Development of cell metabolite analysis on micro fl uidic platform☆
Luyao Lin,Jin-Ming Lin
Department of Chemistry,Beijing Key Laboratory of Microanalytical Methods and Instrumentation,Tsinghua University,Beijing 100084,China
Review Paper
Development of cell metabolite analysis on micro fl uidic platform☆
Luyao Lin,Jin-Ming Lin*
Department of Chemistry,Beijing Key Laboratory of Microanalytical Methods and Instrumentation,Tsinghua University,Beijing 100084,China
A R T I c L E I N F o
Article history:
25 September 2015
Accepted 28 September 2015
Available online 30 September 2015
Micro fl uidic Cell analysis Cellular metabolism Chip-mass spectrometry
Cell metabolite analysis is of great interest to analytical chemists and physiologists,with some metabolites having been identi fi ed as important indicators of major diseases such as cancer.A highthroughput and sensitive method for drug metabolite analysis will largely promote the drug discovery industry.The basic barrier ofmetabolite analysis comes from the interference ofcomplex components in cellbiologicalsystem and low abundance of target substances.As a powerfultoolin biosample analysis, micro fl uidic chip enhances the sensitivity and throughput by integrating multiple functional units into one chip.In this review,we discussed three criticalsteps ofestablishing functionalmicro fl uidic platform for cellular metabolism study.Cell in vitro culture model,on chip sample pretreatment,and microchip combined detectors were described in details and demonstrated by works in fi ve years.And a brief summary was given to discuss the advantages as well as challenges of applying microchip method in cell metabolite and biosample analysis.
©2015 Xi'an Jiaotong University.Production and hosting by Elsevier B.V.All rights reserved.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Contents
1.Introduction........................................................................................................337
2.Cell culture on chip..................................................................................................340
2.1.3D cell culture................................................................................................340
2.2.Cell co-culture................................................................................................340
2.3.Organ on chip................................................................................................341
3.Sample pretreatment on chip..........................................................................................341
3.1.Preconcentration methods......................................................................................342
3.2.Microseparation...............................................................................................342
4.Detection..........................................................................................................343
4.1.ESI.........................................................................................................344
4.2.Paper spray..................................................................................................345
4.3.Maldi.......................................................................................................345
5.Conclusions and perspectives..........................................................................................345
Acknowledgments.......................................................................................................346
Appendix A.Supplementary material.....................................................................................346
References.............................................................................................................346
1.Introduction
Studying cellular metabolites under various environmental stimuli such as drug conditioned culture and multiple cell types interaction,can provide meaningful results for drug discovery and a better understanding of important biological progress,especially those involved in the occurrence of major deceases[1,2]like cancer.Diagnosing methods de fi ning metabolic species as indicators for carcinoma have been wellestablished and applied in hospital.Also,rather than focusing on one single metabolite,the combinatorial study of several or even all metabolites in a certain time section,which is termed metabolomics,is increasingly attracting the interest of scientists[3,4].Following the popularity of genomics and proteomics,metabolomics may provide a newmethodology to interpret the cell biological state.However,there are several challenges remaining in metabolites pro fi ling.Trace amount metabolites secreted from cells often exist in a complex mixture of various components such as proteins,nucleic acids and other biomolecules.The detection of a metabolite requires ef ficient separation and preconcentration scheme,and a highly sensitive detector.Besides,time consumption,chemical reagents and cost have to be balanced to achieve practical usability.
In the industry of drug development,analytical methods for compound metabolic pathway probing in a manner of high throughput,rapidness and great sensitivity have always been in avid demand,given the decades of time,billions of money and intensive labor working spent in getting one drug registered in USFDA.Actually,among several different screening steps concerning compound structure stability and bioactivity in drug discovery,clinical trial causes the largest expense.In the fi nal step of clinical trial,most candidate compounds can be excluded by adverse effects or low ef fi cacy[5,6],thus giving a dead end to all the former testings and relevant investment.And currently,clinical trial cannot be replaced by cell culture or animal experiment, because of the uniqueness and complexity of human body.One solution to reduce the time span and cost is to reconstruct a comparable human body in vitro,at least at the organ level,which is the basic functional unit in drug absorption,distribution,metabolism and excretion(ADME).
Owing to the development of microfabrication technique,the design and miniaturization of fl ow channels can be easily and cheaply completed in laboratory,which enables the wide use of micro fl uidics.Micro fl uidics offers an alternative method to conventional bench top analytical process and exhibits surprising advantages.According to scaling laws,the decrease of reaction volume adversely affects the mass/heat transfer,which means enhancing the reactants exchanging by increasing the surface–volume ratio and shortening the diffusion distance.Therefore, chemical reactions can be more rapidly fi nished in a micro fl uidic reactor.In addition,the perfect matching between micro fl uidic channel dimension and cell size makes it a powerful tool for cell handling and culture,and applicable to researches such as cell positioning,sorting and circulating tumor cell(CTC)capture for cancer diagnosis.The precise control of cell culture microenvironment can be harnessed to mimic in vivo status.Also,the miniaturization largely reduces the reagent and sample consumption,and enables further integration of different functional modes into one chip.This integration of sample introduction or generation,pretreatment,and detection in a portable microchip is highly preferred in the analysis of analytes with low amount,to evade unwanted loss during off-line operation.The concept of lab on a chip(LOC)or micro-total analysis system(μTAS)was proposed to realize above goals,and has been developed with considerable complexity of integration for high-throughput,parallel and fully automated screening.
Therefore,micro fl uidic device is an ideal candidate in pro fi ling cellular metabolites[7–9]and drug screening[10–12]with the ability to cover cell culture,metabolite generation,separation and preconcentration as well as detection on one chip with the assistance from extra detectors like MS and fl uorescence microscopy.In this review,we will go through works concerning critical aspects about application of micro fl uidic device in cellular metabolites analysis in recent years.As mentioned above,on-chip analysis of metabolite can be roughly divided into three steps:cell culture model establishment and metabolites generation,sample pretreatment and detection(Fig.1)[13].Accordingly,our review will have three main sections to demonstrate the corresponding issues.

