Assessment of a six-week computer-based remediation program for social cognition in chronic schizophrenia
Linda K BYRNE*, Lingyi PAN, Marita McCABE David MELLOR Yifeng XU
•Original research article•
Assessment of a six-week computer-based remediation program for social cognition in chronic schizophrenia
Linda K BYRNE1,*, Lingyi PAN2, Marita McCABE1, David MELLOR1, Yifeng XU2
schizophrenia; cognitive rehabilitation; working memory; social cognition; facial affect recognition; controlled trial; China
1. Background
Schizophrenia is associated with impaired social cognition, which in turn is associated with reduced functional outcomes and quality of life.[1]Social cognition broadly refers to the processes involved with decoding social and emotive information, including perspective, Theory of Mind (ToM), affect perception and regulation, and causal attribution.[2]Impairments with affect recognition and processing are considered especially important in affecting other social cognition domains.[3]Measurements of affect recognition are strong predictors of social performance and long-term functional outcomes for people with schizophrenia.[4]
One meta-analysis concludes that impairment in social cognition is a better predictor of long-term outcomes in schizophrenia than impairments in neurocognition.[5]There is also good evidence to suggest that social cognition training is an effective method of improving the social, psychological, and occupational outcomes for schizophrenia. A meta-analysis by Grynszpan and colleagues[6]found a medium effect size (0.64) for improvements in social cognition after computer-based remediation. Further, a recent review of 23 studies examining the efficacy of programs targeting facial affect recognition (FAR) deficits in schizophrenia concluded that they are efficacious in improving both FAR performance and functional status.[7]There is also evidence to suggest that social cognition remediation may be neuro-protective for individuals with schizophrenia. A two-year randomized control trial by Eack[8]found that participants who received Cognitive Enhancement Therapy – which includes social cognition tasks – had significantly greater grey matter preservation in the left hippocampus, parahippocampal gyrus, and the fusiform gyrus compared to participants who received enriched supportive therapy (who showed increased grey matter preservation in the left amygdala). Results such as these support the efficacy of cognitive remediation as an additional therapeutic option in schizophrenia.
Of all the processes included as components of social cognition, facial affect recognition is one of the best researched. Facial affect recognition is largely uninfluenced by medication but can be improved by training and remediation.[7,9,10]Facial affect recognition training – generally involving being asked to identify images of faces displaying emotion – has been shown to be effective for both male and female inpatients and outpatients with schizophrenia or schizoaffective disorder who are taking typical or atypical antipsychotic medication.[9-12]
In addition to the more recent support for social cognitive remediation, neurocognitive remediation is well established as an efficacious intervention for improving cognition in schizophrenia.[13,14]Remediation programs typically require highly trained clinicians, so they are costly in terms of time and funding and, thus,may be difficult to justify in routine practice. However,cognitive remediation programs have been shown to be cost-effective in the short-term,[15]and may also reduce long-term costs when the benefits of improved social and occupational functioning are sustained over time.[16]
Schizophrenia presents a unique and significant global public health issue, and China is no exception.[17]The majority of mental health treatment in China is administered by psychiatrists and psychiatric nurses in specialized psychiatric hospitals, most of which are located in urban centers. This is primarily due to the relatively small number of psychologists, social workers, and occupational therapists who could provide psychosocial support and to the limited training of community-based medical practitioners about the recognition and management of mental disorders.[18]
We have previously demonstrated the efficacy of a pilot program for the remediation of cognitive deficits in schizophrenia in China using a simple, cross-cultural computer-based drill training program.[19]This previous study was implemented in an inpatient setting and resulted in modest improvements on a test of attention.Moreover, there were additional and somewhat unexpected findings of improvements in social functioning and clinical symptoms in the intervention group. Given recent evidence of the importance of remediation of social functioning deficits, we sought to replicate our earlier study using an enhanced computerized remediation program that included facial affect recognition training. Further, we also wanted to explore the efficacy of the program in an outpatient setting. We aimed to assess the suitability of this program by conducting a six-week trial with a sample of outpatients in China. Based on our previous study,we hypothesized that the intervention group would demonstrate significant improvement in attention and,secondarily, that their facial affect recognition scores would improve after training. Based on the findings from the original study, we also hypothesized that the intervention group would improve on a measure of social functioning. Due to the relatively short duration of the intervention (6 weeks), significant changes to clinical symptoms were not expected.
2. Methods
The study, which was conducted at the Shanghai Mental Health Center, received institutional ethics approval. All participants provided written informed consent.
