PAX2稳转细胞系构建及对细胞迁移和侵袭能力的作用研究
·论著·
PAX2稳转细胞系构建及对细胞迁移和侵袭能力的作用研究
李里,宫亮,吴玉斌
作者单位:121000辽宁省锦州市,辽宁医学院附属第一医院风湿免疫科(李里),耳鼻喉科(宫亮);中国医科大学附属盛京医院小儿肾脏风湿免疫科(吴玉斌)
通信作者:吴玉斌,110004 辽宁省沈阳市,中国医科大学附属盛京医院小儿肾脏风湿免疫科;E-mail:772652284@qq.com
【摘要】目的 探讨PAX2稳转细胞系构建及生物学作用。方法选择处于对数生长期大鼠肾小管上皮细胞,实验分为稳转组、空载组、对照组。稳转组采用阳离子脂质体转导将pEGFP-PAX2转入大鼠肾小管上皮细胞,空载组采用同样方法配置空载质粒转染溶液,对照组不添加质粒转染溶液。经G418筛选,建立稳定转染细胞系。通过细胞划痕实验及Transwell实验检测PAX2稳转细胞系的细胞迁移率及细胞侵袭作用。结果应用G418筛选14 d,稳转组大鼠肾小管上皮细胞有明显克隆长出,建立PAX2基因稳定转染肾小管上皮细胞系。稳转组在荧光显微镜下可见绿色荧光,基因融合表达获得成功。稳转组细胞PAX2条带密度与β-action吸光度比值为(1.00±0.04),空载组为(0.83±0.03),对照组为(0.85±0.04),3组吸光度比值比较,差异有统计学意义(F=398.7,P<0.05);其中稳转组吸光度比值高于空载组和对照组,差异均有统计学意义(P<0.05)。细胞划痕后18 h,对照组、空载组和稳转组细胞迁移率分别为(39.34±5.34)%、(40.56±7.54)%和(83.72±7.12)%,3组细胞迁移率比较,差异有统计学意义(F=431.8,P<0.05);其中稳转组细胞迁移率高于对照组和空载组,差异均有统计学意义(P<0.05)。各组细胞培养48 h后,对照组、空载组和稳转组迁移细胞分别为(23.33±3.63)、(22.00±8.14)、(45.67±7.14),3组迁移细胞数比较,差异有统计学意义(F=248.5,P<0.05);其中稳转组迁移细胞数高于对照组和空载组,差异均有统计学意义(P<0.05)。结论pEGFP-PAX2质粒成功转染大鼠肾小管上皮细胞,并成功筛选出高效稳定表达PAX2的稳转细胞系。PAX2稳转增加了肾小管上皮细胞的迁移及侵袭能力。
【关键词】PAX2基因;细胞侵袭;细胞运动;肾小管;上皮细胞;大鼠
基金项目:辽宁省博士科研启动基金计划资助项目(20141136)
【中图分类号】R 692.6
收稿日期:(2015-07-02;修回日期:2015-09-12)
李里,宫亮,吴玉斌.PAX2稳转细胞系构建及对细胞迁移和侵袭能力的作用研究[J].中国全科医学,2015,18(35):4325-4329.[www.chinagp.net]
Li L,Gong L,Wu YB.Study on the construction of PAX2 stably transfected cell lines and their effects on cell migration and invasion ability[J].Chinese General Practice,2015,18(35):4325-4329.
