GLP-1受体激动剂呈葡萄糖依赖性刺激胰岛β细胞胰岛素分泌的机制
刘艳 田秀标 韩颖
·小论坛·
GLP-1受体激动剂呈葡萄糖依赖性刺激胰岛β细胞胰岛素分泌的机制
刘艳 田秀标 韩颖
2型糖尿病的主要治疗目标是控制高血糖,然而传统的治疗药物如胰岛素和磺脲类药物在降低血糖的同时也可以出现低血糖。因此,应在一定的血糖范围内自我调整用药量,避免出现低血糖。低血糖会造成身心障碍,同时可引起心血管事件。因此临床用药应最大限度的降低低血糖风险。胰高血糖素样肽-1受体激动剂呈葡萄糖依赖性的刺激胰岛素分泌,在胰岛素剂量很小或者缺乏,以及血糖浓度不高时,胰高血糖素样肽-1受体激动剂则不会刺激或很少刺激胰岛素分泌。
β细胞;抗糖尿病药物; GLP-1;胰岛素分泌;2型糖尿病
胰岛素和胰高血糖素共同维持血糖的稳定。外源性胰岛素或磺脲类药物治疗,虽然可降低高血糖,但是达到理想的血糖水平后会继续发挥降糖作用,因此会增加低血糖风险[1]。中、重度低血糖可降低患者生活质量[2]。同时,低血糖也与QT间期延长、心律失常和其他心血管不良事件有关[3-4]。因此,对于糖尿病患者来说,降低低血糖的风险非常关键。胰高血糖素样肽(GLP)-1是由肠道L细胞分泌的肠促胰素。与GLP-1受体(GLP-1R) 结合后通过葡萄糖依赖方式诱导胰岛素的分泌。 研究显示,GLP-1R激动剂改善胰岛素分泌的能力取决于血糖水平。当血糖水平降至正常时,GLP-1R激动剂也不再发挥作用。本文对GLP-1R激动剂呈葡萄糖依赖性促进胰岛素分泌的机制进行综述
1 GLP-1R激动剂呈葡萄糖依赖性的促进胰岛素分泌
一项大鼠胰岛素瘤细胞株的体外实验证实,GLP-1R激动剂促进胰岛素分泌的作用是呈葡萄糖依赖性的。GLP-1 (10 nmol/L)诱导的胰岛素分泌量和单独10 mmol/L葡萄糖环境下所诱发的胰岛素最大分泌量较基础水平的胰岛素分泌量分别增加1.5倍和2.5倍,而当同时给予10 mmol/L葡萄糖和GLP-1 (10 nmol/L)所诱发的最大胰岛素分泌量较基线水平增加了6倍[5]。同样,在大鼠胰腺灌注实验中,基线葡萄糖浓度(2.8 mmol/L)时GLP-1 (25 nmol/L)介导的胰岛素分泌量仅轻度增加,当葡萄糖浓度增加到5 mmol/L时,GLP-1介导的胰岛素分泌量较单独的5 mmol/L葡萄糖所诱导的胰岛素分泌量明显增加[6]。另有研究显示,给空腹的健康受试者静脉注射药理浓度的GLP-1(7-36 酰胺),不会引起低血糖[7]。表明GLP-1的促胰岛素分泌作用是葡萄糖依赖性的,并且需要一定浓度的葡萄糖才能发挥作用。
与天然的GLP-1相似,GLP-1R激动剂(如exendin-4)促进胰岛素分泌的作用也需要一定水平的葡萄糖。给健康空腹的受试者分别连续静脉输注艾塞那肽或安慰剂,与安慰剂组相比,在正糖钳夹试验中(5.0 mmol/L),艾塞那肽组胰岛素分泌更多。在同样的受试者中,当血浆葡萄糖浓度降低至低血糖水平(4.0 mmol/L)时,艾塞那肽组的胰岛素分泌量迅速下降到安慰剂组水平[8]。临床中,艾塞那肽治疗引起低血糖的发生率很低[9-10]。同样,艾塞那肽或利拉鲁肽单药治疗或与口服降糖药物联合治疗,在降低糖化血红蛋白之外,重度或轻度低血糖的发生率与安慰剂相比,差异无统计学意义[11-12]。甘精胰岛素和格列美脲与GLP-1R激动剂相比,低血糖的发生率均增加。 另外,磺脲类药物(如格列美脲)可以影响GLP-1R激动剂的葡萄糖依赖性,因此GLP-1R激动剂联用磺脲类药物时低血糖发生率增加[13-16]。
2 葡萄糖诱导的胰岛素分泌