2.1 Participants
As shown in Figure 1, participants were male outpatients at the Shanghai Mental Health Center with a current DSM-IV diagnosis of schizophrenia as determined by the treating clinician based on a clinical interview and referral to the medical records. Potential participants had to be 18-55 years of age and not have a co-morbid substance use disorder. A total of 40 male outpatients were enrolled: 20 were long-term (>12 months) attendees of the hospital’s rehabilitation center(the intervention group), and 20 were outpatients at the hospital not attending the rehabilitation center who responded to flyers advertising the study (the control group). All enrolled patients were clinically stable, regularly taking stable doses of antipsychotic medication, and receiving regular follow-up in the outpatient department. Patients in the intervention group also received a 6-week computerized intervention that included both cognitive training and facial affect recognition (FAR) training (described below). All 40 participants completed baseline and 6-week follow-up assessments of clinical, cognitive,and social functioning.
2.2 Intervention
A description of the development and content of the cognitive remediation Computerized Drill Training (CDT)program administered to the intervention group has been reported previously.[19]It basically involves five simple, repetitive drill training exercises in arithmetic,number lists, pair matching, spatial working memory,and word lists.
The computerized facial affect recognition training module developed for this study utilized posed photographs from the Nanyang Technological University Asian Face Emotion Database, developed by Wong and Cho[20]at the Nanyang Technological University (NTU) in Singapore. This database contains facial photographs of 153 individuals of Asian heritage aged between 20 and 29 years who posed seven different facial expressions(neutral, angry, happy, sad, surprised, afraid, and disgusted) and consented to having these images stored on the database. (There are no reliability estimates for the emotional classification of these photographs yet available, but the database has been used in at least one published study of cross-cultural FAR.[21]) In this study we selected 96 photographs of individuals of Chinese ethnicity from the database that we considered best represented the six emotions (16 photographs of each of the six non-neutral emotions). The training sessions employed 72 photographs of 36 different individuals (12 photographs of each emotion) and the pre-post testing of FAR employed the remaining 24 photographs of 24 different individuals (4 photographs of each emotion).Six individuals appeared in both the training and testing components, but they displayed different emotions in the testing set than they did in the training set.
During the FAR training, the 72 different photographs were displayed in black and white and centered on a computer screen in random order with an equalmix of male and female faces. When a face appeared on the computer screen, participants were required to select which of the six possible emotion labels(happy, sad, afraid, angry, surprised, or disgusted) best described the emotion displayed in the stimulus face.Responses were not timed. In the event of an incorrect response, the correct label subsequently appeared on the screen. Each of the 72 photographs only appeared once during a training session. The FAR component of the computerized training took about 10 minutes to complete.

Figure 1. Flowchart of the study
In this study FAR training was combined with CDT;both separate modules were completed on the same computer and the order of presenting the two modules was randomized. It typically took 45-60 minutes to complete both modules. The training was done in group sessions monitored by two trained psychiatric nurses. Following an initial training session, participants were expected to complete at least 12 computerbased training sessions over a 6-week period. The mean (sd) number of CDT sessions completed by the 20 intervention-group patients was 12.85 (0.59) and the mean number of FAR training sessions completed was 12.60 (0.50). (The number of CDT and FAR sessions completed is slightly different because of technical difficulties during some training sessions.) As all participants in the intervention group completed at least 12 sessions, the 12th training session was considered the ‘f i nal’ session in the analysis.
2.3 Assessments
In order to allow comparison with our previous inpatient study, we used the same outcome measures employed in the earlier study[19]and added pre- and post-measures of facial affect recognition (FAR) to assess the effectiveness of the FAR component that was added to the cognitive remedial training package. Pre and post assessments of intervention and control group participants were conducted individually by either one of the trained psychiatric nurses or by one of the authors (LP). These evaluators were not blind to the group assignment of the individuals they assessed.
2.3.1 Clinical symptoms and social functioning
Participants in both groups were rated on three clinical scales measuring symptoms, global functioning, and social functioning. A Chinese version of the Positive and Negative Syndrome Scales (PANSS)[22]was administered to all participants at baseline and at the end of the trial by an experienced psychiatrist (LP)trained in the consensus rating of this measure.