Study on the Construction of PAX2 Stably Transfected Cell Lines and Their Effects on Cell Migration and Invasion AbilityLILi,GONGLiang,WUYu-bin.DepartmentofRheumatologyandImmunology,theFirstAffiliatedHospitalofLiaoningMedicalUniversity,Jinzhou121000,China
Abstract【】ObjectiveTo investigate the construction and biological effects of PAX2 stably transfected cell lines.MethodsRat renal tubular epithelial cells at exponential phase were selected,and the cells were divided into stably transfected group,empty-loading group and control group.For stably transfected group,cationic liposome transduction was conducted to transfer pEGFP-PAX2 into rat renal tubular epithelial cells;for empty-loading group,cationic liposome transduction was also conducted with empty-loading plasmid transfection solution;for control group,no plasmid transfection solution was added.By G418 selection,stably transfected cell lines were constructed.Cell wound scratch assay and Tanswell test were conducted to measure cell migration rate and cell invasion of PAX2 stably transfected cell lines.ResultsG418 selection was undertaken for 14 days,the cells of stably transfected group had obvious cloning growth,and PAX2 stably transfected cell lines were constructed.Green fluorescence was observed under fluorescence microscope in stably transfected group,and successful gene fusion expression was made.The ratio of the PAX2 strip density to the absorbancy of β-action was (1.00±0.04) for stably transfected group,(0.83±0.03)for empty-loading group and (0.85±0.04)for control group.The three groups were significantly different in optical density ratio(F=398.7,P<0.05);the stably transfected group was higher than empty-loading group in optical density ratio(P<0.05).At 18 hours after