胰岛素主要在致密核心囊泡(最终变成分泌颗粒)中被储存和转运。胞吐过程包括修饰和分泌颗粒融合于细胞膜。该过程由可溶性的NSF附着蛋白受体复合蛋白调节。囊泡膜上的NSF附着蛋白受体即小突触泡蛋白与细胞膜上的NSF附着蛋白受体,突触融合蛋白-1和SNAP25结合,并在细胞膜附近形成一个稳定的复合物,从而利于膜的融合定位,促进胰岛素从胰岛β细胞的分泌[17]。
胰岛素分泌的速率主要取决于含胰岛素颗粒的数量和分泌能力。大部分胰岛素颗粒(95%~99%)存在于储存池,一小部分(1%~5%)存在于待释放池,后者离细胞膜更近[18]。待释放池可进一步分化为立即释放池。立即释放池与电压依赖性钙离子通道(VDCC) 结合后镶嵌在细胞膜上,当VDCC开放,Ca2+内流时迅速准备释放胰岛素。待释放池内容物的释放主要负责胰岛素第一时相的分泌,而相对缓慢的第二时相则反映了待释放池的再填充即颗粒从储存池向待释放池的移动和装填过程。研究显示,胰岛素颗粒的酸化有助于储存池的颗粒移动到待释放池,而颗粒的低pH值可能诱导SNARE蛋白的结构变化,从而促进膜的融合及定位[19]。
3 GLP-1R信号转导与葡萄糖介导的胰岛素分泌
GLP-1R 是一个有7次跨膜区域的G蛋白耦联受体。GLP-1R激活细胞膜结合的腺苷酸环化酶,从而迅速产生cAMP。cAMP的下游效应器分别为蛋白激酶A (PKA)和cAMP 调节的鸟嘌呤核苷酸交换因子(cAMP-GEF)——Epac。研究显示,PKA和Epac在GLP-1介导的胰岛素释放过程中起关键作用[20-22]。
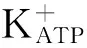
研究显示,肌醇三磷酸受体和兰逆碱受体可控制Ca2+从胞内释放,这些受体的拮抗剂可抑制钙诱导的钙释放[26]。肌醇三磷酸受体的调节性区域包括Ca2+的结合位点,依赖于游离 Ca2+的浓度,可以控制通道的活动。兰逆碱受体同样受Ca2+调节。GLP-1R激动剂通过Ca2+依赖的方式促进钙诱导的钙释放,该过程需要细胞内游离Ca2+的浓度迅速增加,从而调节Ca2+从胞内释放[27]。
GLP-1R活化PKA后不仅促进胞吐颗粒中含有的胰岛素分泌,而且可以调节待释放池的重填,而这两个过程对于第二相胰岛素分泌至关重要。采用穿孔膜片钳全细胞记录法可以让小鼠β细胞膜产生强烈而重复的去极化,从而诱导Ca2+内流,促进胞吐作用。而用forskolin持续增加cAMP浓度,使得Ca2+内流,细胞去极化,通过这种方式产生的胞吐作用持续的时间显著延长。提示cAMP可促进囊泡从储存池流动到待释放池。应用PKA抑制剂仅可抑制部分胞吐作用, 表明cAMP促进胞吐作用是通过依赖和不依赖PKA两种途径来进行的。进一步的研究证实,依赖PKA的cAMP的调节作用是Epac依赖性的。给小鼠使用选择性的Epac激动剂(8CPT-2Me-cAMP),结果小鼠β细胞去极化,胞吐作用明显增加。该效应是在胞吐作用的早期,提示Epac可能增加了待释放池的大小,从而可以更迅速的应答Ca2+内流[28]。Epac和PKA的结合效应与GLP-1R活化过程时一、二相胰岛素分泌增加一致。除促进已经储存的胰岛素释放,GLP-1也可诱导胰岛素基因的转录[29]。
总之,GLP-1R激动剂在血糖升高时促进胰岛素的分泌,当血糖降至正常水平以下时又能停止促泌作用。因此,其除了更好的降低糖化血红蛋白外,低血糖的发生率显著降低。然而GLP-1R激动剂呈葡萄糖依赖性的降糖机制还有待进一步研究。
[1] Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)[J].Diabetes Care,2012,35(6):1577-1596.
