BDNF对大鼠骨髓源神经干细胞诱导分化的影响
※:青海省科技创新能力促进计划项目(2015-ZJ-943Q),青海省科技计划项目(2012-Z-722),青海大学附属医院中青年科研基金项目(ASRF-2014-18)
陈晓娟(1979~),女,回族,青海籍, 医学硕士,主治医师
BDNF对大鼠骨髓源神经干细胞诱导分化的影响
陈晓娟1,肖宗宇2,潘琪3,惠超杰2,侯倩1,才鼎1
(1.青海省人民医院神经内科;2.青海大学附属医院神经外科;3.海南医学院附属医院神经外科)
摘要目的观察脑源性神经生长因子(Brain Derived Neurotrophic Factor,BDNF)对体外培养的Wistar大鼠骨髓源神经干细胞诱导分化的影响。方法运用无血清培养基成功培养Wistar大鼠骨髓源神经干细胞,并对分离获得的悬浮生长的神经细胞球,运用免疫组织化学法检测CD133和Nestin表达情况。采用10%胎牛血清诱导其分化,并添加10 ng/mL BDNF,分化7 d后,采用免疫荧光细胞化学染色方法检测分化后GFAP、Map2、β-tubulin III、Galc的表达情况。结果大鼠骨髓基质细胞在无血清培养基中,呈悬浮状态生长,形成细胞球,经免疫荧光检测,细胞球表达CD133和Nestin。将细胞球转入含血清培养基分化7 d后,表达GFAP的阳性细胞占61.78%±3.54%,Map2阳性细胞占6.28%±0.80%,β-tubulin III阳性细胞占7.43%±1.09%,Galc阳性细胞占2.79%±0.62%。添加10 ng/mL BDNF后,表达GFAP的阳性细胞占62.76%±2.94%,Map2阳性细胞占14.29%±2.45%,β-tubulin III阳性细胞占13.13%±2.42%,Galc阳性细胞占2.97%±0.82%。分别经两独立样本t检测,BDNF组分化为Map2及β-tubulin III阳性细胞,显著高于血清组(P<0.05),但BDNF组分化为GFAP及Galc阳性细胞与血清组相比,无显著性差异(P>0.05)。结论BDNF可显著提高Wistar大鼠骨髓源神经干细胞分化为神经元的比例。
关键词骨髓基质干细胞神经干细胞骨髓源神经干细胞BDNF 分化
中图分类号R332
文献标识码A
DOI:10.13452/j.cnki.jqmc.2015.03.006
AbstractObjectiveTo investigate the inducing effects of BDNF on the differentiation of expression marker of bone marrow mesenchymal stem cells-derived neural stem cells.Methods Wistar rats bone marrow mesenchymal stem cells were cultured in serum-free medium,which was composed of DMEM/F12,20 ng/mL bFGF and 20 ng/mL EGF.Floating neural spheres could be seen in serum-free medium.Expressions of CD133 and Nestin of Neural spheres were detected by immunocytochemistry.Then these neural spheres were randomly divided into two groups,group A were transferred into serum-containing medium(DMEM/F12+10%FBS),group B were cultured in DMEM/F12 medium with 10%FBS and 10ng/mL BDNF. 7 days later,expressions of GFAP、Map2、β-tubulin III、Galc were detected by immunocytochemistry.Results Neural spheres were formed after Wistar rats bone marrow mesenchymal stem cells were cultured in serum-free medium,immunocytochemistry demonstrated these neural sphere cells were positive for CD133 and Nestin. When these neural sphere cells were cultured in DMEM/F12 medium with 10%FBS for 7 days,immunocytochemistry found that these differentiated cells were positive for GFAP,Map2,β-tubulin III and Galc,and the positive rate were as followed,GFAP 61.78%±3.54%,Map2 6.28%±0.80%,β-tubulin III 7.43%±1.09%,Galc 2.79%±0.62%.When 10 ng/mL BDNF was added in the serum-containing medium,the positive rate of these were as followed,GFAP 62.76%±2.94%,Map2 14.29%±2.45%,β-tubulin III 13.13%±2.42%,Galc 2.97%±0.82%.The positive rate of Map2 and β-tubulin III was much higher in the group(serum-containing medium with BDNF)(P<0.05).There was no significant difference in the positive rate of GFAP and Galc between the two groups(P>0.05).Conclusion BDNF could significance increase the rate of neurons when neural stem cells derived from bone marrow of wistar rats were cultured in differentiation condition.