cell scratch was made,the cell migration rates of control group,empty-loading group and stably transfected group were(39.34±5.34)%,(40.56±7.54)% and(83.72±7.12)%,with significant differences among the three groups(F=431.8,P<0.05)and the stably transfected group higher than control group and empty-loading group(P<0.05).At 48 hours of culture,the numbers of migrated cells of control group,empty-loading group and stably transfected group were(23.33±3.63),(22.00±8.14)and(45.67±7.14),with significant differences among the three groups(F=248.5,P<0.05)and stably transfected group higher than control group and empty-loading group(P<0.05).ConclusionpEGFP-PAX2 plasmid is successfully transfected into rat renal tubular epithelial cells,and stably transfected cell lines with highly effective and stable expression of PAX2 are successfully constructed.The stable transfection of PAX2 increases migration and invasion of renal tubular epithelial cells.
【Key words】PAX2 gene;Cell invasion;Cell movement;Kidney tubules;Epithelial cells;Rats
PAX2(Paired Box2)基因是胚胎发育基因,在胚胎发育过程中发挥重要的调控作用。尤其在肾脏,其是肾脏发育过程中前肾、中肾和后肾重要的调控基因。PAX2基因主要通过诱导肾小管上皮细胞转分化发挥调控作用[1-3]。有学者[4-7]发现在病变大鼠体内出现PAX2基因重新表达,并可能调控转分化作用。在体外研究中发现PAX2基因转染肾小管上皮细胞后出现纤维表型标志物的增加以及上皮细胞表型标志物的减少[8]。判定细胞出现上皮细胞转分化的标志除了出现表型的转变外还表现为细胞侵袭及迁移能力的增加。本研究将首先构建PAX2稳转细胞系,进一步探讨PAX2稳转细胞系细胞侵袭及迁移能力,为临床疾病治疗提供实验依据。
1材料与方法
1.1材料大鼠肾小管上皮细胞株(NRK52E),购自上海中科院;pEGFP-PAX2质粒(含Neo抗性基因),购自Clontech 公司;Transwell细胞小室,购自Corning公司。
1.2方法
1.2.1pEGFP-PAX2稳定转染肾小管上皮细胞系的构建及筛选选择处于对数生长期大鼠肾小管上皮细胞,其生长状态良好。分为稳转组、对照组、空载组。将pEGFP-grp78质粒4 μg加入无血清培养液245 μl,再加入Lipofectamine 2000,将上述液体混合,在室温静置30 min。同样方法配置空载质粒转染溶液。对照组不加人质粒转染溶液。在无血清无抗生素的培养液中将肾小管上皮细胞洗涤2次,再将上述转染液与细胞加入培养孔中,在5% CO237 ℃培养箱中培养6 h后,将转染液弃除,再加入胎牛血清继续培养。细胞传代,再用G418的选择培养基筛选,筛选14 d后pEGFP-PAX2重组质粒转染组有克隆形成,建立稳定转染细胞系。
