In vitro–in vivo studies of the quantitative effect of calcium, multivitamins and milk on single dose cipro fl oxacin bioavailability☆
Bishkhi Dey,Prksh Ktkm,Fthi H.Assleh,Bu Ro Chndu, Shnt Kumri Adiki,Anlv Mitr,*
aSchool of Medical Science and Technology,IIT Kharagpur,Kharagpur 721302,India
bFaculty of Pharmacy,University of Zawia,Az Zawiyah,Libya
cDepartment of Pharmaceutical Analysis,Nirmala College of Pharmacy,Guntur,India
Original Article
In vitro–in vivo studies of the quantitative effect of calcium, multivitamins and milk on single dose cipro fl oxacin bioavailability☆
Baishakhi Deya,Prakash Katakamb,Fathi H.Assalehb,Babu Rao Chandub, Shanta Kumari Adikic,Analava Mitraa,*
aSchool of Medical Science and Technology,IIT Kharagpur,Kharagpur 721302,India
bFaculty of Pharmacy,University of Zawia,Az Zawiyah,Libya
cDepartment of Pharmaceutical Analysis,Nirmala College of Pharmacy,Guntur,India
A R T I c L E I N F o
Article history:
16 February 2015
Accepted 26 February 2015
Available online 10 March 2015
Cipro fl oxacin Analytical methodology Bioavailability Bio-waiver Dissolution study
Cipro fl oxacin,commonly used in India as an anti-microbial for prolonged use in chronic and non-speci fi c indications,may affect the bioavailability of the drug.The drug prescribed is commonly taken with multivitamins,calcium and milk.A simple and reliable analytical methodology obtaining a correlation with in vivo urinary excretion studies using UV and HPLC and in vitro dissolution studies(IVIVC)has shown a signi fi cant increase in elimination rate of cipro fl oxacin co-administered with multivitamins, calcium and milk.Appreciable IVIVC results proved that dissolution studies could serve as an alternative to in vivo bioavailability and also support bio-waivers.
©2015 Xi'an Jiaotong University.Production and hosting by Elsevier B.V.All rights reserved.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Development of antimicrobial resistance is a challenging problem in world health scenario,despite the increasing demand for antimicrobials in global pharmaceutical market worths a current value of about$66.5 billions[1].However,antibiotics are one of the most widely used and frequently misused drugs.Due to wide predominance of infectious diseases and emergence of resistant strains,there is an increased inclination among clinicians to prefer newer-generation antibiotics as one of the fi rst-line therapeutic regimens.However,prolonged antibiotic therapy may give rise to some side effects like anemia,dyspepsia,hyperacidity,gastritis, hepatotoxicity and nephrotoxicity,which necessitate co-medications.Such co-medications include common antacids or multivitamins(MVs)which are either prescribed by physicians or selfadvised and that may adversely affect the bioavailability of the antibiotic prescribed.Thus,the anti-microbial effect of the drug is compromised,leading to prolonged consumption of the antibiotic and development of antimicrobial resistance against that particular antibiotic[2–4].
Fluoroquinolones administered orally have been a preferred choice for clinicians in treatment of grave infections for decades. Cipro fl oxacin(CPFX)is a fl uoroquinolone carboxylic acid derivative and broad-spectrum antibiotic active against Gram-positive and Gram-negative organisms.Pharmacokinetically,CPFX undergoes renal and hepatic bio-degradative pathways.After 24 h of administration,approximately 50%of oral dosage or 80%of intravenous dosage appears in the urine either as parent compounds or as metabolites.Renal excretion of CPFX is nearly thrice the glomerular fi ltration rate,highlighting the role of tubular secretion and anionic transport mechanism in the elimination of this carboxy fl uoroquinolone.As a popular oral antibiotic with rapid onset of action,it is used in treatment of numerous infectious diseases af fl icting the skin,bones,gastrointestinal tract(GI-tract),genitourinary tract,and meninges.Mechanistically,CPFX acts by effective inhibition of DNA gyrase preventing DNA replication in susceptible bacteria[5].Patients under prolonged CPFX therapy are reported to suffer from hyperacidity,nausea,vomiting,gastritis,pain in the abdomen,and anemia and hence they consume antacids often without medical supervision.Calcium or magnesium containing antacids is more popular among the consumers than the H2antagonists or proton pump inhibitors.Calcium carbonate tablets or supplements are widely consumed by patients with osteoporosis or bone-related disorders for therapeutic purpose.Similar is the case with intake of calcium-rich foods like milkor yogurt providing dietary supplementation of osteo-calcium. Anemia is another side effect with the sustained use of CPFX and the preventive measure goes to prescribing MVs.Sometimes, secondary disease conditions also necessitate the rational use of MVs along with CPFX.MVs affect the bioavailability of the drug, leading to further research to make it evidence based[2–8].Indians are mainly lacto-vegetarian and use milk as a culturally acceptable measure for various conditions of GI tract disorders and anemia.