KeywordsBone marrow mesenchymal stem cellsNeural stem cells Bone marrow mesenchymal stem cells-derived neural stem cellsBDNFdifferentiation
收稿日期2015-06-03
THE EFFECTS OF BDNF ON THE DIFFERENTIATION
OF BONE MARROW MESENCHYMAL STEM CELLS-DERIVED
NEURAL STEM CELLS
Chen xiaojuan1,Xiao zongyu2,Pan qi3,Hui chaojie2,Hou qian1,Cai ding1
(1.Department of Neurology,People’s Hospital of Qinghai Province,Xining 810000,China;
2.Department of neurosurgery,Affiliated hospital of Qinghai University,Xining 810000,China;
3.Department of neurosurgery,Affiliated hospital of Hainan University,Haikou 570100, China)
脑源性神经生长因子(Brain-derived neurotrophic factor,BDNF)可否促进大鼠BMSCs-NSCs向神经元方向分化?
骨髓基质细胞(bone marrow mesenchymal stem cells,BMSCs)已成为较理想的种子细胞来源之一[1]。近年来,随着神经干细胞(neural stem cells,NSCs)研究的不断深入,部分研究者将BMSCs成功诱导培养出了骨髓源神经干细胞(bone marrow mesenchymal stem cells-derived neural stem cells,BMSCs-NSCs)[2,3]。BDNF是一种具有防止神经元死亡功能的蛋白质,它在体外可保持NSCs的活性和促进其向神经元分化[4,5]。为探讨BDNF对BMSCs-NSCs生长分化的影响,本研究拟通过设计相关实验方案,给出相关答案。
1材料与方法
1.1 实验动物选择
SPF级8周龄Wistar雄性大鼠12只,平均体重200~220 g,由兰州大学实验动物中心提供[合格证号:SCXK(甘)2013-0002]。
1.2 试剂选择
DMEM/F12培养基、0.25%胰蛋白酶为Gibco公司产品。胎牛血清(fetal bovine serum,FBS)为Hyclone公司产品。B27添加剂购自Invitrogen公司。表皮生长因子(epidermal growth factor,EGF)、碱性成纤维细胞生长因子(basic fibroblast growth factor,bFGF)及脑源性神经生长因子(BDNF)为Peprotech公司产品,小鼠抗Nestin抗体、兔抗GFAP抗体、小鼠抗Galc抗体为Chemicon公司产品,兔抗CD133抗体购自Santa Cruz公司,小鼠抗Map2抗体、兔抗β-tubulin III抗体为Abcam公司产品。Alexa Fluor 488山羊抗小鼠IgG、Alexa Fluor 594标记羊抗兔IgG和Alexa Fluor 488山羊抗兔IgG由Molecular Probes公司生产。DAPI封片剂为Vector Laboratories公司产品。
1.3 骨髓源神经干细胞的诱导培养
