Screening of SSR Core Primers for Purity Identification of Pepper(Capsicum)Hybrids
Junjiao GUAN, Qingmei HUANG, Peng ZHANG, Furong MA, Hui ZHANG, Jianhua ZHANG
Quality Standard and Testing Technology Research Institute, Yunnan Academy of Agriculture Sciences, Kunming 650205, China
Pepper (Capsicum) is native to the tropical regions of Central and South America, which has been cultivated for thousands of years,with abundant genetic resources. China is the largest country in cultivation area of pepper. At present, annual cultivation area of pepper in China is 1.3-1.6 million hm2, total output value is 27-30 billion yuan,and the required hybrid seed amount is 975-1 200 t[1].The environment, cultivation measures and excellent varieties are major contributors to the increase in crop yields, and the contribution rate of excellent varieties is higher than 60%.With the development of breeding technologies, pepper varieties increase rapidly, accompanied by prominent variety and seed quality problems in pepper production. In the production practice,low purity and decreased vitality of pepper seeds generally reduce the yield and quality,thus seriously affecting the economic benefits. Therefore, it is particularly necessary to carry out purity detection of pepper seeds.
With the development of molecular marker technology, molecular markers have been applied in purity identification of pepper varieties, among which RAPD markers and SSR markers are commonly used. However,in previous studies,in order to identify the purity of a few hybrids, extensive primers should be screened to find suitable primers[2].Screening a set of core primers suitable for purity identification and constructing standard DNA fingerprints of known varieties are essential for purity identification of known varieties. Based on core primers, suitable primers for purity identification of known varieties can be obtained rapidly. SSR markers exhibit simple operation, high stability and reliability, rich allelic variations and multiple polymorphisms,with broad application prospects in purity detection of hybrid seeds.
The purity of hybrid seeds is mainly identified to detect selfed seedlings with only one pair of primers, which can also detect alien seedlings that require specific primers or primer combi-nations to reduce the probability of occurrence of varieties with identical band type[2].On this basis,core primers suitable for purity identification should have ①high heterozygosity, with a strong ability to distinguish selfed seedlings, thereby easily screening parent-complementary primers; ②high polymorphism,with a strong ability to distinguish alien seedlings. Thus,suitable primers can be easily obtained with only a small number of primers in pre-screening. Accordingly,Wang et al.[2]divided primers into three types: ①high polymorphism and high heterozygosity; ②high or moderate polymorphism and high or moderate heterozygosity; ③ low or moderate polymorphism or low heterozygosity.Specifically, type ①primers are most suitable for purity identification, followed by type ②primers, but type ③primers are generally not recommended as preferred primers for purity identification.
In this study, based on DNA fingerprinting analysis of 100 domestic pepper hybrids, evaluation indexes of core primers suitable for purity identification were determined to screen a set of core primers for purity identification of pepper hybrids and obtain specific primers for specific varieties,aiming at laying the foundation for the construction of standard DNA fingerprints for purity identification of pepper hybrids.
Materials and Methods
Materials
Experimental materials Totally 100 pepper hybrids were used as experimental materials,including major varieties bred by the Institute of Vegetables and Flowers in Chinese Academy of Agricultural Sciences, Hunan Xiangyan Seed Industry Co., Ltd.,Jiangsu Academy of Agricultural Sciences,Haihua Co.,Ltd.,Hebei Academy of Agricultural Sciences, Chinese Academy of Tropical Agricultural Sciences,Hunan Vegetable Research Institute,and Sichuan Academy of Agricultural Sciences, which can basically represent the current situation of new pepper varieties in China.
SSR primers According to the core primer screening principle proposed by Zhao et al.[3], 17 pairs of primers with good polymorphism, stability and repeatability were obtained by online primary screening and laboratory secondary screening, including Es330,Es363, Epms923, Hpms1 -214,Epms697, Es110, Es120, Es285,Es292, Es297, Es395, Hpms1 -106,Epms712, Es417, Es321, Es64 and Hpms1-5[4-6]. These 17 pairs of SSR primers were used for further evaluation and screening to obtain suitable primers for purity identification. The 5’end of sense strand of primers was labelled with fluorescence labels TAMRA, HEX, ROX and 6-FAM, respectively, to detect the size of amplified fragments with different allelic variations using DNA analyzer.
Methods
DNA extraction Tender tissues were collected from five randomly selected seedlings of each variety to extract genomic DNA with modified CTAB method proposed by Zhou et al[7].