The current research work aims at detecting the quantitative effect of calcium(Ca),MVs and milk on the elimination of singledose CPFX in the urine of human volunteers by UV and HPLC. Further research will be extended to see CPFX interactions with Ca, milk and MVs by in vitro dissolution studies and establish in vitro–in vivo correlation(IVIVC)which is a predictive mathematical treatment describing the relationship between an in vitro property of a dosage form(e.g.extent of drug release)and a relevant in vivo response(e.g.amount of drug absorbed)[9–11].In cipro fl oxacin assessment,urinary samples were preferred to blood samples considering volunteer's compliance,convenient methodology, concentration of drugs in the urine being higher than in the blood, detection that can be made by simple analytical methods of UV, lack of protein in the urine of a healthy volunteer obviating the need for denaturation step,and no extra sample pretreatment(the only caution is to look after urine dilutions)[12–16].A novel applicational aspect of the current research was to establish IVIVC using dissolution test as a surrogate for in vivo CPFX interactions and bioavailability studies,which can waive the need for expensive human trials and hence is a suitable substitute for bioequivalence studies[9–11,15,16].
2.Materials and methods
2.1.Chemicals and reagents
All solvents used were of HPLC grade and reagents were of analyticalgrade.HPLC grade water was used.Reagents as speci fi ed like phosphoric acid,triethylamine and acetonitrile were purchased from Merck and Sigma(Mumbai,India).CPFX was a gift sample from M/s East India Pharmaceuticals Ltd.,Kolkata and Lome fl oxacin(internal standard,IS)from Macleods(Mumbai,India).CPFX fi lm coated tablets(500 mg),calcium tablets(500 mg) and multivitamin syrup(Polybion®)were purchased from the local market.Packed fresh cow milk was obtained from local dairy.Each 5 mL of Polybion syrup contained vitamin B1,2 mg;vitamin B2, 2.5 mg;vitamin B6,0.75 mg;nicotinamide,15 mg;pantothenyl alcohol,3 mg;and vitamin-B12,2 mg.The syrup speci fi cation of MV content was 16.03 for 100 mL,34.28 for 250 mL,and 52.27 for 400 mL.
2.2.Instrumentation
UV spectrophotometric determinations were done in Thermo Scienti fi c(Mumbai,India).HPLC analyses were carried out in Shimadzu LC 10 ADVp(Japan)with UV detector,and dissolution studies were performed using USP TypeІІdissolution test apparatus(Labindia DS 8000,Mumbai,India).
2.3.Volunteer selection and urine collection
The study protocol followed the ethical principles for biomedical research involving human volunteers(ICMR 2009).Healthy adult human volunteers(four males and females each)of 25–35 years were selected(lottery)from a pool of 25 volunteers residing locally with the body weight of 65–78 kg.Study time was winter season.Volunteers were clinically assessed by physicians of School of Medical Science and Technology,IIT Kharagpur.Anthropometric status,blood biochemistry and hematology values were within the normal limits in all these subjects.Smokers'alcoholics,those with enzyme de fi ciencies,particularly renal and hepatic insuf fi ciencies,children,pregnant and lactating women, non-cooperative and inconsistent individuals were excluded from the study.Written consents of eight volunteers were obtained after explaining them the research protocol in vernacular.The volunteers were asked to maintain standard dietary conditions (avoiding high caloric and junk food),de fi nite water(1 L)intake, normal physical activities,and avoidance of strenuous exercises and work overloads in the ensuing days of study.
The volunteers were given one CPFX tablet(500 mg)on the fi rst day;one CPFX tablet(500 mg)and vitamin B-complex (Polybion,15 mL)on the third day(out of four days of trial,a gap of one day was kept between each day of trial so as to ensure complete renal clearance of CPFX);one CPFX tablet(500 mg)and calcium tablet(Calbon 500,Saga Lab;calcium carbonate 1.25 g)on the fi fth day;and one CPFX tablet(500 mg)and 250 mL of cowmilk immediately after tablet intake on the seventh day and no other food or drink was ingested for the next 6 h on the seventh day.Midstream urine samples of the eight volunteers were collected in sterilized vials properly labeled on four days of drug intake maintaining the same time intervals(2,4,8,12,and 16 h), stored in refrigerator and protected from light.
