.本研究对Sf9细胞的核纤层进行了初步分析,我们正利用cDNA末端快速扩增技术(rapid-amplificatioin of cDNA ends,RACE)来克隆Dm0-likelamin基因全长,并通过电镜深入观察核纤层的精密结构,以探讨杆状病毒感染Sf9细胞后核纤层的分布、数量的变化,分析核纤层对子代病毒核衣壳出核的影响.此外,Sf9细胞与其他物种的Lamin在进化上的关系,将在克隆Sf9Dm0-likelamin基因全长并分析内含子结构的差异后得知.Lamin是真核细胞普遍存在的一个重要结构蛋白.Dm0-like Lamin在草地贪夜蛾生长发育中的功能及作用机制也是一个值得研究的问题.
参考文献(References):
[1]Yoon B C, Jung H, Dwivedy A,etal. Local translation of extranuclear Lamin B promotes axon maintenance.Cell, 2012,148(4):752-764.
[2]Taimen P, Pfleghaar K, Shimi T,etal. A progeria mutation reveals functions for lamin A in nuclear assembly, architecture, and chromosome organization.ProceedingsoftheNationalAcademyofSciencesoftheUnitedStatesofAmerica, 2009,106(49):20788-20793.
[3]Reddy K L, Zullo J M, Bertolino E,etal. Transcriptional repression mediated by repositioning of genes to the nuclear lamina.Nature, 2008,452(7184):243-247.
[4]祁燃,许楠,张传茂.高等动物细胞核膜和核纤层结构、功能及动态变化调控机制.中国科学:生命科学,2013,43(10):802-814.
Qi R, Xu N, Zhang C M. Dynamic structure and function of the nuclear envelope and lamina in mammalian cells.ScienceChina:LifeSciences, 2013,43(10):802-814. (in Chinese with English abstract)
[5]Naetar N, Korbei B, Kozlov S,etal. Loss of nucleoplasmic LAP2α-lamin A complexes causes erythroid and epidermal progenitor hyperproliferation.NatureCellBiology, 2008,10(11):1341-1348.
[6]Quarta G, Syrris P, Ashworth M,etal. Mutations in the LaminA/Cgene mimic arrhythmogenic right ventricular cardiomyopathy.EuropeanHeartJournal, 2012,33(9):1128-1136.
[7]Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging.Science, 2006,312(5776):1059-1063.
[8]Lenz-Böhme B, Wismar J, Fuchs S,etal. Insertional mutation of the drosophila nuclear laminDm0 gene results in defective nuclear envelopes, clustering of nuclear pore complexes, and accumulation of annulate lamellae.TheJournalofCellBiology, 1997,137(5):1001-1016.
[9]李岩,李建远.核纤层蛋白的研究进展.现代生物医学进展,2013,13(3):561-563.
Li Y, Li J Y. Update of the studies on Lamin.ProgressinModernBiomedicine, 2013,13(3):561-563. (in Chinese with English abstract)
[10]孙帅,赵菊梅,张元,等.核纤层蛋白基因与新疆不同种族特发性扩张型心肌病相关性研究.中国实用内科杂志,2014,34(7):698-701.
Sun S, Zhao J M, Zhang Y,etal. Association ofLMNAgene with idiopathic dilated cardiomyopathy in Uygurs and Hans people in China.ChineseJournalofPracticalInternalMedicine, 2014,34(7):698-701. (in Chinese with English abstract)
[11]何谦,张淑群,赵丽华,等.核基质蛋白Lamin A/C在正常乳腺和乳腺癌组织中的差异表达.中国肿瘤临床,2010,37(9):495-498.
He Q, Zhang S Q, Zhao L H,etal. The differential expression of Lamin A/C in normal human breast tissue and in breast carcinoma tissue.ClinicalOncologyandCancerResearch, 2010,37(9):495-498. (in Chinese with English abstract)
[12]Butin-Israeli V, Adam S A, Goldman A E,etal. Nuclear lamin functions and disease.TrendsinGenetics, 2012,28(9):464-471.
[13]Belt E J T, Fijneman R J A, van den Berg E G,etal. Loss of lamin A/C expression in stage Ⅱ and Ⅲ colon cancer is associated with disease recurrence.EuropeanJournalofCancer, 2011,47(12):1837-1845.
[14]Hocevar B A, Burns D J, Fields A P. Identification of protein kinase C (PKC) phosphorylation sites on human lamin B. Potential role of PKC in nuclear lamina structural dynamics.JournalofBiologicalChemistry, 1993,268(10):7545-7552.
[15]Kuga T, Nozaki N, Matsushita K,etal. Phosphorylation statuses at different residues of lamin B2, B1, and A/C dynamically and independently change throughout the cell cycle.ExperimentalCellResearch, 2010,316(14):2301-2312.
[16]Bao X, Zhang W, Krencik R,etal. The JIL-1 kinase interacts with lamin Dm0 and regulates nuclear lamina morphology of drosophila nurse cells.JournalofCellScience, 2005,118(21):5079-5087.
[17]Schreiber K H, Kennedy B K. When lamins go bad: Nuclear structure and disease.Cell, 2013,152(6):1365-1375.
[18]Gerace L, Huber M D. Nuclear lamina at the crossroads of the cytoplasm and nucleus.JournalofStructuralBiology, 2012,177(1):24-31.
[19]Simpson-Holley M, Colgrove R C, Nalepa G,etal. Identification and functional evaluation of cellular and viral factors involved in the alteration of nuclear architecture during herpes simplex virus 1 infection.JournalofVirology, 2005,79(20):12840-12851.
[20]Reynolds A E, Liang L, Baines J D. Conformational changes in the nuclear lamina induced by herpes simplex virus type 1 require genesUL31 andUL34.JournalofVirology, 2004,78(11):5564-5575.
[21]Muranyi W, Haas J, Wagner M,etal. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina.Science, 2002,297(5582):854-857.
[22]Hamirally S, Kamil J P, Ndassa-Colday Y M,etal. Viral mimicry of Cdc2, cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress.PLoSPathogens, 2009,5(1):e1000275.
[23]Lee C P, Huang Y H, Lin S F,etal. Epstein-barr virus BGLF4 kinase induces disassembly of the nuclear lamina to facilitate virion production.JournalofVirology, 2008, 82(23):11913-11926.
[24]Knudson D, Harrap K. Replication of a nuclear polyhedrosis virus in a continuous cell culture ofSpodopterafrugiperda: Microscopy study of the sequence of events of the virus infection.JournalofVirology, 1976,17(1):254-268.
[25]Anderson I G, Prior H C. Baculovirus infections in the mud crab,Scyllaserrata, and a freshwater crayfish,Cheraxquadricarinatus, from Australia.JournalofInvertebratePathology, 1992,60(3):265-273.
[26]Nishimura T, Favre D, Durrenberger M,etal. Nuclear export of recombinant baculovirus nucleocapsids through small pore or nuclear-pore-like structure in Sf9 cells.OkajimasFoliaAnatJapon, 1994,71:83-97.
[28]Peter A, Stick R. Evolution of the lamin protein family: What introns can tell.Nucleus, 2012,3(1):44-59.
[29]Melcer S, Gruenbaum Y, Krohne G. Invertebrate lamins.ExperimentalCellResearch, 2007,313(10):2157-2166.