无菌条件下取大鼠双侧股骨和肱骨,用10 mL DMEM/F12培养基冲洗骨髓腔,收集骨髓组织悬液,以含10%FBS的DMEM/F12重悬,接种于25 cm2培养瓶,37 ℃、5%CO2、饱和湿度条件下培养。收集第四代BMSCs,经0.25%胰酶消化,制备单细胞悬液,以含20 ng/mL bFGF、20 ng/mL EGF及1:50的B27添加剂的DMEM/F12无血清培养基重悬,接种密度设为2×105个活细胞/mL,隔天换半液一次并添加相应剂量生长因子。
1.4 大鼠骨髓源性神经干细胞CD133和Nestin检测
选取在无血清培养基中呈悬浮生长的状态良好的大鼠骨髓源神经细胞球,去除培养基,采用4%多聚甲醛固定30 min,0.3%Triton X-100破膜10 min,10%山羊血清37 ℃封闭60 min,不冲洗;滴加一抗CD133(1:200)和Nestin(1:100),置湿盒、4 ℃过夜;滴加二抗:Alexa Fluor 594标记羊抗兔IgG(1:200)和Alexa Fluor 488标记羊抗小鼠IgG(1:200),37 ℃孵育1 h;然后使用DAPI对细胞核进行复染并封片。以上各步骤前后均使用0.01 mol/L PBS冲洗(3次×5min)。
1.5 大鼠骨髓源性神经干细胞的分化
收集生长状态良好的大鼠骨髓源性神经球,或将神经球消化制备为单细胞悬液,调整细胞密度为2×105个活细胞/mL,重悬于新的培养瓶。实验分两组,血清组:将细胞接种于含血清培养基(DMEM/F12+10% FBS);BDNF组:在血清组的基础上,加入10 ng/mL BDNF,分化7 d后,采用免疫荧光细胞化学法对GFAP、Map2、β-tubulin III、Galc的表达情况进行检测,并使用DAPI对细胞核进行复染。在荧光显微镜下,随机观察10个高倍视野并拍照,分别计算每个视野呈GFAP、Map2、β-tubulin III、Galc阳性细胞数,并计算每个视野呈DAPI蓝染的细胞核总数,按下列公式分别计算各标志物阳性细胞百分率:阳性细胞百分率=阳性细胞数/细胞总数×100%。
1.6 统计学处理
采用SPSS 19.0统计软件,结果以均数±标准差表示,组间比较采用两独立样本t检验,视P<0.05为差异具有统计学意义。
2结果
2.1 大鼠骨髓源神经干细胞的培养及鉴定
大鼠骨髓基质细胞在含血清培养基中呈贴壁生长,细胞形态以长梭形为主,体积较小、大小基本一致,增殖速度较快。将第四代大鼠BMSCs转入含20 ng/mLbFGF、20 ng/mLEGF及B27添加剂(150)的DMEM/F12培养基后,其增殖方式发生改变,细胞呈悬浮球状聚集生长,球体呈圆形或椭圆形,球体内细胞呈圆形,有较强的折光性,增殖速度较慢。经免疫组织化学检测,所形成的细胞球表达CD133和Nesitn(图1)。

大鼠骨髓源神经干细胞Nestin(绿色、图A)、CD133(红色、图B)呈阳性,细胞核以DAPI复染(蓝色、图C),荧光显微镜标尺为50 μm
Expressions of Nestin(A)and CD133(B)of bone marrow mesenchymal stem cells-derived neural stem were detected by immunocytochemistry.The nuclei were counterstained with DAPI(blue), Scale bar=50 μm
图1大鼠骨髓源神经干细胞Nestin和CD133的免疫荧光检测图
Figure 1Expressions of Nestin and CD133 of BMSC-NSCs by immunocytochemistry
2.2 大鼠骨髓源神经干细胞的分化及鉴定