[2] Pettersson B, Rosenqvist U, Deleskog A, et al. Self-reported experience of hypoglycemia among adults with type 2 diabetes mellitus (Exhype)[J]. Diabetes Res Clin Pract,2011,92(1):19-25.
[3] Johnston SS, Conner C, Aagren M, et al. Evidence linking hypoglycemic events to an increased risk of acute cardiovascular events in patients with type 2 diabetes[J].Diabetes Care,2011,34:1164-1170.
[4] Yakubovich N, Gerstein HC. Serious cardiovascular outcomes in diabetes: the role of hypoglycemia[J].Circulation,2011,123(3):342-348.
[5] Montrose-Rafizadeh C, Egan JM, Roth J. Incretin hormones regulate glucose-dependent insulin secretion in RIN 1046-38 cells: mechanisms of action[J].Endocrinology,1994,135(2):589-594.
[6] Göke R, Wagner B, Fehmann HC,et al. Glucose-dependency of the insulin stimulatory effect of glucagon-like peptide-1 (7-36) amide on the rat pancreas[J].Res Exp Med (Berl),1993,193(2):97-103.
[7] Qualmann C, Nauck MA, Holst JJ,et al. Insulinotropic actions of intravenous glucagon-like peptide-1 (GLP-1) [7-36 amide] in the fasting state in healthy subjects[J].Acta Diabetol,1995,32(1):13-16.
[8] Degn KB, Brock B, Juhl CB, et al. Effect of intravenous infusion of exenatide (synthetic exendin-4) on glucose-dependent insulin secretion and counterregulation during hypoglycemia[J]. Diabetes,2004,53(9):2397-2403.
[9] Bergenstal RM, Wysham C, MacConell L, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial[J].Lancet,2010,376(9739):431-439.
[10] Russell-Jones D, Cuddihy RM, Hanefeld M, et al. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind study[J].Diabetes Care, 2012,35(2):252-258.
[11] Apovian CM, Bergenstal RM, Cuddihy RM, et al. Effects of exenatide combined with lifestyle modification in patients with type 2 diabetes[J]. Am J Med,2010,123(5):468.e9-468.e17.
[12] Liutkus J, Rosas Guzman J, Norwood P, et al. A placebo-controlled trial of exenatide twice-daily added to thiazolidinediones alone or in combination with metformin[J].Diabetes Obes Metab,2010,12(12):1058-1065.
[13] Blevins T, Pullman J, Malloy J, et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes[J]. Clin Endocrinol Metab,2011,96(5):1301-1310.
[14] Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study[J].Lancet,2008,372(9645):1240-1250.
[15] Pencek R, Brunell SC, Li Y,et al.Use of concomitant glucose-lowering therapies and associated treatment results observed in clinical trials of exenatide twice-daily[J].Endocr Pract,2012,18(2):227-237.
[16] Blevins T, Han J, Nicewarner D, et al. Exenatide is non-inferior to insulin in reducing HbA1c: an integrated analysis of 1423 patients with type 2 diabetes[J].Postgrad Med,2010,122(3):118-128.
[17] Rorsman P, Renström E. Insulin granule dynamics in pancreatic β cells[J].Diabetologia,2003,46(8):1029-1045.
[18] Rorsman P, Eliasson L, Renström E, et al. The cell physiology of biphasic insulin secretion[J].News Physiol Sci,2000,15(2):72-77.