1.2.2稳定转染细胞系鉴定在荧光显微镜下观察各组细胞,蓝色激光激发,随机选一视野,发出绿色荧光的细胞并摄像。PAX2 mRNA的表达检测:在24孔板中加入PBS漂洗2次,取1×106细胞加Trizol 1.0 ml,按试剂盒操作说明用三氯甲烷分离、采用异丙醇RNA沉淀、将RNA再溶解,提取细胞总RNA。按照试剂盒说明书配制20 μl RT反应体系;PCR反应:引物设计:上游:5′-CAACGGTGAGAAGAGGAAACGAG-3′,下游:5′-TAATGCTGCTGGGTGAAGGTGTC-3′,β-actin作为内参照,按照试剂盒说明配制50 μl PCR反应体系,结束后进行琼脂凝胶电泳检测并摄像;经图像分析仪测定吸光度值,即检测基因产物吸光度值与内参照吸光度的比值为A值。以对照组和空载组的肾小管上皮细胞作为对照。
1.2.3细胞划痕试验细胞铺板:前1天将培养瓶中细胞用0.25%胰蛋白酶消化,接种1×105细胞至6孔培养板中,当细胞融合至80%~90%时开始进行实验。弃去培养基,应用200 μl pipette tip使培养板出现划痕,应用培养基清洗细胞表面2次,再加入培养基中,在镜下观察10、18 h,并于100倍镜下拍照记录。并计算各组迁移率。迁移率用来描述细胞迁移修复速度,具体计算方法:迁移率=(0 h细胞划痕宽度-10 h细胞划痕宽度或18 h细胞划痕宽度)/0 h细胞划痕宽度×100%。
1.2.4Transwell实验将50 μl的Matrigel(1 μg/μl)平铺在Transwell的8 μm孔径的聚碳酸酯微孔滤膜上,37 ℃放置1 h,室温干燥过夜。将600 μl含20%胎牛血清的NIH3T3细胞的上清加入Transwell下室,将200 μl对照组、空载组和稳转组细胞悬液加入上室,每种细胞设3个复孔,培养48 h后取出小室,将膜上层未穿越细胞拭去,用多聚甲醇固定30 min,苏木精染色15 min,PBS洗去多余苏木精,镜下计数穿到膜背细胞数。计数3个不同视野,取平均值(×200)。
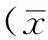
2结果
2.1稳定转染细胞系的构建及筛选应用G418筛选14 d,稳转组大鼠肾小管上皮细胞有明显克隆长出,建立了PAX2基因稳定转染肾小管上皮细胞系(见图1)。
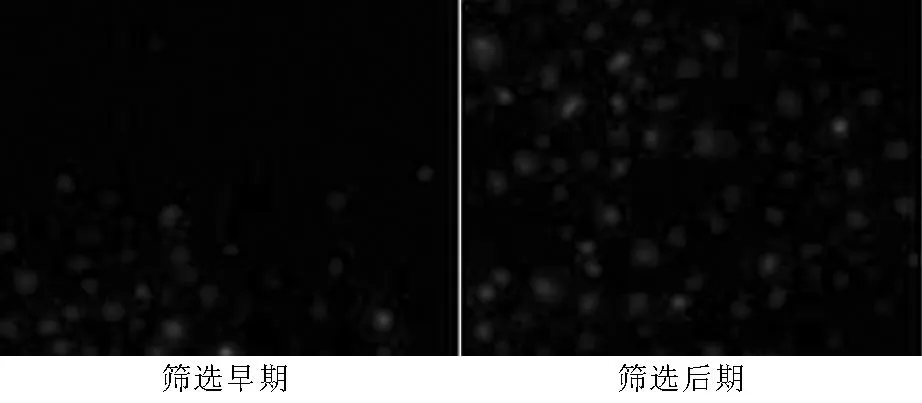
图1 PAX2基因转染肾小管上皮细胞的G418筛选(×200)
Figure 1G418 selection of renal tubular epithelial cells after PAX2 gene transfection
2.2稳定转染细胞系鉴定
2.2.1各组细胞绿色荧光表达稳转组及空载组在荧光显微镜下均可见绿色荧光,对照组未见绿色荧光(见图2),基因融合表达获得成功。
2.2.2各组细胞PAX2 mRNA表达稳转组细胞PAX2条带密度与β-action吸光度比值为(1.00±0.04),空载组为(0.83±0.03),对照组为(0.85±0.04)。3组吸光度比值比较,差异有统计学意义(F=398.7,P<0.05);其中稳转组吸光度比值高于空载组和对照组,差异均有统计学意义(P<0.05,见图3)。
2.3PAX2稳定转染后肾小管上皮细胞运动迁移改变细胞划痕后10 h,对照组、空载组和稳转组细胞迁移率分别为(23.00±3.10)%、(24.33±4.02)%和(43.12±7.13)%。3组细胞迁移率比较,差异有统计学意义(F=501.6,P<0.05)。细胞划痕后18 h,对照组、空载组和稳转组细胞迁移率分别为(39.34±5.34)%、(40.56±7.54)%和(83.72±7.12)%。3组细胞迁移率比较,差异有统计学意义(F=431.8,P<0.05);其中稳转组细胞迁移率高于对照组和空载组,差异均有统计学意义(P<0.05,见图4)。