In order to get reproducible urinary excretion studies,water loading was done by ingesting 400 mL of water to the volunteers after overnight fasting prior to the trial to promote diuresis and enable collection of suf fi cient urine samples[12].Before drug administration,the volunteers were instructed to ensure complete urination;1 h after water loading,the urine sample collected at this time was taken as the blank sample.The volunteers were instructed to take CPFX with 200 mL of water,followed by another 200 mL given at hour intervals for the next 4 h.The same procedures were used for the four days of drug intake of trials and the volunteers were instructed for complete emptying of the bladders to collect suf fi cient urine samples[12,15].
2.4.UV spectrophotometric analysis of urine samples
The UV analysis of the urine samples collected from the volunteers at different time intervals was carried out in the following manner.Urine samples(0.4 mL)collected at different time intervals were diluted with 20 mL of methanol:water(80:20,v/v).The samples were scanned using the same diluents(methanol:water, 80:20,v/v)as blank and the wavelength range was fi xed between 200 and 400 nm[16–21].
For the quantitative estimation of CPFX release under different study conditions,0.4 mL of urine was diluted to 20 mL.The volume of urine collected in each case was 50 mL.UV absorbance values of 0.4 mL of diluted urine samples at different time intervals(2,4,8,12,and 16 h)were plotted in the standard calibration curve of CPFX and intrapolated to get the corresponding concentration of CPFX.Thus,the amount of CPFX present in 50 mL of urine samples was calculated by unitary method.
2.5.HPLC analysis of the urine samples
HPLC analysis was carried out usinginertsil ODS C18column, (150mm×4.6mm,5μm)maintaining a column temperature of 20°C,injection volume of 10μL with a fl ow rate of 1.2 mL/min, and a run time of 10 min.The UV detector was set at a wavelength of 278 nm.
A 7 mL of triethylamine was diluted in 1000 mL of HPLC grade water and then adjusted to a pH of 3.0 with phosphoric acid,wellmixed and degassed.The mobile phase consisted of a fi ltered and degassed mixture of 0.025 M H3PO4(adjusted to a pH of 3.0 with triethylamine)and acetonitrile in the ratio of 87:13(v/v).
In HPLC studies,quantitative estimation of CPFX release under different study conditions was determined according to the following equation:

where Atis the average area of major peak in test chromatogram, Asis the average area of major peak in standard CPFX chromatogram,Wtis the wt of test taken in mg(to get from HPLC calibration curve),Wsis the wt of std CPFX taken in mg(11/30/60 mg),P is the purity of working standard(99.5%),Dtis the dilution of test samples(50 times diluted),Dsis the dilution of standard solution, and Awis the average wt of tablet(500 mg).
2.6.Dissolution studies
Absorption and hence bioavailability of the drug greatly depend on the dissolved state of the drug.Therefore,dissolution rate is a critical step in the performance of a drug and its dosage forms. This step of a drug is found to be affected by co-administered drugs and other food ingredients[9,10,14,22–26].
In vitro CPFX interaction with calcium,MVs and milk was studied in USP TypeІІdissolution test apparatus with certain modi fi cations of the reported literature[24,27–31].Brie fl y,dissolution study of CPFX tablets was carried out in 500 mL of dissolution media under simulated gastric and intestinal pH conditions(pH 1.2 and 6.8,respectively),thermostated at 37±5°C and operated at 100 rpm.5 mL of aliquots of dissolution media were withdrawn at predetermined time intervals(5,15,30,45,60,90 and 120 min) and their absorbance was measured at 276 nm in UV spectrophotometer.
For testing the action of calcium on the dissolution behavior of CPFX,calcium tablet was added to the dissolution mediumwith an appropriate concentration of CPFX at the start of the experiment while other conditions remained the same.Aliquots of the dissolution medium were withdrawn and CPFX concentrations were determined spectrophotometrically at 276 nm.
In vitro milk–CPFX interaction studies were conducted in 500 mL dissolution media,and other conditions remained the same as above.One FDA glass(250 mL)of low fat milk was added to the dissolution media with an appropriate concentration of CPFX at the start of the experiment;CPFX concentrations were determined spectrophotometrically at 276 nm.
In vitro MVs–CPFX interaction studies were conducted in 500 mL of dissolution media,and other conditions remained as before.A 55 mL of Polybion syrup was added to the dissolution media with an appropriate concentration of CPFX at the start of the experiment;CPFX concentrations were determined spectrophotometrically at 276 nm.In all the above in vitro dissolution studies,the aliquots withdrawn were not replenished with fresh equivalent amount of dissolution media.
Cumulative percentage of CPFX release pro fi les was characterized by the area under the dissolution curves(AUC)up to a time‘t’in simulated gastric and intestinal fl uids(SGF and SIF)under different study conditions.The AUC for each dissolution pro fi le was calculated by the trapezoidal rule[32–34].