[19] Barg S, Eliasson L, Renstr m E, et al. A subset of 50 secretory granules in close contact with L-type Ca2+channels accounts for first-phase insulin secretion in mouse β cells[J].Diabetes,2002,51(Suppl 1):S74-S82.
[20] Chepurny OG, Leech CA, Kelley GG,et al. Enhanced Rap1 activation and insulin secretagogue properties of an acetoxymethyl ester of an Epac-selective cyclic AMP analog in rat INS-1 cells: studies with 8-pCPT-2′-O-Me-cAMP-AM[J].Biol Chem,2009,284(16):10728-10736.
[21] Kelley GG, Chepurny OG, Schwede F, et al. Glucose-dependent potentiation of mouse islet insulin secretion by Epac activator 8-pCPT-2'-O-Me-cAMP-AM[J].Islets,2009,1(3):260-265.
[22] Seino S, Takahashi H, Fujimoto W, et al. Roles of cAMP signalling in insulin granule exocytosis[J].Diabetes Obes Metab,2009,11(Supp14):180-188.
[23] Kang G, Leech CA, Chepurny OG, et al. Role of the cAMP sensor Epac as a determinant of KATPchannel ATP sensitivity in human pancreatic β-cells and rat INS-1 cells[J].J Physiol,2008,586(5):1307-1319.
[24] MacDonald PE, Wheeler MB. Voltage-dependent K+channels in pancreatic β cells: role, regulation and potential as therapeutic targets[J]. Diabetologia,2003, 46(8): 1046-1062.
[25] Suga S, Kanno T, Nakano K, et al. GLP-1(7-36)amide augments Ca2+current through L-type Ca2+channel of rat pancreatic beta-cell in a cAMP-dependent manner[J].Diabetes,1997,46(11):1755-1760.
[26] Kang G, Chepurny OG, Rindler MJ, et al. A cAMP and Ca2+coincidence detector in support of Ca2+-induced Ca2+release in mouse pancreatic β cells[J]. J Physiol,2005, 566: 173-188.
[27] Kang G, Holz GG. Ampli cation of exocytosis by Ca2+-induced Ca2+release in INS-1 pancreatic β cells[J].J Physiol,2003,546 (Pt 1): 175-189.
[28] Eliasson L, Ma X, Renström E, et al. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic β-cells[J].J Gen Physiol,2003, 121(3): 181-197.
[29] Kemp DM, Habener JF. Synergistic effect of dimethyl sulfoxide on glucagon-like peptide 1 (GLP-1)-stimulated insulin secretion and gene transcription in INS-1 cells: characterization and implications[J].Biochem Pharmacol,2002,64(4):689-697.
MechanismofGLP-1receptoragonistsinthestimulationofinsulinsecretionofisletβcellinaglucose-dependentmanner
LiuYan*,TianXiubiao,HanYing.
*GraduateSchoolofTianjinMedicalUniversity,Tianjin300070,China
The aim to treat type 2 diabetes is to control the high blood glucose. However, the traditional drugs such as insulin and sulfonylureas can not only lower the blood glucose, but also cause hypoglycemia.Therefore, the drug dosage should be adjusted frequently in order to maintain the blood glucose in a certain range and to avoid hypoglycemia. Hypoglycemia can cause physical and mental disorders, as well as cardiovascular events. Therefore, great efforts should be made in clinical medication to reduce the risk of hypoglycemia. Glucagon-like peptide -1 receptor agonists can stimulate the secretion of insulin in a blood glucose-dependent manner. When the insulin dose is very low or deficient, or the concentration of blood glucose is not high, glucagon like peptide-1 receptor agonists will not or rarely stimulate insulin secretion.
β cells;Anti diabetic drugs;GLP-1;Insulin secretion;Type 2 diabetes mellitus
(IntJEndocrinolMetab,2015,35:66-69)
10.3760/cma.j.issn.1673-4157.2015.01.017
300070 天津医科大学研究生院(刘艳);300280 天津海滨人民医院内分泌科(刘艳,田秀标, 韩颖)
2014-10-10)