2.4PAX2稳定转染对肾小管上皮细胞侵袭能力的影响各组细胞培养48 h后,计数膜下表面的细胞数。对照组、空载组和稳转组迁移细胞分别为(23.33±3.63)、(22.00±8.14)、(45.67±7.14),3组迁移细胞数比较,差异有统计学意义(F=248.5,P<0.05);其中稳转组迁移细胞数高于对照组和空载组,差异均有统计学意义(P<0.05,见图5)。
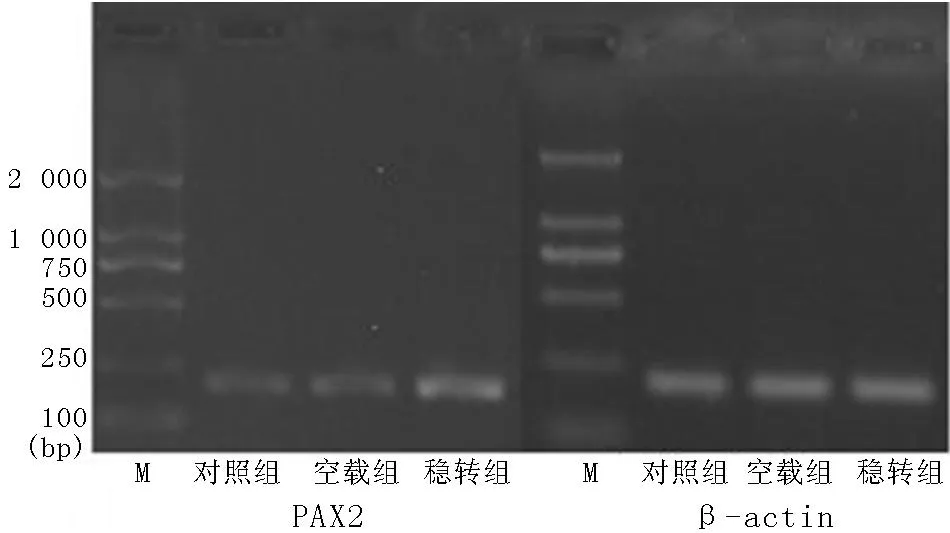
图3 3组细胞PAX2 mRNA表达的RT-PCR电泳图
Figure 3RT-PCR eelectrophoretogram of PAX2 mRNA expression of the three groups
3讨论
PAX2基因为胚胎发育基因,既往研究表明PAX2在病理肾脏出现重新表达,认为其可能通过调控肾小管上皮细胞转分化调控疾病发生,但具体机制不清楚。肾小管上皮细胞转分化发生中重要的一步即细胞出现了迁移及侵袭能力的增强[9],故本研究通过建立PAX2稳转细胞系,探讨PAX2转染是否导致细胞的侵袭及迁移能力增强,为进一步探讨PAX2基因调控肾小管上皮细胞转分化提供依据。
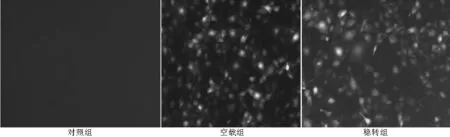
图2 荧光显微镜观察pEGFP-PAX2在肾小管上皮细胞中的表达(×200)
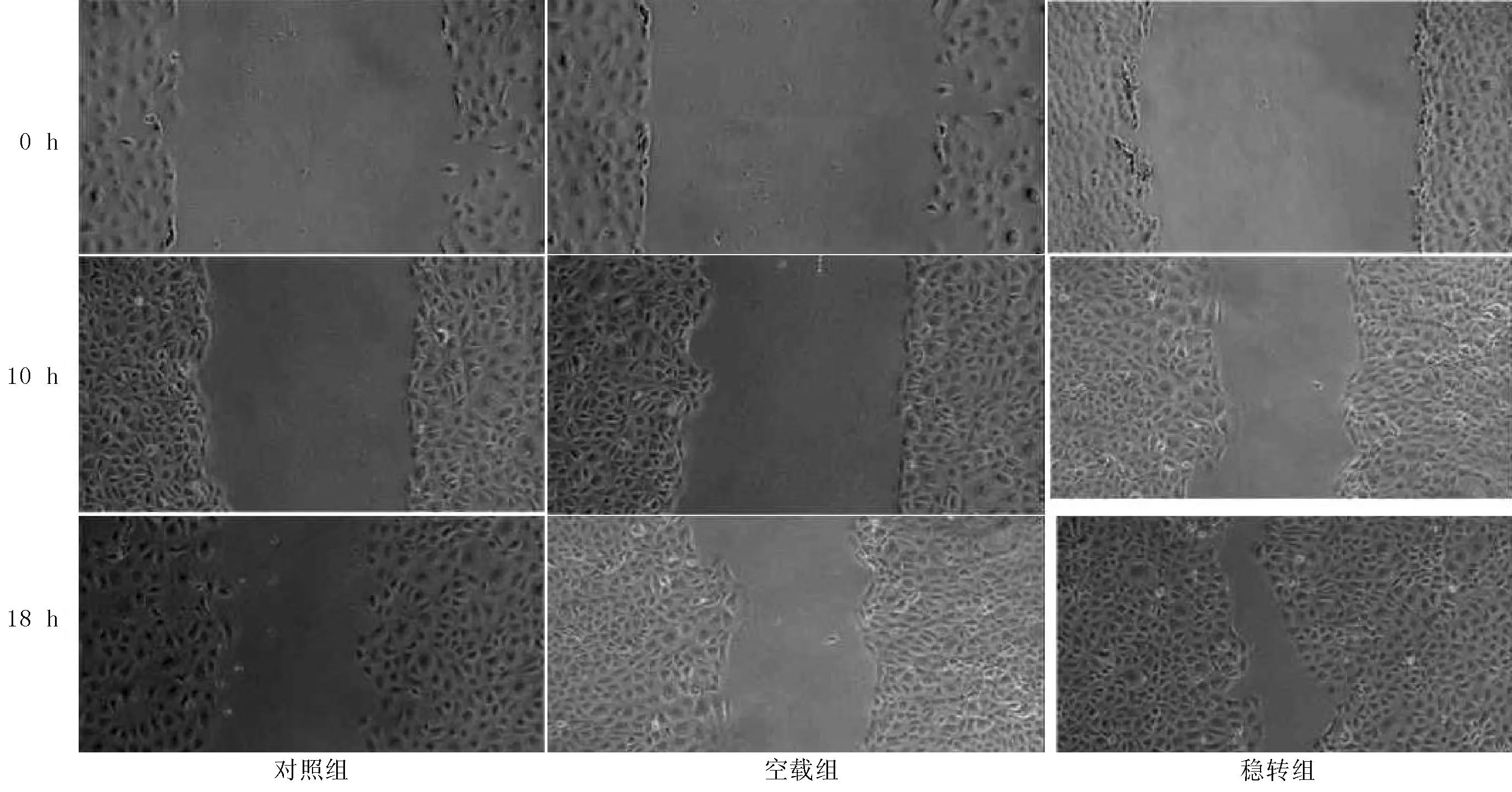
图4 3组0 h、10 h、18 h细胞迁移率比较
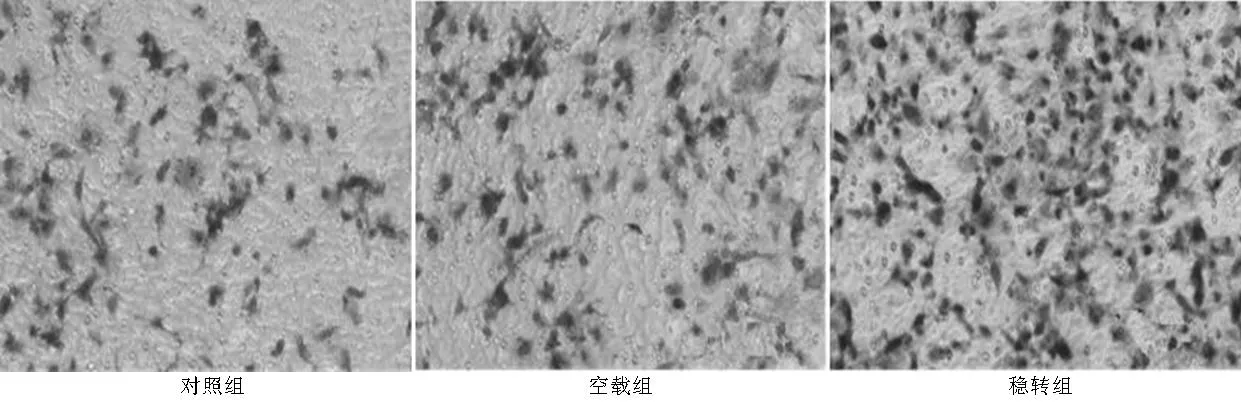
图5 3组细胞侵袭能力比较(×200)
本实验应用脂质体介导转染法将PAX2质粒转染入大鼠肾小管上皮细胞,G418是目前广泛应用的稳定转染筛选试剂[10],本实验应用该试剂进行了大鼠肾小管上皮细胞筛选,获得了稳定转染PAX2基因的肾小管上皮细胞系。通过荧光显微镜观察及RT-PCR鉴定,稳定转染PAX2组荧光表达明显增加,PAX2 mRNA表达也明显增加,其表达较对照组及空载组差异有统计学意义,证明稳定转染细胞系构建成功。同时也表明脂质体介导转染法可以成功进行基因的稳定表达转染。
细胞迁移实验是通过细胞受伤后的自动修复能力来评估,如单层细胞在受伤24 h内,其只靠细胞移动来愈合修复,并不会出现反应性修复增生[11],故本实验应用细胞划痕实验评估细胞迁移运动能力。结果表明稳转组细胞迁移速度较空载组及对照组明显增快,表明过表达PAX2可明显增加肾小管上皮细胞迁移能力。本试验通过目前较为常用的Transwell实验探讨细胞侵袭能力,细胞在含有Matrigel的聚碳酸酯微孔中穿过,是在模拟体内细胞穿过细胞外基质的过程,可以反映细胞的侵袭能力[12],本研究结果表明稳转组穿透的细胞数较空载组及对照组明显增加,说明PAX2可明显增加细胞侵袭能力。
本文价值:
本研究首次通过构建PAX2稳转细胞系探讨其生物学作用,发现PAX2转染可以增加肾小管上皮细胞的迁移及侵袭能力,这可能是PAX2基因诱导肾小管上皮细胞转分化的重要机制,为临床疾病的治疗提供实验依据。
参考文献
[1]Huang B,Pi L,Chen C,et al.WT1 and Pax2 re-expression is required for epithelial-mesenchymal transition in 5/6 nephrectomized rats and cultured kidney tubular epithelial cells[J].Cells Tissues Organs,2012,195(4):296-312.
[2]Zhou TB,Qin YH,Lei FY,et al.Association of PAX2 with cell apoptosis in unilateral ureteral obstruction rats[J].Ren Fail,2012,34(2):194-202.
[3]Li L,Wu Y,Zhang W.PAX2 re-expression in renal tubular epithelial cells and correlation with renal interstitial fibrosis of rats with obstructive nephropathy[J].Ren Fail,2010,32(5):603-611.