Fig.1.Micro fl uidic device for cellular metabolism analysis[13].
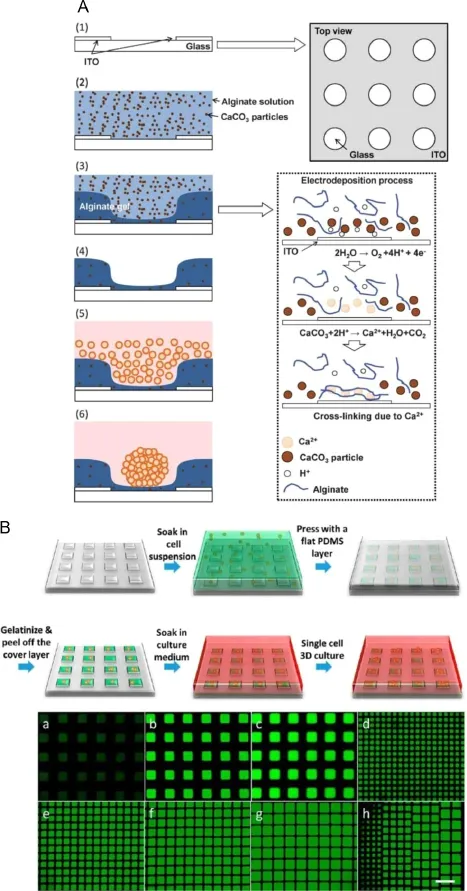
Fig.2.Schematics of building 3D cell culture environment.(A)Fabrication of alginate microwellarray by electrodeposition[25];(B)Fabrication of microcollagen array by PDMS templet and gelation[26].
2.Cell culture on chip
The fi rst step towards developing micro fl uidic platform of pro fi ling cellular metabolites is to establish a similar cell culture environment on chip compared to in vivo condition[14,15].As we all know,cells are basic building blocks constituting our body. However,in our body,the higher hierarchy structures can be much more complicated.Cells in vivo exist in a complex microenvironment consisting of various soluble cellular factors and insoluble extracellular matrix(ECM).These components regulate cell behaviors such as migration,mitosis and apoptosis by ligand–receptor interaction,phosphorylation,opening up the downstream signal pathway as well as affecting transcription factors.The gradient of cytokines and ECM compose the chemical microenvironment.Besides,cell–cell interaction within same or different cell types is another essential part of microenvironment.Cell–cell interaction can be regulated by secreting cellular factors or direct contact,and abnormal cell–cell interaction is an important cause of critical diseases like autoimmunity and cancer metastasis.The last component which can be easily ignored is the mechanical microenvironment.Cells in different tissues and organs may face distinct mechanical forces.Red blood cell(RBC)and leukocyte should endure the large shear forces by blood fl ow,and epithelium cells on the interfaces of alveolus-capillary can be stretched by the inhalation and expiration during breath.Huh et al.[16]developed a polydimethylsiloxane(PDMS)based in vitro lung model with porous fi lm as alveolus interfaces to accommodate epithelial and endothelial cells on opposite site,and mimicked the breathing stretch by adding two pressure tuning side channels.When vacuum was applied to side channels,the elastic PDMS walls generated deformation and gave pulling force on the cell culture fi lm, thus resulting in the stretch similar to an actual lung.The model was applied to investigate the cell toxicity of nanoparticles.Also, other micro fl iudic devices have been developed to systematically study the effects of mechanicalmicroenvironments,such as fl uidic shear stress[17]and surface strain[18,19],with tunable magnitude of forces upon monolayer of endothelial cells.Results revealed that cell orientation and formation of cell skeleton were signi fi cantly in fl uenced by mechanical stress.Actually,2D endothelium is especially suitable for the investigation of membrane stress due to its tendency to form integrated monolayer and easy combination with fl uorescence imaging and other optical observation methods.With the assistance from pressure-mediated microvalve and precisely controlled micro fl uidic system,regionally selective and magnitude determined mechanical stimuli are easily achieved.Several strategies are provided to realize the construction in vitro culture model after the basic elements of which were reviewed.
2.1.3D cell culture
Conventional dish culture of cell gives 2D monolayer structure, where cells adapt to show fl at morphology.While in vivo,cells are more likely“fl oating”in the ECM,supported by the various structural proteins like collagen,laminin and fi bronectin,thus presenting more extensive shapes.The differences of 3D and 2D culture are not limited in the morphology.Evidence shows shapes under 2D culture,cells lose functionality[20],go dedifferentiated [21]and exhibit different expression levels of several important genes[22].In contrary,3D culture helps cells maintain phenotypes even after weeks of in vitro culture[23,24].One scheme to implement 3D culture is to accommodate cells in biocompatible hydrogen such as agarose,alginate,matrigel and collagen,supplemented with ECM components for a better cell attachment. Ozawa et al.[25]developed an alginate 3D model for embryonic stem cells and HepG2 culture based on electrodeposition of alginate gel(Fig.2A).Indium tin oxide(ITO)electrode is selectively patterned on substrate surface,upon which the electrolysis of water produces H+,dissolving the CaCO3particles in alginate solution,thus forming microwells of alginate gel.Embryonic bodies and HepG2 spheroids were formed in 3D culture,which demonstrated the validity of culture platform.Guan et al.[26]developed a facile and rapid method to generate a large microcollagen array for long-term cell culture and screening of drug resistance heterogeneity(Fig.2B).Cell culture chamber was fabricated by fulfi lling PDMS microwells with cell collagen suspension,and sequentially gelatinized to form 3D culture gel.Long-term culture was maintained as long as 30 days at single cell level,with the ability to retrieve any cell of interest for further examination. Collagen based 3D microtissue culture models for cell viability assessment[27]or immunoassay of cytotoxicity[28]under drug stimulation were also reported,which were compatible to microplate reader.This platform provides a high throughput and quantitative method for drug screening and better prediction of drug ef fi cacy at the tissue level.