2.7.Comparison of dissolution data
The dissolution pro fi les were further analyzed by difference factor(f1)and similarity factor(f2).Difference factor(f1)is the percentage difference between two curves at each point and is a measurement of the relative error between the two curves.The similarity factor(f2)is a logarithmic reciprocal square root transformation of the sum of squared error and is a measurement of the similarity in the percent dissolution between the two curves[35–37].The following equations were used to calculate f1 and f2 values:

where n is the number of points,Rtis the dissolution value of the reference product at time t,and Ttis the dissolution value for the test product at time t.For dissolution curves to be considered similar,f1 should be close to zero and f2 should be close to 100. Generally,f1 value ranges up to 15(0–15)and f2 values are greater than 50(50–100),which ensures equivalence between the two curves.
In this research,CPFX was used as the standard substance and CPFX+Ca,CPFX+MVs and CPFX+milk were used as the reference substances.Comparison was made for both in vitro SIF and SGF conditions using f2 values.The more the deviation from the standard value,the greater the accuracy of the experimental methodology.
2.8.IVIVC studies
IVIVC is a predictive mathematical treatment describing the relationship between in vitro properties of a dosage form(e.g. extent of drug release)and a relevant in vivo response(plasma drug concentration or amount of drug absorbed).IVIVC has drawn the attention and is worthy of consideration by pharmaceutical industries,academics and regulatory sectors.If in vitro tests can suitably re fl ect bioavailability data,it is helpful to waive bioequivalence studies on healthy volunteers.Among the three main levels(A,B and C)of IVIVC,level A(the highest grade)relates the entire in vitro dissolution curve to in vivo concentration pro fi le (in vivo absorption curve)so that in vitro dissolution can serve as a surrogate marker for in vivo drug actions study;level B of IVIVC utilizes Statistical Moments Analysis to compare the mean of any parameter of in vitro dissolution(e.g.mean dissolution time,MDTinvitro)with the mean of any in vivo parameter(e.g.mean residence time,MRTinvivo);level C of IVIVC establishes a single point correlation between an in vitro dissolution parameter(e.g.time to release 50%or 90%of the active drug,T50%or T90%)and corresponding in vivo parameter(e.g.Cmax,Tmaxor AUC)[9–11,32,38–41].
In this study,IVIVC was determined by plotting the percentage of CPFX released(CPFXrel)in vitro(from dissolution studies) against the cumulative percentage of CPFX excreted in urine for a suf fi cient period of time which is directly related to the total amount of CPFX absorbed.
3.Results
The mean(±SD)values of various parameters of the eight volunteers were determined as follows:age,27.3±4.16 years; weight,69.5±8.01 kg;height,170.3±6.45 cm;body surface area (BSA),1.7±6.95 m2;systolic blood pressure,123±0.82mmHg; and diastolic blood pressure,80±0.91 mmHg.Linearity details of the standard calibration curves of CPFX for UV,HPLC and dissolution studies are presented in Table 1.The quantitative elimination of CPFX at hourly intervals(2,4,8,12,and 16 h)was determined from the standard calibration curve of UV and HPLC studies and is shown in Figs.1–3.The peak areas(±SD)values ofCPFX eliminated at hourly intervals in the urine ofeight volunteers as detected in HPLC analysis are provided in Table 2.The pharmacokinetic parameters determined from the urinary excretion data obtained from UV and HPLC studies are summarized in Tables 3 and 4.The in vitro cumulative CPFX elimination in SGF and SIF under different study conditions is shown in Tables 5 and 6 and Suppl.Figs.1 and 2.Comparisons of dissolution pro fi les under different study conditions both in SGF and SIF were made based onf2 values.f2 values in SGF were 35.81(CPFX+Ca),29.77 (CPFX+milk)and 31.31(CPFX+MVs),and in SIF they were found to be 36.36(CPFX+Ca),31.2(CPFX+milk)and 32.16(CPFX+MVs). For IVIVIC studies,the correlation coef fi cient of CPFX release both in vitro(dissolution studies)and in vivo(urinary excretion studies) are presented in Table 7 and Suppl.Figs.3–6.

Table 1 Data of calibration curve of CPFX for UV,HPLC and dissolution studies.

Fig.1.Quantitative elimination of CPFX at hourly intervals under different study conditions(UV studies).

Fig.2.Quantitative elimination of CPFX at hourly intervals under different study conditions(HPLC studies).

Fig.3.Cumulative elimination of CPFX under different study conditions(HPLC studies).

Table 2 Peak area values of CPFX eliminated in the urine ofthe 8 volunteers in HPLC studies under different conditions.