SSR amplification The total PCR reaction volume was 20 μl, containing 0.25 mmol/L dNTPs, 0.3 mmol/L forward and reverse primers, 0.4 U of Taq DNA polymerase, 1×PCR buffer(containing Mg2+), and 50 ng of DNA template. SSR fluorescent primers were synthesized by Shanghai Sangon Biological Engineering Technology and Services Co., Ltd. The PCR amplification was started with initial denaturation at 94 ℃for 4 min, followed by 35 cycles of denaturation at 94 ℃for 45 s, annealing at 55 ℃for 45 s, and extension at 72 ℃for 45 s;the amplification was completed by holding the reaction mixture at 72 ℃for 10 min to allow complete extension of PCR products. PCR amplification was performed using AB9700 amplifier.
Electrophoresis assay PCR products were denatured and separated using ABI 3130 sequencer, and each reaction was repeated twice.
Statistical analysis By using the fragment analysis software of DNA analyzer, data of allelic variations of each sample at each locus were recorded. Pepper is a diploid species.Due to the co-dominant characteristics of SSR markers, allelic variations at homozygous loci were recorded as X/X; allelic variations at heterozygous loci were recorded as X/Y. Where, X and Y indicated the sizes of two different allelic fragments at the loci; the smaller fragment was ahead of the larger one. Invalid allelic variations were recorded as 0/0.
The polymorphism of primers was ev aluated by polymorphism information content(PIC):
Where, Piand Pjrepresented the frequency of the ithand jthalleles, respectively;i ≠j.
The heterozygosis degree of primer loci was evaluated by heterozygosity:
Heterozygosity=Number of varieties with heterozygous band pattern at the locus / Total number of experimental varieties.
Results and Analysis
Screening of primers suitable for purity identification
By using 17 pairs of SSR primers,DNA fingerprint of 100 pepper varieties was constructed. According to related data, genotype frequency, basic information of genotype distribution,polymorphism and heterozygosity of primers were recorded and analyzed. As shown in Table 1, polymorphism and heterozygosity of SSR primers of pepper were relatively low;PIC value and heterozygosity were not exactly cooperative. For instance,Epms697 exhibited high PIC value(0.51)and low heterozygosity(0.23).
According to the classification standards, SSR primers were divided into three types:①high polymorphism and high heterozygosity (Hpms1-214,Es395 and Hpms1-5);②high or moderate polymorphism and high or moderate heterozygosity (Es330, Es363,Epms923,Es120 and Es64);③low or moderate polymorphism or low heterozygosity(Epms697, Es110, Es285,Es292, Es297, Hpms1 -106,Epms712,Es417 and Es321).