将大鼠骨髓源神经干细胞从含bFGF、EGF等生长因子的无血清培养基中移出,并转入含10%FBS的DMEM/F12含血清培养基后,细胞球或单细胞均呈贴壁生长,长出突起,形似神经元样或胶质细胞样。分化7 d后,行免疫荧光细胞化学检测,血清组表达GFAP的阳性细胞占61.78%±3.54%,Map2阳性细胞占6.28%±0.80%,β-tubulin III阳性细胞占7.43%±1.09%,Galc阳性细胞占2.79%±0.62%。BDNF组表达GFAP、Map2、β-tubulin III和Galc的阳性细胞比率分别为62.76%±2.94%、14.29%±2.45%、13.13%±2.42%和2.97%±0.82%。分别经两独立样本t检验,BDNF组分化为Map2及β-tubulin III阳性细胞显著高于单纯血清组(P<0.05),但BDNF组分化为GFAP及Galc阳性细胞与单纯血清组相比,无显著性差异(P>0.05)(图2、表1)。
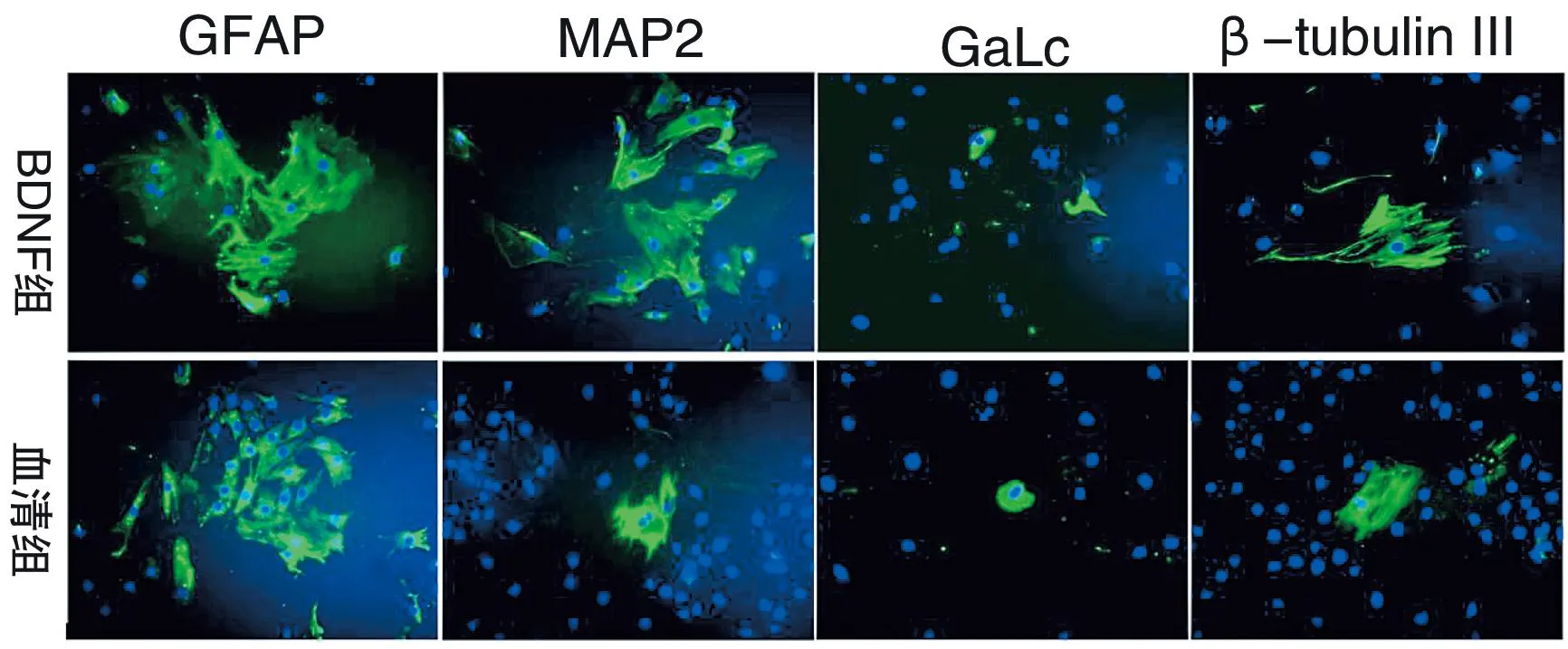
将大鼠骨髓源神经球转入含血清培养基后,大部分细胞呈GFAP阳性,少部分细胞呈Map2、Galc和β-tubulin III阳性。细胞核以DAPI复染(蓝色).荧光显微镜,放大倍数:×200
After BMSC-NSCs were cultured in serum-containing medium for 7 days,these cells derived from BMSCs-NSCs were positive for GFAP,MAP2,Galc and β-tubulin III.×200
图2大鼠骨髓源神经干细胞分化后GFAP,MAP2,Galc和β-tubulin III的表达情况
Figure 2Expression of GFAP,MAP2,Galc and β-tubulin III on the differentiation of BMSCs-NSCs
表1 BDNF对骨髓源神经干细胞分化的影响( ± s)
Table 1 The effects of BDNF on the differentiation of BMSC-NSCs( ± s)

表1 BDNF对骨髓源神经干细胞分化的影响( ± s)
组别例数GFAP阳性率Map2阳性率Galc阳性率β-tubulinIII阳性率BDNF组962.76±2.9414.29±2.452.97±0.8213.13±2.42血清组961.78±3.546.28±0.802.79±0.627.43±1.09t 0.637 9.312 0.524 6.432P 0.533 0.000 0.607 0.000
表1显示,BDNF能显著提高大鼠骨髓源神经干细胞分化为Map2及β-tubulin III阳性细胞的比例,但两组间GFAP与Galc表达阳性率无显著差异(P>0.05)。
3讨论
BMSCs是一种存在于骨髓非造血组织中的多能干细胞,它具来源丰富、取材简单、培养增殖容易、增殖能力强等特点,目前已证实骨髓基质细胞在特定的培养条件下可分化成多种细胞,如神经干细胞、神经元细胞、神经胶质细胞、成纤维细胞、成骨细胞、软骨细胞、脂肪细胞、肌肉细胞和血管内皮祖细胞等[6-8]。近年来的研究表明,BMSCs于体外通过诱导分化可获得BMSCs- NSCs[2,3]。BMSCs-NSCs的成功培养,避免了神经干细胞或胚胎干细胞等其他干细胞涉及的来源有限、取材困难、免疫排斥及伦理等问题。因此,BMSCs- NSCs在应用神经干细胞治疗人类某些神经系统疾病及损伤方面具有良好的临床应用前景。