Droplet technique in micro fl uidic has always been highlighted especially in the fi eld of biosample analysis,due to its precise control of de fi ned volume,minimized reagent consumption and prevention in cross contamination.Micro fl uidic droplet is broadly applied in constructing 3D culture models with clever art of hydrogel gelation.In a recent work from Utech et al.[29],calcium ethylene diamine tetraacetic acid(Ca-EDTA)chelate was mixed with alginate solution as the dispersed phase.After the alginate droplet was made at nozzle by fl ow focusing,H+in oil phase gradually diffused into the microdroplet and released Ca2+which further solidi fi ed the alginate.Besides,the gelation process can also be conveniently controlled by sequential adjustment of the temperature for agarose or matrigel based droplet,or selective exposure to ultraviolet(UV)for PEG hydrogel.Micro fl uidic droplet technique realizes monodispersed,and highly uniformed gel droplet for cell encapsulation at the single cell level,providing a platform for high throughout cell culture and screening.
Combined with microarray presentation,parallel experiments can be performed in multi-parameters drug screening.Du et al. [30]reported a droplet based micro fl uidic system for drug combination screening(Fig.3).In this system,droplet is con fi ned in the array of microwells which are covered by a layer of oil to isolate each droplet.Sequential operations such as cell seeding, drug addition and stimulation,and fl uorescence probing can be completed by a tapered capillary.The system was validated by exploring the effects of drug combination based on cell viability testing.
2.2.Cell co-culture
Any tissue or organ with certain function in human body is composed of large cell populations and multiple cell types.Interactions between multiple cell types constitute a stable and functional organism,while the absence of proper intercellular interaction may severely alter the behavior of cells.It has been reported that complex interaction between cancer and endothelial cells mediated by cell factors such as vascular endothelialgrowth factor (VEGF)and transforming growth factor-β(TGF-β)would increase the drug resistance of tumor and induce angiogenesis[31,32]. Therefore,in the aspect of drug metabolite pro fi ling and ef fi ciency testing,data from single cell type experiment can be rather limited.Under this circumstance,cell co-culture model is being paid intensive attention to improve the traditional screening platform. In a work of our group,a CaSki cells and human umbilical vein endothelial cells(HUVECs)co-culture model under different levels of oxygen(5%and 15%)was developed to study the cell-cell communication in cervical cancer development(Fig.4)[33].VEGF165was semi-quantitatively analyzed by functional nucleic acid and chromogenic system online,and other metabolism related species such as reactive oxygen species(ROS)were also studied.Another work by Mao et al.[13]suggested a cell co-culture model to emulate the epinephrine communication between 293 and L-02 cells by connecting two cell culture chambers in the dynamic on-chip culture.When acetylcholine is infused into the culture system,293 cells on the upstream are stimulated and thus produce epinephrine,which will affect L-02 cells on the downstream chamber and enhance its glucose secretion.The fi nal metabolite,glucose,as well as signaling molecule,epinephrine,was successfully detected by electrospray ionization-quadrupole-time of fl ight-mass spectrometry(ESI-Q-TOF-MS).This work provides a useful platform to study the in fl uence of cell-cell communication upon metabolic pathway.
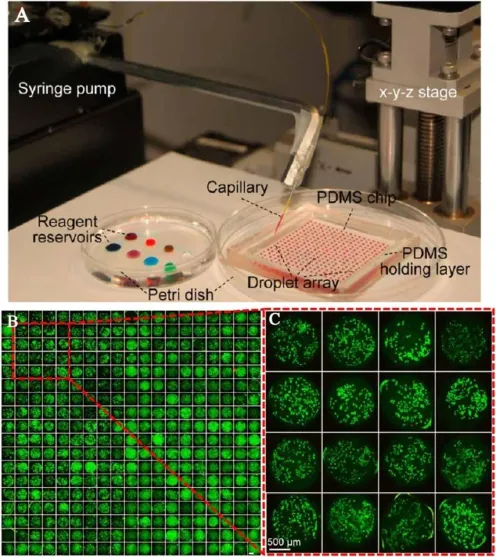
Fig.3.Cell culture droplet array for drug combination screening.(A)Photo of device set up;(B),(C)Fluorescence images of cell culture array with A549 cells[30].
2.3.Organ on chip
Organ is the basic functional unit in drug metabolism,and the ADME process concerning drug ef fi ciency is actually carried out at the organ level.To have a systematic view of drug metabolic pathway,one has to construct a model more closely emulating organ functions rather than a single or a few cell types co-culture, which is termed“organ on chip”or even“body on chip”[34–36]. Among all kinds of organs,liver is the most frequently investigated one because of its high metabolic activity in drug metabolism. Zhang et al.[37]reported an in vitro liver model with the functionality of metabolizing the prodrug capecitabine to produce an anti-cancer intermediate(Fig.5A).HepG2 was cultured to emulate the liver function,while MCF-7 cell was a representative of tumor. Prodrug and its intermediate were detected by on-line solid phase extraction(SPE)and mass spectrometry(MS)with a limit of detection(LOD)of 10 nM.Imura et al.[38]developed a more complicated biosystem to assess the drug absorption of intestine and metabolism of liver as well as its bioacitivity on target cells(Fig. 5B).Caco-2,HepG2 and MCF-7 were cultured on chip to represent the modules of intestine,liver and tumor,respectively.The platform provides an ef fi cient assessment to the oral administrated medicine.