Table 3 Pharmacokinetic parameters of urinary excretion data by UV.

Table 4 Pharmacokinetic parameters of urinary excretion data by HPLC.

Table 5 In vitro data of CPFX release in SGF(dissolution studies).
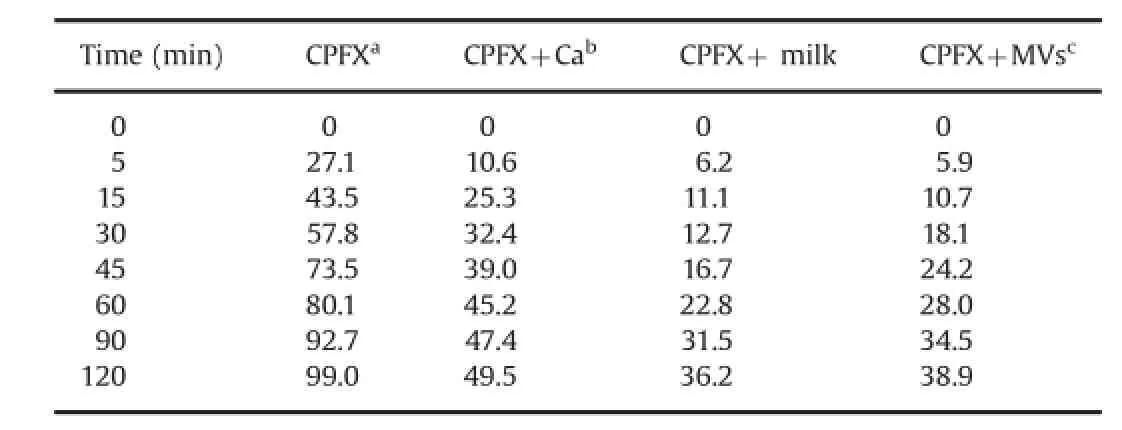
Table 6 In vitro data of CPFX release in SIF(dissolution studies).

Table 7Correlation coef fi cient(R2)values obtained from IVIVC data of CPFX release under different conditions.
4.Discussion
Our research data showed that elimination of CPFX(either cumulative release or at hourly intervals)increased signi fi cantly when co-administered with MVs,Ca-tablets and milk as evident by in vivo UV and HPLC urinary excretion studies and in vitro dissolution studies.Elimination of CPFX was markedly increased in the order of MVs>milk>calcium in contrast to CPFX release when administered alone.
Analyses of pharmacokinetic parameters showed that an increase in kavalues increased the Cmaxand vice versa.In contrast, Tmaxvalues were found longer with smaller kavalues and shorter with larger kavalues.However,kavalues did not show any in fl uence on the AUC.
The similarity factor,f2 values,ranged from 29.77 to 36.36, suggesting the accuracy of our in vitro experimental methodology [35–37].From the point of IVIVC studies,our research data showed R2values ranging from 0.651 to 0.999 under different study conditions,thus exhibiting a good correlation.Estimation of CPFX elimination under different conditions and determination of the IVIVC based on urinary excretion data were found to be simple, reliable and cost-effective.The simultaneous dissolution studies could serve as a bio-waiver for expensive human trials and hence this method could be a suitable substitute for bioequivalence studies.Dissolution studies can serve as a surrogate for in vivo bioavailability and support bio-waivers.