研究表明[9,10],采用含bFGF和EGF的无血清培养基可成功将BMSCs诱导培养为BMSCs-NSCs。在本实验中,我们选用Wistar大鼠,取大鼠骨髓,以含10%胎牛血清的DMEM/F12进行培养,在成功培养BMSCs后。我们将BMSCs转入含bFGF、EGF的无血清培养基中后,呈悬浮球状聚集生长。CD133和Nestin为目前公认的神经干细胞特异性标志物,通过免疫荧光检测,发现我们所培养出的细胞球均表达CD133、Nestin。因此,通过无血清悬浮培养法,我们成功培养了大鼠骨髓源神经干细胞。
低噪声放大器的稳定性非常重要,对其进行精准的判定是不可或缺的。一般而言,采用k检验的方法[13]对稳定性进行判定,如式(4)所示:
多向分化潜能是神经干细胞的主要特征,其可分化为中枢神经系统内神经元、星形胶质细胞、少突胶质细胞等细胞[11]。在本实验中,我们将呈悬浮球状聚集生长的大鼠骨髓源细胞球转入含10%FBS的含血清培养基中后,其失去悬浮增殖状态,呈贴壁生长,贴壁细胞呈现多种形态,形似神经元样或胶质细胞样。细胞球分化7 d后,免疫荧光检测发现已分化细胞可表达GFAP、Map2、Galc和β-tubulin III,证明大鼠骨髓源神经球具备多向分化潜能,可分化为表达星形胶质细胞、神经元及少突胶质细胞特异性标志物的终末分化细胞,但在已分化细胞中,大部分细胞为GFAP阳性的星形胶质细胞,而表达成熟神经元标志物Map2和未成熟神经元标志物β-tubulin III 的细胞仅为6.28%±0.80%和7.43%±1.09%,表达少突胶质细胞标志物Galc的细胞比较也较低,为2.79%±0.62%。因此,在含10%FBS的培养条件下,BMSCs-NSCs主要向星形胶质细胞分化,而分化为神经元样细胞的数量较少。
BDNF是一种具有防止神经元死亡功能的蛋白质,属于神经营养因子家族成员之一,它可促进中枢神经系统的发育并维持其正常功能。在中枢神经系统中,其广泛分布在大部分脑区,上丘、大脑皮层和海马,它对神经细胞的发育、存活和修复起着至关重要的作用[4,5]。有学者报道,BDNF在不同来源的神经干细胞分化过程中均显示出重要作用,其可诱导NSCs向神经元方向分化[12]。Ahmed[13]等发现BDNF能促进胚鼠纹状体来源的神经干细胞分化为Map2阳性细胞数量,并促进神经突起生长。Yoo等[14]发现,BDNF对人胚中脑来源的神经干细胞有显著保护作用,并可提高其向神经元分化比率。为探索在体外条件下,BDNF是否可促使Wistar大鼠BMSCs-NSCs向神经元方向分化,我们将BMSCs-NSCs转入含10%胎牛血清的DMEM/F12培养基后,添加10 ng/mL BDNF。分化7 d后,进行免疫组化检测,发现其分化为神经元(Map2、β-tubulin III)的比例大大提高,Map2和β-tubulin III的阳性率分别达到了14.29%±2.45%和13.13%±2.42%,经统计学分析,BDNF组分化为Map2和β-tubulin III阳性细胞的比较显著高于血清组。但经统计学分析,BDNF组和血清组间,星形胶质细胞(GFAP)及少突胶质细胞(Galc)的阳性细胞比率却无显著性差异,表明在体外分化条件下,BDNF可显著促进Wistar大鼠骨髓源神经干细胞向神经元方向分化。由于本实验仅从体外实验进行检测,BDNF虽可促进大鼠BMSCs-NSCs向Map2和β-tubulin III阳性细胞方向分化,但这些已分化细胞是否具神经元的功能,有待进一步研究。
综上所述,采用无血清悬浮培养法成功培养了Wistar大鼠骨髓源神经干细胞,将其转入含10%胎牛血清的DMEM/F12培养基中进行分化,并添加10 ng/mL BDNF,BDNF可显著促进Wistar大鼠骨髓源神经干细胞向神经元分化。
参考文献
[1]Mahmood A,Lu D,Chopp M.Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain[J].Neurosurgery,2004,55(5):1185-1193.