The Clinical Global Impression Scale (CGI) is a wellrecognized tool of global functioning that yields three measures; severity of illness, global improvement, and an efficacy index. It has been widely used with Chinese individuals in Taiwan[23,24]and in mainland China.[25,26]The Severity of Illness item in the scale requires the clinicians to rate the severity of illness at the time of assessment on a 7-point scale from 1 (normal, not at all ill) to 7 (among the most severely ill). Thus, a lower score indicates better functioning. For this study, only the Severity of Illness item of the CGI was used.
The Personal and Social Performance Scale (PSP)[27]is a 100-point rating scale that assesses four domains of social functioning: a) socially useful activities, including work and study; b) personal and social relationships; c)self-care, and; d) disturbing and aggressive behaviors.The total score is divided into three levels: 71-100 is classified as ‘mild difficulties’; 31-70 as ‘disabled’,and 0-30 as ‘poor’ (i.e., requiring intensive support or supervision). A Chinese version of the PSP[28]has demonstrated good internal consistency (Cronbach’s alpha=0.84), test-retest reliability (intra-class correlation coefficient=0.95) and inter-rater reliability (kappa=0.82),as well as robust construct validity.
2.3.2 Neurocognitive assessment
The brief cognitive assessments included in this study,assessed by trained psychiatric nurses, included a measure of verbal working memory and a measure of verbal memory and learning. A Chinese version of the letter-number sequencing task (LNST)[29]was used to assess verbal working memory; the total score was used as the variable of interest. The verbal memory task employed was Form B of the Hong Kong List Learning Test (HKLLT),[30]a Chinese verbal learning and memory test that has been well validated in a variety of populations including individuals with schizophrenia.[31]Form B of the HKLLT yields information across four trials in a blocked condition where the words are semantically related; the total score for the test was used as the memory variable in this analysis.
2.3.3 Social cognitive assessment
Twenty-four photographs (4 for each of six emotions)from the NTU Asian Face Emotion Database (see above) were used at baseline and at the end of the 6-week trial to assess pre-post changes in facial affect recognition (FAR) in both groups. (These were different from the 72 photographs used in the training sessions with the intervention group.) Participants were shown the black and white photographs one at a time in a booklet and asked to circle the emotion that they thought the face was making. The number of correct assignments for each of the six emotions (scored 0-4)and for all 24 pictures (scored 0-24) was recorded for each respondent.
3. Data Analysis
Group differences in demographic characteristics and in baseline clinical, cognitive, and FAR variables were assessed using analysis of variance (ANOVA). At the end of the 6-week trial, within-group changes in clinical,cognitive, and FAR outcomes were assessed using paired sample t-tests,[33]and between-group differences in the changes over the 6 weeks were assessed using analysis of co-variance (ANCOVA) which adjusted for the baseline values of the outcome variables. In the ANCOVA for each outcome variable, Levene’s tests were all non-significant (p>0.05), indicating that the assumption of the equality of variances was met.
To assess the magnitude of any treatment effects and the magnitude of changes in the treatment-as-usual control group, we calculated group-specific standardized effect sizes for each variable of interest by dividing the difference of the pre-trial and post-trial means (for each group) by the standard deviation of the difference scores.[34]To examine any learning across the 12 sessions of the training program in the intervention group,mean performance at each session was charted and paired sample t-tests assessed differences between the fi rst and last session for each of the cognitive and FAR component tests.
4. Results
All 40 enrolled participants completed both the baseline assessment and the 6-week follow-up assessment. As shown in Table 1, there were no significant differences between groups in age, educational attainment,duration of illness, or daily chlorpromazine-equivalent[32]dosage of antipsychotic medication.

Table 1. Characteristics of the sample
4.1 Clinical, social, and neurocognitive results
The results of the clinical, social, and cognitive measures are shown in Table 2. At baseline the intervention group had significantly more severe positive symptoms of psychosis than the control group (as assessed by the PANSS positive symptom subscale). Both of the groups showed a small, but statistically significant decrease in the PANSS negative symptom subscale score over the 6-week trial. The intervention group also had a statistically significant decrease in the PANSS positive and general psychopathology subscale scores (see Table 2). However, at the end of the trial there were no significant differences in the clinical measures between the groups and there were no significant differences in the magnitude of change in these measures between the two groups.
Based on the results of the single measure of overall social functioning employed in the study (PSP),there were no significant differences between groups at baseline, but the intervention group experienced a much larger (statistically significant) improvement in social functioning than the control group over the 6-week trial. By the end of the trial, social functioning was significantly better in the intervention group than in the control group. However, at the end of the study the proportion of subjects in the three functional categories in the intervention group (0.0% poor, 97.6% disabled,2.4% mild difficulties) was not significantly different from the corresponding proportions in the control group(0.0% poor, 100.0% disabled, and 0.0% mild difficulties)(Mann-Whitney U=190.0, p=0.799).