Organ on chip or body on chip is a potential tool which may greatly promote the clinical treatment,especially point-of-care diagnosis and personal specialized therapy.However,one challenge remaining to be solved is how to accommodate dozens of cell populations and sub-cell populations into an in vitro model with proper proportion and position[35].For example,there are entities of hepatic cells,vascular endothelial cells and preicytes, and other immune related lymphocytes in liver.The culture technique for some cell types is still immature and unsatisfactory. But hopefully,with the assistance of newly emerging methods such as bioprinting[39,40],one day we may construct an“organ”with more complex architectures and more power of control.
3.Sample pretreatment on chip

Fig.4.Cell co-culture micro fl uidic system under different oxygen gradients.(A)Oxygen induced cell-cell communication;(B)Illustration of microchip structure; (C)Microvalve constituted by micropillars;(D)Two-layer cellco-culture device under low oxygen condition;(E)Schematics of cell secretion detection[33].
After the construction of biomodel on chip and drug stimulation,the following procedure is to detect drug metabolites with the assistance of an external detector.However,the sample pretreatment has to be carried out on microchip in advance,because the complex compositions among culture medium may severely inhibit the ionization ef fi ciency and interfere the detection of a target molecule of low abundance or trace amount.Considering the limited volume of analysis system,pretreatment,roughly divided as preconcentration and separation,must have low sample consumption.And on-line pretreatment is preferred to increase the integration and automation of the platform,while avoiding unnecessary sample loss occurred in off-line method.
3.1.Preconcentration methods
Our group has published a series of works employing micro-SPE columns integrated to cell culture chip for establishment of drug cellular testing platform[41],study of cell-cell communication[13]and drug metabolism[42,43].Commercialized SPE beads were infused and immobilized in a microchamber with shrinking ends to receive the upstream cell culture medium with drug metabolites.Analytes were captured and thus desalted by the microextraction process.When elution buffer was added,the dissolved metabolite was online analyzed by ESI-MS.Highly parallel experiments can be performed on the same chip by arranging multiple cell cultures and SPE channels to investigate the effects of different concentrations of a drug.
Another method developed by our group realized the extraction of cell metabolites from culture medium via microdialysis (Fig.6A)[44].A homemade microdialysis hollow fi ber was immersed into cell culture medium,and as dialysate fl owed into the fi ber,glucose from culture media diffused through dialysis interface.The other end of the fi ber came to paper spray ionization for MS detection.With the continuously fl owing dialysate and generating droplets,on-line monitoring at glucose level in cell culture medium was achieved with the shortest time intervalof 1.5 s.This work demonstrated a potential platform with label free MS detection for the monitoring of cellular culture system.
3.2.Microseparation
Chromatography is a highly ef fi cient method for the separation and identi fi cation of analytes among complex matrix.There are standards of drug metabolite analysis based on the mature gas chromatography-mass spectrometry(GC-MS)or liquid chromatography-mass spectrometry(LC-MS)techniques.For microseparation,microchip electrophoresis(MCE)assumes the most successful miniaturization of chromatography,including operations of sample introduction,derivatization,separation and detection in a con fi ned small area of few square centimeters.MCE is applicable to analysis of genes,amino acids,proteins and even single cells. The principle of MCE is based on the employment of electroosmotic fl ow,the rate of which can be controlled by modifying inner surface or adjusting buffer composition,thus realizing the separation of substances with different motilities.Frequently used detecting methods coupled to CE separation include UV absorbance,fl uorescence and electrochemical sensor.Normally,absorbance test requires an enough high concentration of analyte,or a large molar extinction coef fi cient,which is often inapplicable to cell metabolite analysis.Although fl uorescence and electrochemicalmethod are frequently used in metabolite detection,each of them has their own advantages.Fluorescence provides signi fi cantly better sensitivity even at the single molecule level,but speci fi c probes are necessary to label the targets.For electroactive substances,electrochemical method is label-free without requirement of derivatization or extra bulky device which increases the portability of the device.For instance,electrochemical method is an ideal tool for saccharides analysis,because there is no functional group for the addition of fl uorophore,neither do saccharides have large molar extinction coef fi cient.Our group reported a microchip-CE method for assay of multiplex cell metabolism related proteins(Fig.6B)[45].Aptamers for recognition of plateletderived growth factor-BB(PDGF-BB),VEGF165and thrombin were designed to have different lengths,which modulate the electrophoretic mobility of respective proteins by speci fi c bonding.Theemployment of SYBR gold staining increases the sensitivity while reduces the matrix interference.There are other works concerning the application of MCE in metabolites probing with combination of electrochemical[46]or ESI-MS detection[47].The sensitivity and quantitative ability of CE can be further strengthened via modi fi cation of sample introduction manner such as inkjet direct sampling.(Fig.7)[48].
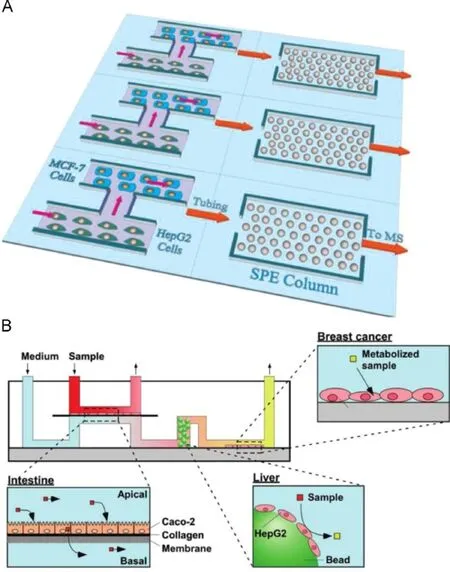
Fig.5.Schematics of constructing on chip organ model.(A)Multiple parallel channels for cell culture,metabolism and metabolite extraction in an in vitro liver model[37]; (B)Assessment of drug absorption,metabolism and effect on tumor by intestine-liver-cancer modelon chip[38].