By using primers Hpms1-214,Es395 and Hpms1-5,among 100 pepper hybrids,97 varieties had heterozygous band pattern, while three varieties(Super No.16,Xingshu 201,Bola No.4) exhibited no heterozygous band pattern. These three varieties were further detected with Es 64, and heterozygous banding pattern was ob-served.

Table 1 Basic information of 17 pairs of SSR primers
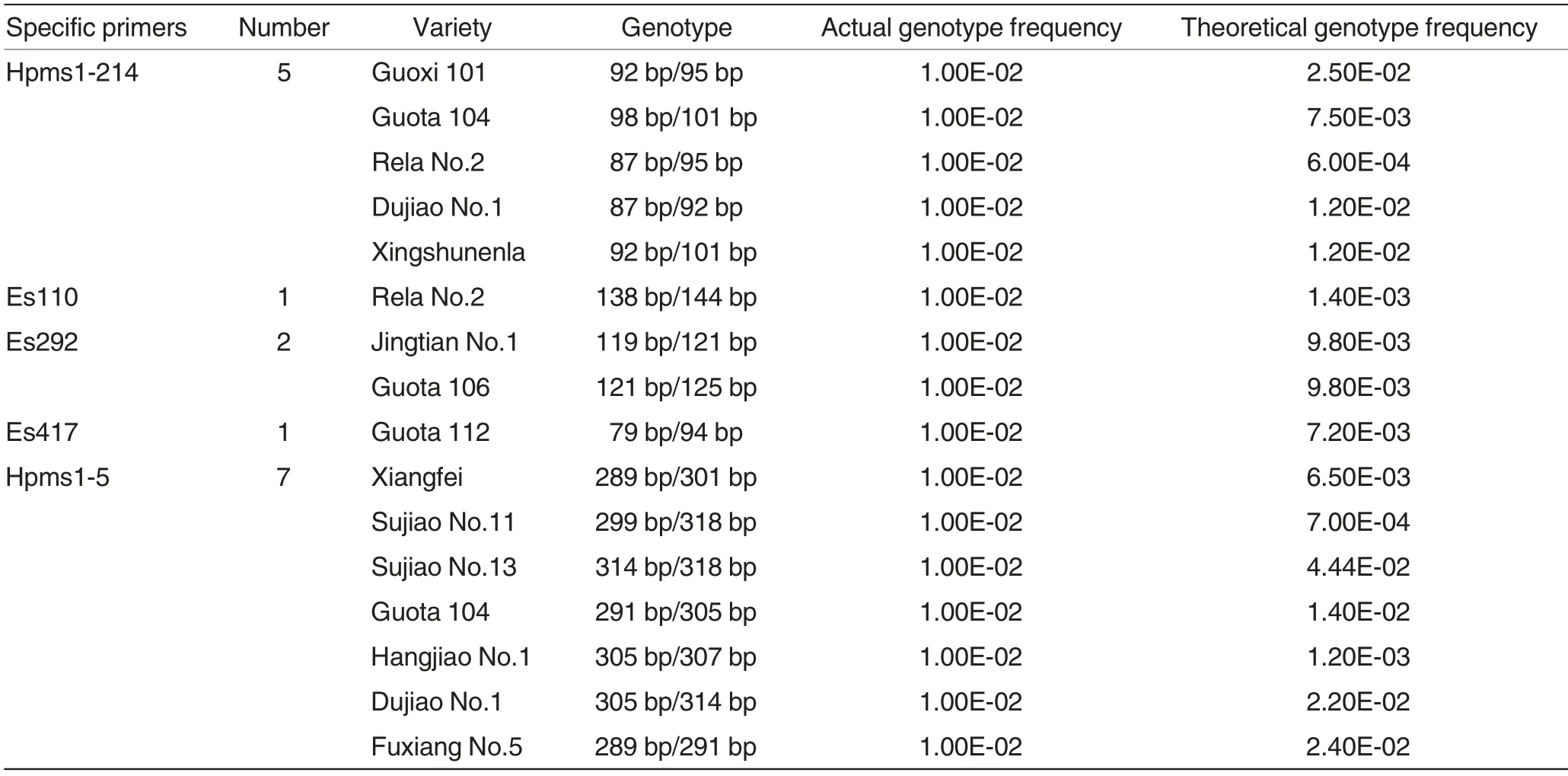
Table 2 Pepper varieties with specific primers and corresponding genotypes
Determination of specific primers for purity identification
Within a range of specific varieties, when the genotype of a particular variety only appears once (called specific band pattern)by using a single primer or when the genotype frequency is predicted to be lower than a certain value according to the genotype frequency table, the primer can be used as a specific primer within the specific range. In addition, heterozygous genotype is required if the specific primers are used for purity identification.
The detection results of 100 pepper hybrids using 17 primers were shown in Table 2. To be specific, the genotype of 14 varieties only appeared once with specific primers; Hpms1-214 and Hpms1-5 were the most frequently used specific primers(five and seven times, respectively), but Es395 was not used as a specific primer. According to the results, Es395 had high polymorphism and heterozygosity,while Es292 had low polymorphism and heterozygosity, suggesting that specific primers exhibited no direct linear relationship with the polymorphism and heterozygosity of primers but demonstrated a greater relationship with the uniformity of the occurrence frequency of different alleles. The calculation results indicated that the actual genotype frequencies of these characteristic patterns were 1.00E-02(1/100), while the highest and lowesttheoretical genotype frequencies were 2.50E-02 and 6.00E-04, respectively.The theoretical genotype frequencies of seven varieties were higher than the actual genotype frequencies.
The theoretical minimum genotype frequency and corresponding genotype of each primer were shown in Table 3. According to the results,the minimum genotype frequency of a single primer was 1.00E-04. Considering genotypes with predicted genotype frequencies below 1.00E-02 (1/100) as specific genotypes, Es-120, Es285, Es297 and Epms712 could not be used as specific primers for identification of any variety, while other 13 primers might be used as specific primers for identification of varieties with specific genotypes.