[2]Lee J,Elkahloun A G,Messina S A,et al.Cellular and genetic characterization of human adult bone marrow-derived neural stem-like cells:a potential antiglioma cellular vector[J].Cancer Res,2003,63(24):8877-8889.
[3]Tang Y,Cui Y C,Wang X J,et al.Neural progenitor cells derived from adult bone marrow mesenchymal stem cells promote neuronal regeneration[J].Life Sci,2012,91(19-20):951-958.
[4]Chang D J,Lee N,Choi C,et al.Therapeutic effect of BDNF-overexpressing human neural stem cells(HB1.F3.BDNF)in a rodent model of middle cerebral artery occlusion[J].Cell Transplant,2013,22(8):1441-1452.
[5]Yang Z,Qiao H,Sun Z,et al.Effect of BDNF-plasma-collagen matrix controlled delivery system on the behavior of adult rats neural stem cells[J].J Biomed Mater Res A,2013,101(2):599-606.
[6]Black I B,Woodbury D.Adult rat and human bone marrow stromal stem cells differentiate into neurons[J].Blood Cells Mol Dis,2001,27(3):632-636.
[7]Kao R,Lu W,Louie A,et al.Cyclic AMP signaling in bone marrow stromal cells has reciprocal effects on the ability of mesenchymal stem cells to differentiate into mature osteoblasts versus mature adipocytes[J].Endocrine,2012,42(3):622-636.
[8]Ratajczak J,Wysoczynski M,Zuba-Surma E,et al.Adult murine bone marrow-derived very small embryonic-like stem cells differentiate into the hematopoietic lineage after coculture over OP9 stromal cells[J].Exp Hematol,2011,39(2):225-237.
[9]陈静娟,李华,许志恩,等.无血清神经培养基体外培养大鼠骨髓源性神经干细胞[J].中国组织工程研究与临床康复,2010,14(19):3441-3445.
[10]高健,刘振华,姜晓丹,等.骨髓源性神经干细胞诱导分化为多巴胺能神经元的实验研究[J].中华神经医学杂志,2006,5(4):349-352.
[11]Stoll E A,Makin R,Sweet I R,et al.Neural Stem Cells in the Adult Subventricular Zone Oxidize Fatty Acids to Produce Energy and Support Neurogenic Activity[J].Stem Cells,2015,33(7):2306-2319.
[12]罗媚,龙大宏,谷海刚,等.脑源性神经营养因子体外诱导神经干细胞向神经元分化[J].解剖学研究,2008,30(3):190-192,213,封3.
[13]Ahmed S,Reynolds B A,Weiss S.BDNF enhances the differentiation but not the survival of CNS stem cell-derived neuronal precursors[J].J Neurosci,1995,15(8):5765-5778.
[14]Yoo Y M,Kim Y J,Lee U.The change of the neuron-glia differentiation rate in human neural precursor cells(HPCs)and Ad-BDNF-/-GDNF-infected HPCs following the administration of a neurotoxin[J].Neurosci Lett,2005,387(2):100-104.