Another miniaturization by integrating LC column into a microchip combined with MS detection also demonstrates a great capacity for metabolite analysis,which can be termed microchip-LC-MS.Compared with conventional LC,microchip-LC separation is much faster and costs lower sample consumption,thus largely increasing the throughput.Sainiemiet al.[49]reported a silicon LC system on chip with micropillars functionalized with SiO2or C18coating as a basic separation unit.Separation can be completed in 5 min with good sensitivity of MS or fl uorescence detection.Also, microchip based liquid chromatography system has been well established and commercialized by several companies[50].
4.Detection
As mentioned above,commonly used detectors for micro fl uidic cellular analysis platform include fl uorescence microscopy, electrochemical and MS methods.High sensitivity,good speci ficity,low cost and short time period are preferred characteristics for a detector,especially in microanalysis of complex system(for a detailed and comprehensive discussion about detectors suitable for biospecies detection,readers can refer to previously published review[51]).But sometimes,the size of detector may determine the usability of a platform,if portability is in high favor.For example,electrochemical method can be miniaturized to a microelectrode,which can be selectively patterned in micro fl uidic channels and realize region con fi ned detection.Several works reported a patch type metabolite monitor with an array of microelectrodes on needle tip for non-enzymatic detection of interstitial glucose level[52]or lactate level[53].Although in animal models, the method may be short-lived compared with in vitro experiments,it is still a potential complement to disease therapy like diabetes.Besides,the integration of features in nano scale may greatly increase the detection performance by exhibiting large contact area and thus gaining high probability in capturing target molecular or target cells[54].But in general,MS is the most powerful tool in metabolite analysis,due to the abundant structural information given in a MS spectrogram.With the ability to complete simultaneous detection of multiple analytes by different charge-to-mass ratios,the analytical capacity of MS coverscompounds and biomolecules from small molecules to peptides, proteins and nucleic acids.Recently,the rapid development of high resolution MS pushes LOD to fmol.Besides,fast speed of over one hundred times full-spectrum scanning per second is compatible and applicable to probe biochemical reactions completed in milliseconds inside cell.As for drug metabolite identi fi cation,the composing elements and structural information of drug compound can be easily obtained by multiple determining methods [55,56].In microchip-MS combination,one critical issue is the interfaces between microchip fl ow and MS ionization region. Therefore,ionization methods such as ESI,paper spray and matrix assisted laser deposition ionization(MALDI)are carefully reviewed to address above problem.
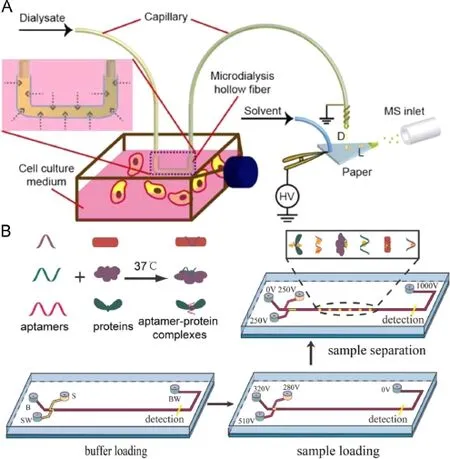
Fig.6.Cell metabolism related sample preconcentration and microseparation methods.(A)Microdialysis to extract glucose for paper spray ionization from cell culture medium[44];(B)Cell metabolism proteins assay based on tunable aptamer and microchip-CE[45].

Fig.7.Drop-by-drop sample introduction by ink jet for CE method.(A)Illustration of ink jet sample introduction to CE;(B)NBD-F derivatization of amino acids by drop-bydrop sample introduction[48].
4.1.ESI

Fig.8.Microchip-MS interfaces with multichannel set up for concentration depended screening.(A)Combination of microchip to ESI-MS detection[41]; (B)Combination of microchip to paper spray MS detection[64].
ESI method enables direct ionization of target molecule in liquid buffer,and is often conveniently combined with LC separation.For microchip-MS method,the fl ow rate of micro fl uidic dynamic culture system is comparable to nano fl ow ESI,and via external connecting silicon fused capillary,sample can be directly introduced from microchip into ESIwith on-line SPE channels(Fig. 8A)[41].Severalworks also reported microchip based emitters for electrospray composed of PDMS[57]or glass material[58,59].The integration of electrospray emitter onto microchip gives better combination to other on chip functional blocks such as MCE,and further increases the sensitivity as well as throughput by densely arrangement of multiple nozzles.Mao et al.[60]introduced a multi-nozzle emitter array consisting of 96 10-nozzle emitters in a circular array on a silicon chip.The platform provided advantages such as no cross-contamination and highly parallel experiments.
4.2.Paper spray
Paper spray is a facile,cheap and highly ef fi cient ionization method for qualitative and quantitative MS analysis of complex samples such as dried blood[61],fruit juice[62]and body metabolites[63].Paper spray presents great matrix tolerance with simple sample introducing fashion.Buffer with analyte is directly dripped onto paper surface,and as the paper gets wet,the analyte driven by electric fi eld gradually moves to the tip and spray.In the whole process,electro-inert substances remain still,thus realizing a separation of target molecule from matrix.Our group has developed a multichannel microchip device with a movable capillary to sequentially aspirate different samples in different channels (Fig.8B)[64].The sample was transported in droplet and separated by air plug until it fell onto the paper surface and sprayed. The process was in full automation and controlled by the computer,which made the device a potential platform for high throughput screening of biomolecular interaction or cell metabolite under drug stimulation.