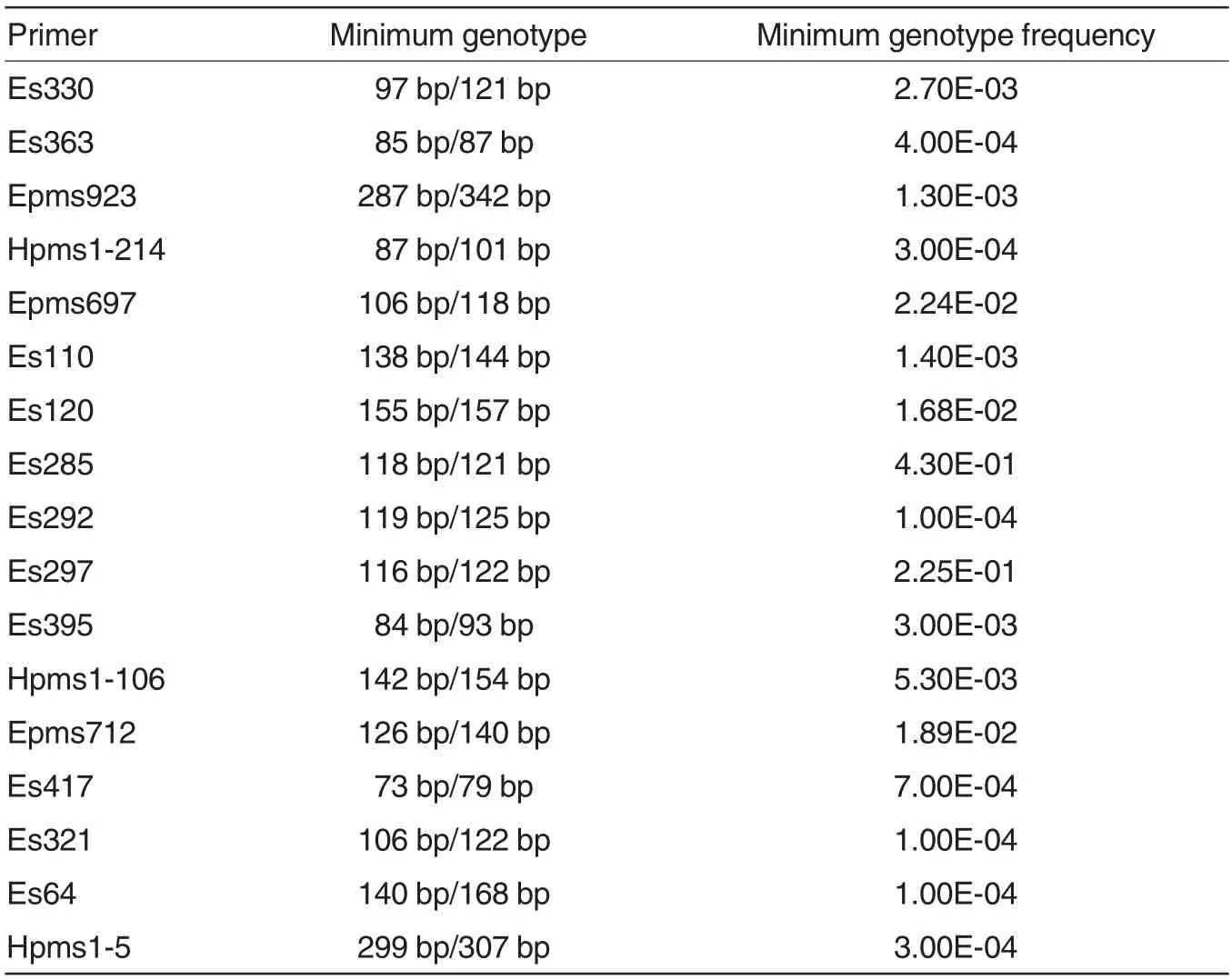
Table 3 Genotypes with the minimum genotype frequencies
Discussion
Application of SSR markers in variety identification and purity detection of pepper hybrids
Currently, SSR markers have been widely used for variety identification,DNA fingerprinting and molecular marker assisted breeding. However, due to narrow genetic background of pepper, SSR markers demonstrate low polymorphism (the maximum PIC is 0.66) and low heterozygosity (the highest heterozygosity is 0.67)in pepper hybrids,which are difficult to distinguish more pepper hybrids and are not conducive to purity detection of hybrids. Therefore, it is necessary to develop more polymorphic markers.
Screening of core primers for purity identification of pepper hybrids
Core primers with high polymorphism and heterozygosity are suitable for purity identification. Among the 17 primers evaluated in this study,Hpms1-214,Es395 and Hpms1-5 exhibit the best overall performance. By using these three primers, 97% of the experimental varieties have heterozygous band pattern,which is consistent with the requirements for detection of selfed seedlings. Moreover, these three primers are highly polymorphic with a strong ability to identify hybrids.Therefore, these three primers can be recommended as the preferred core primers for purity identification of pepper hybrids. Es330, Es363, Epms923,Es120 and Es64 exhibit moderate performance and can basically meet the requirements, which can be recommended as candidate core primers for purity identification to identify varieties those can not be identified with Hpms1-214, Es395 and Hpms1-5.However, other nine primers demonstrate low polymorphism and heterozygosity, which are generally not recommended as primers for purity identification. Thus, considering the ability of primers to distinguish selfed seedlings,these eight primers can fully meet the requirements for purity identification.Actually,the purity of the vast majority of varieties can be identified with 2-3 primers(such as Hpms1-214 and Es395). From the perspective of detection efficiency, generally 100 individuals of each sample are detected;increasing the number of primers may double the workload. Therefore, selecting core primers can greatly improve the detection efficiency.