The two measures used to assess neurocognitive functioning in the study had different results. The results for the LNST, which assesses verbal working memory,were similar in the two groups at baseline, improved slightly over the 6-week trial in both groups, and remained similar between groups at the end of the trial.Results for the HKLLT, which assesses verbal learning and memory, indicated that the intervention group had significantly poorer verbal learning skills than the control group at baseline and that these skills got significantly worse over the 6-week intervention. However, verbal learning also deteriorated in the control group over the 6-week trial, so after adjusting for baseline values the difference between groups in verbal learning at the end of the trial was no longer statistically significant.

Table 2. Result of the clinical, social functioning, and neurocognitive measures before and after the 6-week trial in the intervention and control groups
4.2 Results of facial emotion recognition tests
Results of the facial emotion recognition tests are shown in Table 3. Neither the total recognition score nor any of the 6 sub-scores were significantly different at baseline between the two groups. In the intervention group there was a significant improvement in the total score over the 6-week intervention, but after adjusting for baseline values the difference between the intervention group and control group at the end of the trial was not statistically significant (though there was a trend[p=0.09] in favor of the intervention group). Among the six sub-scores, both the recognition of sad faces and of fearful faces improved substantially in the intervention group (effect size >0.50), but when compared with the change in the control group, the only statistically significant improvement was in the recognition of fearful faces.

Table 3. Result of the facial emotion recognition tests before and after the 6-week trial in the intervention and control groups
4.3 Changes in cognitive scores and emotional recognition scores during the 12 training sessions in the intervention group
Figure 2 displays the mean performances for the intervention group across the 12 training sessions on the fi ve cognitive drill training tasks. Comparison of the mean (sd) score in the 12th session with that in the 1st session using paired t-tests had the following results:pair matching (t=-4.53, p<0.001) ; arithmetic (t=0.25,p=0.805), number list (t=-1.27, p=0.227), spatial working memory (t=0.47, p=0.647), and word lists (t=-0.33,p=0.198). Figure 3 displays the mean performance (i.e.,number of correctly identified faces) for the intervention group for each of the six different types of facial expressions in the FAR test over the 12 training sessions.Comparison of the mean number of correct responses in the 1stsession with that in the 12thsession, did not indicate significant improvement in the recognition of any of the six emotions. The mean (sd) total number of correct identif i cations in trial 1 was 10.5 (1.9) compared to 10.9 (2.7) in trial 12 (paired t=-0.69, p=0.495).

Figure 2. Mean performance on cognitive skills during the 12 training sessions conducted over 6 weeks in the intervention group(n=20)
5. Discussion
5.1 Main findings
The current study was designed to replicate our previous research using a simple user-friendly, computerbased drill training program that could be applied in a relative short time period in different cross-cultural seゆngs to enhance the performance of individuals with schizophrenia on tasks of memory, attention, and social functioning. We failed to replicate the gains that we found previously in an inpatient sample,[19]and we were unable to replicate the research of others who have used more sophisticated interventions[16]to improve attention and working memory. In fact, the intervention group performed significantly worse on a measure of verbal memory after the intervention than at baseline.Four of the five specific skills repeatedly practiced by individuals in the intervention group failed to show any improvement during the 12 training sessions over 6 weeks. This was a surprising finding given our prior study and the now substantial evidence for the effectiveness of cognitive remediation in schizophrenia.We speculate that there may be cohort-specific issues at play that resulted in the largely null findings. A recent paper[35]suggests that intrinsic motivation mediates the relationship between neurocognition and functional outcomes and that both the instructional method and the motivation of participants is important. It is possible that the current group lacked motivation to pay sufficient attention to the training tasks during the intervention.
The intervention group showed small but measurable improvements on the Personal and Social Performance (PSP) scale, a measure of social functioning. Although the improvements were not enough to improve the classification range for the group(most intervention group participants remained in the‘disabled’ range), it does give tentative support to the notion that involvement in remediation programs can have a positive effect on social functioning. Medalia’s recent review[36]concluded that cognitive remediation has the greatest effect on social functioning when it is part of an comprehensive psychosocial rehabilitation program, as was the case in this study. The rehabilitation program at the Shanghai Mental Health Center in which intervention group patients participated in has been shown to be effective in improving psychosocial outcomes for participants,[37]so this may have contributed to the modest improvement in social functioning seen over the 6 weeks of the current study.