4.3.Maldi
MALDI is well tolerant to salt and non-volatile components of buffer system.But in the region of molecular weight under 500 Da, fragments from matrix composition signi fi cantly raise up the background noises which are unfavorable to small molecule analysis[65].Due to the vacuum condition required by MALDI,the online coupling of microchip to MALDIis rather dif fi cult.Instead,offline methods of depositing samples onto MALDI target plate after microchip operation are well developed.A series of works about constructing a micro-bioreactor by covalently immobilizing trypsin onto glass fi ber[66]or microchannel inner surface have been reported[67,68].Proteins such as hemoglobin and cytochrome c were digested by trypsin and further collected for deposition to MALDI target and MS detection.With the assistance of microfl uidic reactor,time period for proteolysis can be reduced to less than 5 s.The platform demonstrated great capacity for identi fication and analysis of complex protein samples.Mikkonen et al. [69]reported an open microchannel for charged sample preconcentration and direct usage as MALDI target plate.Peptides of cytochrome c digested by trypsin were preconcentrated at different positions of microchannel,and determined by pH gradient of water electrolysis.MALDI-MS detection was carried out by depositing matrix and enhancing crystallization into channel.This work provides a direct combination of microchip-MS method,thus evading the possible cross contamination and sample loss.
5.Conclusions and perspectives
Construction of cell in vitro model,on chip sample pretreatment and detection are three critical parts concerning a successful integrated microchip method for cellular metabolism study.Since the appearance of micro fl uidic chip,lab on chip method has always been paid intensive attention,especially in the fi eld of biosample analysis.Micro fl uidic platform has been proved an ideal candidate for cell metabolism analysis with at least four advantages:
i.The micro scale of channelgeometry provides precise controlof cell culture microenvironment and delicate cell manipulation scheme;
ii.On-line separation and preconcentration methods avoid the loss of sample and increase the detection sensitivity;
iii.Microchip interfaces are compatible with various detectors such as MS,fl uorescence microscopy and electrochemical sensor with little or without necessary further modi fi cation,which strengthens the capability of metabolite detection;
iv.With integrated functions and multiple parallel channels,micro fl uidic platform allows operation in full automation with high throughput.
However,there are stillchallenges before a wider application of micro fl uidic platform come to drug screening and cellular events study.How can we establish a proper cell culture model with perfect simulation of tissue or organ functions meanwhile allowing transparent and thorough investigation of the system?How will the separation and detection method be developed to probe components inside cells of less content but with important biological signi fi cance?Addressing these questions may require hard work and wisdom of generations.But actually,micro fl uidic platform has already revolutionized the way of bioanalysis and life analysis.
Acknowledgments
This work was fi nancially supported by National Natural Science Foundation of China(Nos.81373373,91213305,21227006) and CERS–China Equipment and Education Resources System (No.CERS-1-75).
Appendix A.Supplementary material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpha.2015.09.003.
References
[1]R.Zenobi,Single-cell metabolomics:analytical and biological perspectives, Science 342(2013)1243259.
[2]A.R.Fernie,R.N.Trethewey,A.J.Krotzky,et al.,Metabolite pro fi ling:from diagnostics to systems biology,Nat.Rev.Mol.Cell Biol.5(2004)763–769.
[3]G.J.Patti,O.Yanes,G.Siuzdak,Innovation:metabolomics:the apogee of the omics trilogy,Nat.Rev.Mol.Cell Biol.13(2012)263–269.
[4]M.Baker,Metabolomics:from small molecules to big ideas,Nat.Methods 8 (2011)117–121.
[5]J.Arrowsmith,Trialwatch:phase IIfailures:2008–2010,Nat.Rev.Drug Discov. 10(2011)328–329.
[6]J.Arrowsmith,Trialwatch:phase IIIand submission failures:2007–2010,Nat. Rev.Drug Discov.10(2011)87.
[7]J.H.Sung,M.L.Shuler,In vitro microscale systems for systematic drug toxicity study,Bioprocess Biosyst.Eng.33(2010)5–19.
[8]J.R.Kraly,R.E.Holcomb,Q.Guan,et al.,Review:micro fl uidic applications in metabolomics and metabolic pro fi ling,Anal.Chim.Acta 653(2009)23–35.
[9]Q.Zhuang,S.Wang,J.Zhang,et al.,Nephrocyte–neurocyte interaction and cellular metabolic analysis on membrane-integrated micro fl uidic device,Sci. China Chem.(2015)1–8,in press.
[10]M.H.Wu,S.B.Huang,G.B.Lee,Micro fl uidic cell culture systems for drug research,Lab Chip 10(2010)939–956.
[11]P.Neuži,S.Giselbrecht,K.Länge,et al.,Revisiting lab-on-a-chip technology for drug discovery,Nat.Rev.Drug Discov.11(2012)620–632.
[12]P.S.Dittrich,A.Manz,Lab-on-a-chip:micro fl uidics in drug discovery,Nat.Rev. Drug Discov.5(2006)210–218.
[13]S.Mao,J.Zhang,H.Li,et al.,Strategy for signaling molecule detection by using an integrated micro fl uidic device coupled with mass spectrometry to study cell-to-cell communication,Anal.Chem.85(2013)868–876.
[14]K.Ziółkowska,R.Kwapiszewski,Z.Brzózka,Micro fl uidic devices as tools for mimicking the in vivo environment,New J.Chem.35(2011)979.
[15]J.H.Sung,M.L.Shuler,Microtechnology for mimicking in vivo tissue environment,Ann.Biomed.Eng.40(2012)1289–1300.
[16]D.Huh,B.D.Matthews,A.Mammoto,et al.,Reconstituting organ-level lung functions on a chip,Science 328(2010)1662–1668.
[17]X.Zhang,D.J.Huk,Q.Wang,et al.,A micro fl uidic shear device that accommodates parallel high and low stress zones within the same culturing chamber,Biomicro fl uidics 8(2014)054106.
[18]Q.Wang,X.Zhang,Y.Zhao,A multiplexed micromechanical cell stimulator for studying magnitude-dependent cell responses,Micro fl uid.Nano fl uid.18 (2014)415–425.