Screening of specific primers for purity identification of specific varieties
For specific varieties, selfed seedlings and alien seedlings can be detected simultaneously with only one primer, thus greatly reducing the cost of purity identification[8].
Specific primers for purity identification of a particular variety should have parent-complementary band pattern to detect selfed seedlings and low genotype frequencies to distinguish alien seedlings. Especially, parentcomplementary primers can be used to detect selfed seedlings (relatively common in actual identification). In addition,specific primers are generally selected from the recommended core primers and demonstrate high polymorphisms, which still have certain ability to distinguish alien seedlings,and a single primer can basically meet the needs of general purity identification.Only under necessary conditions can the number of primers be increased.
The results show that a variety can be detected with multiple specific primers. Based on different detection purposes, different primers are used.In subsequent studies, specific primers of more varieties should be screened,aiming at realizing rapid and low-cost purity identification of pepper hybrids.
Conclusion
According to the polymorphism and heterozygosity, Hpms1-214,Es395 and Hpms1-5 were determined as preferred core primers for purity identification of pepper hybrids. By using these three core primers, 97 pepper hybrids (accounting for 97% )demonstrated heterozygous band pattern. Es330, Es363, Epms923,Es120 and Es64 were determined as candidate core primers for purity identification of pepper hybrids. Specific primers of 14 varieties were obtained,which could be used to further screen parent-complementary primers of each pepper hybrid.
[1]BAI ZB(白占兵),LI XF(李雪峰),DAI WZ(戴雄泽). The identification system of hot pepper hybrid purity by SSR molecular marker (利用SSR 分子标记建立辣椒纯度鉴定体系)[J]. Journal of China Capsicum (辣椒杂志), 2010(1):32-34.
[2]WANG FG(王凤格),ZHAO JR(赵久然),WANG L (王璐),et al.Determination of SSR core primers for maize hybrid purity identification(适于玉米杂交种纯度鉴定的SSR 核心引物的确定)[J].Journal of Agricultural Biotechnology(农业生物技术学报),2007,15(6):964-969.
[3]ZHAO JR (赵久然),WANG FG (王凤格), GUO JL (郭景伦), et al. Series of research on establishing DNA fingerprinting pool of Chinese new maize cultivars Ⅱ. Confirmation of a set of SSR core primer pairs(中国玉米新品种DNA指纹库建立系列研究Ⅱ. 适于玉米自交系和杂交种指纹图谱绘制的核心引物的确定)[J].Journal of Maize Sciences(玉米科学),2003,11(2):3-5,8.
[4]MINAMIYAMA Y, TSURO M, HIRAI M.An SSR-based linkage map of Capsicum annuum[J]. Mol Breeding, 2006,18:157-169.
[5]LEE JM, NAHM SH, KIM YM, et al.Characterization and molecular genetic mapping of microsatellite loci in pepper[J]. Theor Appl Genet, 2004, 108: 619-627.
[6]HUANG HH,ZHANG ZH,ZHANG ZHH,et al. Analysis of SSRs information in Capsicum spp. from EST database[J].Agricultural Sciences in China,2006,10(10):1532-1536.
[7]ZHOU CY(周春阳),XIE LB(谢立波),LI JF(李景富),et al.The extraction of genomic DNA from hot pepper (辣椒叶片基因组DNA 提取方法的研究)[J].Journal of China Capsicum(辣椒杂志),2011(3):35-37.
[8]YASHITOLA J, THIRUMURUGAN T,SUNDARAM RM. Assessment of purity of rice hybrids using microsatellite and STS markers[J]. Crop Science, 2002,42(4):1369-1373.
 Agricultural Science & Technology2015年10期
Agricultural Science & Technology2015年10期
- Agricultural Science & Technology的其它文章
- Research Advances in Gene Regulation and Genetic Improvement of Fish Feeding
- Instrucions for Authors
- Cambridge Scientific Abstracts (CSA)
- Overview of Pharmaceutical Research on the Poria with Hostwood of Traditional Chinese Medicine
- Molecular Marker Assisted Selection for Fusarium Wilt Resistance Breeding in Watermelon(Citrullus lanatus)
- Study on Relative Soil and Water Conservation Benefits of Ridge Tillage in Different Terrain Conditions in the Black Soil Area of Northeast China