Prolonged antibiotic therapy causes anemia,gastric irritation or acidity;thus,antacids and multi-vitamin formulations are the commonly co-administered drugs with antibiotics[1,5,8].Calcium either in the form of carbonate or chloride is a constituent of many marketed antacid formulations[2,3,42,43].Multivitamin formulations,especially with mineral contents,are found to reduce the bioavailability of fl uoroquinolones[4,5,8].It is a common practice of many patients to intake drugs with drinks like milk, yogurt or juices other than water.Moreover,calcium-enriched foods and dairy products are often included in the breakfast menu of patients undergoing treatment[6,7,24].Our research fi ndings showed that the bioavailability of CPFX was signi fi cantly reduced by co-administration of MVs followed by that of milk and calcium. Calcium or its salts chelates or binds with CPFX,preventing its absorption and thereby reducing its bioavailability.However,milk was found to inhibit absorption of CPFX more than calcium alone mostly because of CPFX interactions with the milk matrix which decreased CPFX absorption by complexation with metal ions present in milk and by adsorption at the surface of milk protein molecules mostly casein(forms~84%of total milk protein),thus providing additional inhibitory effect and preventing absorption of CPFX[24].Structurally,each CPFX molecule has one R-COOH group which readily interacts with Ca2+ions(also other poly cations)[41].The R–COOH group of CPFX becomes negatively charged,releasing H+ion,determined by its p Kavalue.The p Kafor carboxyl groups ranges between 1.8 and 2.4[12,13].Thus,a pH above this p Kawould result in a greater percentage of the carboxyl groups on CPFX being ionized.Depending on other mechanisms like intake of antacids containing metals like calcium,H2antagonists,proton pump inhibitors;the type of food ingested by the patient;and the pH of the stomach which mostly ranges from 1 to 6,it is likely that the p Kaof the carboxyl group will be exceeded [13,41].However,if the patient takes only CaCO3containing formulations with CPFX on an empty stomach,CaCO3can interact with gastric HCl to form CaCl2,CO2and H2O,thus increasing the gastric pH value.Therefore,the magnitude of change in gastric pH, whether it is from CaCO3itself or from combination of CaCO3and other medications,can in fl uence the amount of ionized carboxyl groups found on CPFX.Owing to such ionization,the exposed and negatively charged groups on CPFX bind with the positively charged Ca2+ions that are present in the medication or supplement administered,referred to as chelation.The degree or signi fi cance of this interaction is,however,dependent on the time of exposure to one another and the pH of the environment at that time[2,13,24,26,41–44].A 2 h separation of the calcium containing products and the CPFX can avoid this interaction.The same logic can be applied for MVs necessitating a gap of about 4 h between CPFX and intake of MV.If the co-administration of other drugs or food ingested decreases absorption or bioavailability of CPFX,itpotentially increases the risk of infection treatment failure and a drug producing sub-therapeutic actions for a long time leads to development of antimicrobial resistance against that particular antibiotic.Thus,the potency of a renowned antibiotic becomes greatly hindered due to drug–drug and drug–food interactions.
5.Conclusions
Drug–food and drug–drug interactions are matters of concern since the therapeutic ef fi cacy and bioavailability of renowned drugs get greatly suppressed.Moreover,severe adverse drug reactions may also be precipitated.Our current research showed a signi fi cant reduction in the bioavailability of popular antibiotic CPFX on interactions with MVs,calcium tablets and milk.Thus,to prevent sub-therapeutic effects and protect against development of anti-microbial resistance,patients should consider taking CPFX and other Ca-containing medicaments at a lag period of 2 h and above.The same solution can be applicable to other dairy and milk products.For MVs,a lag period of at least 4 h is recommended.
The UV-HPLC analytical methodology followed the estimation of CPFX elimination under different study conditions was found to be simple,reliable and cost-effective.Determination of IVIVC based on urinary excretion data showed that dissolution studies can serve as a bio-waiver for expensive human trials and hence the developed methods can be suitable substitutes for bioequivalence studies.
Acknowledgments
The authors deeply acknowledge the instrument and laboratory facilities of Phytochemical Research and Development,Kolkata and Central Research Facilities of IIT Kharaghpur.Special thanks and acknowledgments go to the volunteers who participated in the trial.Also thanks to the companies that helped with the research and to all other people who made the research possible.
Appendix A.Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.jpha.2015.02.003.
References
[1]The Global Antibacterials Market:R&D Pipelines,Market Analysis and Competitive Landscape,Arrow head Publishers,2007,pp.1–126.
[2]R.W.Frost,K.C.Lasseter,A.J.Noe,et al.,Effects of aluminium hydroxide and calcium carbonate antacids on the bioavailability of cipro fl oxacin,Antimicrob. Agents Chemother.36(1992)830–832.
[3]J.Shimada,K.Shiba,T.Oguma,et al.,Effect of antacid on absorption of the quinolone lome fl oxacin,Antimicrob.Agents Chemother.36(1992)1219–1224.
[4]R.E.Polk,D.P.Healy,J.Sahai,et al.,Effectofferrous sulphate and multivitamins with zinc on absorption of cipro fl oxacin in normal volunteers,Antimicrob. Agents Chemother.33(1989)1841–1844.
[5]A.L.VanWert,C.Srimaroeng,D.H.Sweet,Organic anion transporter 3(Oat3/ Slc22a8)interacts with carboxy fl uoroquinolones and deletion increases systemic exposure to cipro fl oxacin,Mol.Pharmacol.74(2008)122–131.
[6]P.J.Neuvonen,K.T.Kivisto,P.Lehto,Interference of dairy products with the absorption of cipro fl oxacin,Clin.Pharmacol.Ther.50(1991)498–502.
[7]S.F.Aman,F.Hassan,B.S.Naqvi,et al.,Studies offood drug interactions,Pak.J. Pharm.Sci.23(2010)313–320.
[8]V.S.Srinivasan,Bioavailability of nutrients:a practical approach to in vitro demonstration of the availability of nutrients in multivitamin–mineral combination products,J.Nutr.131(2001)1349S–1350S.