[19]Q.Wang,X.Zhang,Y.Zhao,Micromechanical stimulator for localized cell loading:fabrication and strain analysis,J.Micromech.Microeng.23(2013) 015002.
[20]F.Pampaloni,E.G.Reynaud,E.H.K.Stelzer,The third dimension bridges the gap between cellculture and live tissue,Nat.Rev.Mol.Cell Biol.8(2007)839–845. [21]K.Bhadriraju,C.S.Chen,Engineering cellular microenvironments to improve cell-based drug testing,Drug Discov.Today 7(2002)612–620.
[22]A.Birgersdotter,R.Sandberg,I.Ernberg,Gene expression perturbation in vitro–a growing case for three-dimensional(3D)culture systems,Semin.Cancer Biol.15(2005)405–412.
[23]C.E.Semino,J.R.Merok,G.G.Crane,et al.,Functional differentiation of hepatocyte-like spheroid structures from putative liver progenitor cells in threedimensional peptide scaffolds,Differentiation 71(2003)262–270.
[24]E.Cukierman,R.Pankov,D.R.Stevens,et al.,Taking cell-matrix adhesions to the third dimension,Science 294(2001)1708–1712.
[25]F.Ozawa,K.Ino,T.Arai,et al.,Alginate gelmicrowell arrays using electrodeposition for three-dimensional cell culture,Lab Chip 13(2013)3128–3135.
[26]Z.Guan,S.Jia,Z.Zhu,et al.,Facile and rapid generation of large-scale microcollagen gelarray for long-term single-cell3D culture and cellproliferation heterogeneity analysis,Anal.Chem.86(2014)2789–2797.
[27]H.Zhao,L.Zhou,Q.Zhang,et al.,Bi-content micro-collagen chip provides contractility-based biomechanical readout for phenotypic drug screening with expanded and pro fi led targets,Lab Chip 15(2015)3481–3494.
[28]X.Yan,J.Wang,L.Zhu,et al.,A ready-to-use,versatile,multiplex-able threedimensional scaffold-based immunoassay chip for high throughput hepatotoxicity evaluation,Lab Chip 15(2015)2634–2646.
[29]S.Utech,R.Prodanovic,A.S.Mao,et al.,Micro fl uidic generation of monodisperse,structurally homogeneous alginate microgels for cell encapsulation and 3D cell culture,Adv.Healthc.Mater.4(2015)1628–1633.
[30]G.S.Du,J.Z.Pan,S.P.Zhao,et al.,Cell-based drug combination screening with a micro fl uidic droplet array system,Anal.Chem.85(2013)6740–6747.
[31]P.Carmeliet,Angiogenesis in life,disease and medicine,Nature 438(2005) 932–936.
[32]T.L.Whiteside,The tumor microenvironment and its role in promoting tumor growth,Oncogene 27(2008)5904–5912.
[33]X.Lin,Q.Chen,W.Liu,et al.,Oxygen-induced cell migration and on-line monitoring biomarkers modulation of cervical cancers on a micro fl uidic system,Sci.Rep.5(2015)9643.
[34]N.K.Inamdar,J.T.Borenstein,Micro fl uidic cell culture models for tissue engineering,Curr.Opin.Biotechnol.22(2011)681–689.
[35]E.W.Esch,A.Bahinski,D.Huh,Organs-on-chips at the frontiers of drug discovery,Nat.Rev.Drug Discov.14(2015)248–260.
[36]M.Baker,Tissue models:a living system on a chip,Nature 471(2011) 661–665.
[37]J.Zhang,J.Wu,H.Li,et al.,An in vitro liver model on micro fl uidic device for analysis of capecitabine metabolite using mass spectrometer as detector, Biosens.Bioelectron.68(2015)322–328.
[38]Y.Imura,K.Sato,E.Yoshimura,Micro total bioassay system for ingested substances:assessment of intestinalabsorption,hepatic metabolism,and bioactivity,Anal.Chem.82(2010)9983–9988.
[39]D.B.Weibel,W.R.Diluzio,G.M.Whitesides,Microfabrication meets microbiology,Nat.Rev.Microbiol.5(2007)209–218.
[40]B.Derby,Printing and prototyping oftissues and scaffolds,Science 338(2012) 921–926.
[41]D.Gao,H.Li,N.Wang,et al.,Evaluation of the absorption of methotrexate on cells and its cytotoxicity assay by using an integrated micro fl uidic device coupled to a mass spectrometer,Anal.Chem.84(2012)9230–9237.
[42]Q.Chen,J.Wu,Y.Zhang,et al.,Qualitative and quantitative analysis of tumor cellmetabolism via stable isotope labeling assisted micro fl uidic chip electrospray ionization mass spectrometry,Anal.Chem.84(2012)1695–1701.
[43]S.Mao,D.Gao,W.Liu,et al.,Imitation ofdrug metabolism in human liver and cytotoxicity assay using a micro fl uidic device coupled to mass spectrometric detection,Lab Chip 12(2012)219–226.
[44]W.Liu,N.Wang,X.Lin,et al.,Interfacing microsampling droplets and mass spectrometry by paper spray ionization for online chemicalmonitoring ofcell culture,Anal.Chem.86(2014)7128–7134.
[45]X.Lin,Q.Chen,W.Liu,etal.,Assay of multiplex proteins from cellmetabolism based on tunable aptamer and microchip electrophoresis,Biosens.Bioelectron.63(2015)105–111.
[46]R.A.Saylor,E.A.Reid,S.M.Lunte,Microchip electrophoresis with electrochemical detection for the determination of analytes in the dopamine metabolic pathway,Electrophoresis 36(2015)1912–1919.
[47]N.Nordman,T.Sikanen,M.E.Moilanen,et al.,Rapid and sensitive drug metabolism studies by SU-8 microchip capillary electrophoresis-electrospray ionization mass spectrometry,J.Chromatogr.A 1218(2011)739–745.