[4]Cohen T,Loutochin O,Amin M,et al.PAX2 is reactivated in urinary tract obstruction and partially protects collecting duct cells from programmed cell death[J].Am J Physiol Renal Physiol,2007,292(4):F1267-1273.
[5]Yi ZW,Zhu CP,Dang XQ,et al.PAX2 expression of pathological kidney in children with nephrotic syndrome and acute glomerulonephritis[J].Journal of Clinical Research,2005,22(11):1526-1530.(in Chinese)
易著文,朱翠平,党西强,等.肾病综合征和急性肾炎患儿肾组织PAX2的表达及临床意义[J].医学临床研究,2005,22(11):1526-1530.
[6]Geng WM,Yi ZW,He XJ,et al.Function of PAX2 in tubular epithelium transdifferentiation[J].Journal of Clinical Pediatrics,2007,25(4):284-287.(in Chinese)
耿文茂,易著文,何小解,等.PAX2在肾小管上皮细胞转分化中的作用[J].临床儿科杂志,2007,25(4):284-287.
[7]Pi L,Jiang T,Ouyang J,et al.Role of PAX2 gene in renal tubular epithelial cell transdifferentiation[J].Central China Medical Journal,2007,31(1):7-10.(in Chinese)
皮蕾,姜傥,欧阳涓,等.PAX2基因在肾小管上皮细胞转分化中的作用[J].华中医学杂志,2007,31(1):7-10.
[8]Li L,Wu Y,Yang Y.Paired box 2 induces epithelial-mesenchymal transition in normal renal tubular epithelial cells of rats[J].Mol Med Rep,2013,7(5):1549-1554.
[9]Rastaldi MP.Epithelial-mesenchymal transition and its implications for the development of renal tubulointerstitial fibrosis[J].J Nephrol,2006,19(4):407-412.
[10]Liu Y.Epithelial to mesenchymal transition in renal fibrogenesis:pathologic significance,molecular mechanism,and therapeutic intervention[J].J Am Soc Nephrol,2004,15(1):1-12.
[11]Rastaldi MP,Ferrario F,Giardino L,et al.Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies[J].Kidney Int,2002,62(1):137-146.
[12]Yang J,Liu Y.Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis[J].Am J Pathol,2011,159(4):1465-1475.
(本文编辑:贾萌萌)