[9]I.E.Shohin,G.V.Ramenskaya,A.Y.Savchenko,Developing in vitro–in vivo correlation for Trimetazidine,Indapamide and Cipro fl oxacin extended release solid oral dosage forms,Int.J.Pharm.Biosci.2(2011)P573–P580.
[10]M.D.Faiyazuddin,S.Ahmad,G.Mustafa,et al.,Bio-analytical approaches, bioavailability assessment,and bioequivalence studies for waiver drugs: in vivo and in vitro perspective,Clin.Res.Regul.Affair 27(2010)1–10.
[11]E.A.Bamigbola,Correlation of in vitro dissolution pro fi les with in vivo pharmacokinetic parameters of some commercial brands of aspirin tablets marketed in Nigeria,in:A.Noreddin(Ed.),Readings in Advanced Pharmacokinetics-Theory,Methods and Applications,InTech,2012,pp.251–266.
[12]S.Sadray,H.Tajerzedeh,A.Mohajer,et al.,Ef fi cacy of urine samples in bioavailability study of Ranitidine,DARU 11(2003)1–6.
[13]M.Kamberi,K.Tsutsumi,T.Kotegawa,et al.,In fl uences of urinary pH on cipro fl oxacin pharmacokinetics in humans and antimicrobialactivity in vitro versus those of spar fl oxacin,Antimicrob.Agents.Chemother.43(1999) 525–529.
[14]A.El-Kattan,M.Varma,Oral absorption,intestinal metabolism,and human oral bioavailability,in:J.Paxton(Ed.),Topics on Drug Metabolism,InTech, 2012,pp.1–34.
[15]M.E.Olivera,R.H.Manzo,H.E.Junginger,et al.,Biowaiver monographs for immediate release solid oral dosage forms:cipro fl oxacin hydrochloride, J.Pharm.Sci.100(2011)22–33.
[16]M.A.Noman,H.O.Kadi,High performance liquid chromatography assay with UV detection for the determination of cipro fl oxacin in plasma,Gr.J.Phys.Sci.2 (2012)20–26.
[17]M.Amini,M.Khanavi,A.Shafee,Simple high performance liquid chromatographic method for determination of cipro fl oxacin in human plasma,Iran. J.Pharm.Res.2(2004)99–101.
[18]M.Kamberi,K.Tsutsumi,T.Kotegawa,et al.,Determination of cipro fl oxacin in plasma and urine by HPLC with ultraviolet detection,Clin.Chem.44(1998) 1251–1255.
[19]N.M.Kassab,A.K.Singh,E.R.M.Kedor-Hackmam,et al.,Quantitative determination of cipro fl oxacin and nor fl oxacin in pharmaceutical preparations by high performance liquid chromatography,Brazil.J.Pharm.Sci.41(2005) 507–513.
[20]M.N.Qureshi,I.U.Rahman,G.A.Marwat,Comparative analysis of cipro fl oxacin in different pharmaceutical products by high performance liquid chromatograph,Sci.Tech.Dev.31(2012)69–73.
[21]N.Sultana,M.S.Arayne,F.Hussain,In vitro monitoring of cipro fl oxacin antacids interactions by UV and HPLC,Pak.J.Pharm.Sci.18(2005)23–31.
[22]K.U.Shah,G.M.Khan,Regulating drug release behavior and kinetics from matrix tablets based on fi ne particle sized ethyl cellulose ether derivatives: an in vitro and in vivo evaluation,Sci.World J.20(2011)1–8.
[23]P.Tangri,I.Dutt,Development and validation of dissolution procedures for cipro fl oxacin,Curr.Pharm.Res.2(2012)553–556.
[24]K.Papai,M.Budai,K.Ludanyi,et al.,In vitro food drug interaction study:which milk component has a decreasing effect on the bioavailability of cipro fl oxacin? J.Pharm.Biomed.Anal.52(2010)37–42.
[25]M.K.Khan,M.F.Khan,H.Khan,et al.,Bioavailability ofcipro fl oxacin tablets in humans and its correlation with the dissolution rates,Pak.J.Pharm.Sci.22 (2009)329–334.
[26]B.M.Lomaestro,G.R.Bailie,Effect of staggered dose of calcium on the bioavailability of cipro fl oxacin,Antimicrob.Agents Chemother.35(1991) 1004–1007.
[27]M.A.Kalam Azad,A.Ullah,A.H.M.Mahbub Latif,et al.,Bioequivalence and pharmacokinetic study of two oral formulations of cipro fl oxacin tablets in healthy male volunteers,J.Appl.Res.7(2007)150–157.