[48]F.Chen,Y.Rang,Y.Weng,et al.,Drop-by-drop chemicalreaction and sample introduction for capillary electrophoresis,Analyst 140(2015)3953–3959.
[49]L.Sainiemi,T.Nissilä,R.Kostiainen,et al.,A microfabricated micropillar liquid chromatographic chip monolithically integrated with an electrospray ionization tip,Lab Chip 12(2012)325–332.
[50]S.L.Lin,H.Y.Bai,T.Y.Lin,et al.,Micro fl uidic chip-based liquid chromatography coupled to mass spectrometry for determination of small molecules in bioanalytical applications,Electrophoresis 33(2012)635–643.
[51]Y.Liu,S.Wang,Y.Song,et al.,Biospecies Capture and detection at low concentration,Micro.Nanosyst.4(2012)254–272.
[52]S.J.Lee,H.S.Yoon,X.Xuan,et al.,A patch type non-enzymatic biosensor based on 3D SUS micro-needle electrode array for minimally invasive continuous glucose monitoring,Sens.Actuator B:Chem.(2015)10.1016/j.snb.2015.08.013.
[53]J.R.Windmiller,N.Zhou,M.C.Chuang,et al.,Microneedle array-based carbon paste amperometric sensors and biosensors,Analyst 136(2011)1846–1851.
[54]S.Wang,Y.Wan,Y.Liu,Effects of nanopillar array diameter and spacing on cancer cell capture and cell behaviors,Nanoscale 6(2014)12482–12489.
[55]H.Li,Y.Zhang,J.Lin,Recent advances in coupling techniques of micro fl uidic device-mass spectrometry for cellanalysis,Sci.Sin.Chim.44(2014)777.
[56]C.-H.Lin,X.Lin,L.Lin,et al.,Development of LC-MS method for analysis of paclitaxel-inhibited growth and enhanced therapeutic response in human glioblastoma cells,Chin.Chem.Lett.(2015)10.1016/j.cclet.2015.03.007.
[57]X.Sun,R.T.Kelly,K.Tang,et al.,Membrane-based emitter for coupling micro fl uidics with ultrasensitive nanoelectrospray ionization-mass spectrometry,Anal.Chem.83(2011)5797–5803.
[58]P.Hoffmann,U.Häusig,P.Schulze,et al.,Micro fl uidic glass chips with an integrated nanospray emitter for coupling to a mass spectrometer,Angew. Chem.Int.Ed.46(2007)4913–4916.
[59]J.Wu,S.Wang,Q.Chen,et al.,Cell-patterned glass spray for direct drug assay using mass spectrometry,Anal.Chim.Acta 892(2015)132–139.
[60]P.Mao,H.T.Wang,P.Yang,et al.,Multinozzle emitter arrays for nanoelectrospray mass spectrometry,Anal.Chem.83(2011)6082–6089.
[61]H.Wang,J.Liu,R.G.Cooks,et al.,Paper spray for direct analysis of complex mixtures using mass spectrometry,Angew.Chem.Int.Ed.49(2010)877–880.
[62]W.Liu,S.Mao,J.Wu,et al.,Development and applications of paper-based electrospray ionization-mass spectrometry for monitoring of sequentiallygenerated droplets,Analyst 138(2013)2163–2170.
[63]J.Liu,H.Wang,N.E.Manicke,et al.,Development,characterization,and application of paper spray ionization,Anal.Chem.82(2010)2463–2471.
[64]W.Liu,Q.Chen,X.Lin,et al.,Online multi-channelmicro fl uidic chip-mass spectrometry and its application for quantifying noncovalent protein-protein interactions,Analyst 140(2015)1551–1554.
[65]D.Gao,H.Liu,Y.Jiang,et al.,Recent advances in micro fl uidics combined with mass spectrometry:technologies and applications,Lab Chip 13(2013) 3309–3322.
[66]H.Bao,S.Liu,L.Zhang,et al.,Ef fi cient sample proteolysis based on a microchip containing a glass fi ber core with immobilized trypsin,Microchim.Acta 179 (2012)291–297.
[67]H.Bao,L.Zhang,G.Chen,Immobilization of trypsin via graphene oxide-silica composite for ef fi cient microchip proteolysis,J.Chromatogr.A 1310(2013) 74–81.
[68]H.Fan,F.Yao,S.Xu,et al.,Microchip bioreactors based on trypsin-immobilized graphene oxide-poly(urea-formaldehyde)composite coating for ef fi cient peptide mapping,Talanta 117(2013)119–126.
[69]S.Mikkonen,M.K.Rokhas,J.Jacksén,et al.,Sample preconcentration in open microchannels combined with MALDI-MS,Electrophoresis 33(2012) 3343–3350.
5 September 2015
in revised form
☆Peer review under responsibility of Xi'an Jiaotong University.*
.
E-mail address:jmlin@mail.tsinghua.edu.cn(J.-M.Lin).
 Journal of Pharmaceutical Analysis2015年6期
Journal of Pharmaceutical Analysis2015年6期
- Journal of Pharmaceutical Analysis的其它文章
- Thermal stability and hydration behavior of ritonavir sulfate:A vibrational spectroscopic approach☆
- Identi fi cation,synthesis and characterization of process related des fl uoro impurity of ezetimibe and HPLC method validations☆
- Quanti fi cation of tolvaptan in rabbit plasma by LC–MS/MS:Application to a pharmacokinetic study☆
- Antimicrobial and antiproliferative prospective of kosinostatin–a secondary metabolite isolated from Streptomyces sp.☆
- Optimization,validation and application of an assay for the activity of HMG-CoA reductase in vitro by LC–MS/MS☆
- In vitro–in vivo studies of the quantitative effect of calcium, multivitamins and milk on single dose cipro fl oxacin bioavailability☆