[28]T.S.Oishi,M.A.Haque,I.Dewan,et al.,Comparative in vitro dissolution study of some cipro fl oxacin generic tablets under bio-waiver conditions by RP-HPLC, Int.J.Pharm.Sci.Res.2(2011)3129–3135.
[29]M.S.Islam,T.Haque,R.Jahangir,et al.,In vitro release kinetic study of Cipro fl oxacin HCl from Methocel K15M CR,Methocel K4M CR,and Methocel K4M premium matrix tablets,Stamford J.Pharm.Sci.2(2009)37–43.
[30]U.Golla,B.K.Nalla,R.Talla,etal.,Formulation and in vitro evaluation ofgastroretentive drug delivery system ofcipro fl oxacin hydrochloride,D.Pharm.Sin.2 (2011)33–39.
[31]E.Akpabio,C.Jackson,C.Ugwu,et al.,Quality controland in vitro bioequivalence studies on four brands of cipro fl oxacin tablets commonly sold in Uvo Metropolis Nigeria,J.Chem.Pharm.Res.3(2011)734–741.
[32]K.C.Yeh,K.C.Kwan,A comparison of numerical integrating algorithms by trapezoidal,lag range,and Spline approximation,J.Pharmacokinet.Biopharm. 6(1978)79–98.
[33]H.Cheng,W.R.Gillespie,W.J.Jusko,Mean residence time concepts for nonlinear pharmacokinetic systems,Biopharm.Drug Dispos.15(1994)627–641. [34]E.M.Landaw,D.Katz,Comments on mean residence time determination,J. Pharmacokinet.Biopharm.13(1985)543–547.
[35]T.S.Oishi,I.Nimmi,S.M.A.Islam,Comparative in vitro bioequivalence analysis of some generic tablets of Atorvastatin,a BCS class IIcompound,Bangladesh Pharm.J.14(2011)61–66.
[36]M.C.Ma,R.P.Lin,J.P.Liu,Statistical evaluations of dissolution similarity,Statist. Sin.9(1999)1011–1027.
[37]E.Demirturk,L.Oner,Evaluation of in vitro dissolution pro fi le comparison methods of immediate release Gliclazide tablet formulations,J.Fac.Pharm.25 (2005)1–10.
[38]N.Watari,L.Z.Benet,Determination of mean input time,mean residence time, and steady state volume of distribution with multiple drug inputs,J.Pharmacokinet.Biopharm.17(1989)593–599.
[39]D.O.Chanter,The determination of mean residence time using statistical moments:is it correct?J.Pharmacokinet.Biopharm.13(1985)93–100.
[40]M.Weiss,The relevance of residence time theory to pharmacokinetics,Eur.J. Clin.Pharmacol.43(1992)571–579.
[41]H.Cheng,W.R.Gillespie,Volumes of distribution and mean residence timeof drugs with linear tissue distribution and binding and nonlinear protein binding,J.Pharmacokinet.Biopharm.24(1996)389–402.
[42]J.Sahai,D.P.Healy,J.Stotka,et al.,The in fl uence of chronic administration of calcium carbonate on the bioavailability of oralcipro fl oxacin,Br.J.Clin. Pharmacol.35(1993)302–304.
[43]L.Rambout,J.Sahai,K.Gallicano,et al.,Effect of bismuth subsalicylate on cipro fl oxacin bioavailability,Antimicrob.Agents Chemother.38(1994)2187–2190.
[44]Y.Mizuki,I.Fujiwara,T.Yamaguchi,Pharmacokinetic interactions related to the chemical structures of fl uoroquinolones,J.Antimicrob.Chemother.37 (Suppl.A)(1996)41–55.
5 December 2013
in revised form
☆Peer review under responsibility of Xi'an Jiaotong University.
.Tel.:+91 9475258298;fax:+91 32 22279970. E-mail address:analavamitra@gmail.com(A.Mitra).
 Journal of Pharmaceutical Analysis2015年6期
Journal of Pharmaceutical Analysis2015年6期
- Journal of Pharmaceutical Analysis的其它文章
- Development of cell metabolite analysis on micro fl uidic platform☆
- Thermal stability and hydration behavior of ritonavir sulfate:A vibrational spectroscopic approach☆
- Identi fi cation,synthesis and characterization of process related des fl uoro impurity of ezetimibe and HPLC method validations☆
- Quanti fi cation of tolvaptan in rabbit plasma by LC–MS/MS:Application to a pharmacokinetic study☆
- Antimicrobial and antiproliferative prospective of kosinostatin–a secondary metabolite isolated from Streptomyces sp.☆
- Optimization,validation and application of an assay for the activity of HMG-CoA reductase in vitro by LC–MS/MS☆