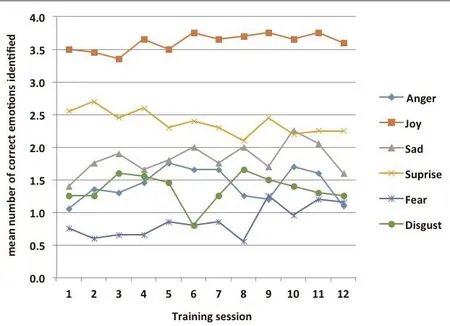
Figure 3. Mean performance on facial affect recognition test during the 12 training sessions conducted over 6 weeks in the intervention group (n=20)
In our previous study we also found significant improvements in the PANSS scores of the intervention group.[19]This finding was replicated here, but the improvements were modest and not significantly greater than those seen in the control group. Engagement in a relatively intensive remediation program may have a positive impact on symptomatology .
This study added a facial emotion recognition component to the training sessions that was not included in the previous study. Over the 12 training sessions the recognition of facial emotions by individuals in the intervention group did not improve significantly for any of the six facial emotions, but the pre-post change in the FAR test (which used different faces than used in the training sessions) did show a statistically significant improvement in the overall emotional recognition rate in the intervention group and a statistical trend of a greater improvement in the intervention group than in the control group(p=0.09). Considering recognition of the six specific emotions, only the recognition of fear and sadness substantially improved over the six weeks of training in the intervention group (effect size >0.50), and only the recognition of fearful faces improved significantly more in the intervention group than in the control group.This may be related to the fi nding that the recognition of negative emotions of fear, sadness and disgust have been found to be differentially more impaired than the recognition of other emotions in individuals with schizophrenia[38-41]and in relatives of people with schizophrenia.[42]
5.2 Limitations
There are several limitations to this study that will need to be addressed in future studies. a) The sample size was relatively small (although comparable to other studies utilizing drill and practice training[43-45]) so some of the negative findings may have been due to Type II errors. b) The sample was limited to males who were currently using antipsychotic medications and being treated at a single specialized psychiatric hospital. It is unclear how generalizable the results are to other individuals with schizophrenia. c) Assignment to the intervention and control group was not randomized; all intervention group subjects were currently participating in a rehabilitation program while all control group subjects were not. d) The clinicians who assessed outcomes were not blind to the group assignment of the patients they were evaluating. e) The photographs used in training and testing of FAR were limited to individuals 20-29 years of age. Whether or not the ability to recognize the facial emotions of individuals in these ages is representative of the ability to recognize the facial emotions of individuals at other ages (whom individuals with schizophrenia frequently need to interact with) is unknown. f) The test-retest reliability and other psychometric measures of the outcome variable used to assess the effectiveness of FAR training(the number of correctly identified facial emotions)has never been tested so the validity of this measure remains unknown. g) No adjustment was made for the IQ of participants. h) A single measure was used to assess social functioning; a more comprehensive assessment will be needed to confirm the finding of improved social functioning with the remediation training. i) Several of the remediation training tasks were aimed at spatial working memory but this was not assessed before and after the intervention.
5.3 Importance
The results of this study highlight the fact that to be effective cognitive remediation for individuals with schizophrenia needs to be conducted in a specific type of setting in which participants are actively engaged in the process. This study is one of the first attempts to integrate FAR training as part of a short, computeradministrated cognitive remediation program in China. The results suggest (but do not prove) that this type of training could improve overall recognition of facial emotions (particularly fear) by individuals with schizophrenia, but more work on developing the content, duration, and frequency of the training and on testing the resultant training modules in larger,more diverse samples will be needed before this can be recommended as a standard part of cognitive remediation programs.
Acknowledgement
We would like to thank the participants and their families for their time.
Funding
This research was supported by funds awarded to Dr. Linda Byrne from the Faculty of Health at Deakin University, Melbourne, Australia and the Australia-China Science and Research Fund.
Conflict of interest statement
The authors declare no conflict of interest related to the manuscript.
Informed consent
All participants in the study signed written informed consents for participating in the study.
Ethical review
The institutional review board of the Shanghai Mental Health Center and the Human Research Ethics Committee of Deakin University approved this study.
1. Swartz MS, Perkins DO, Stroup TS, Davis SM, Capuano G,Rosenheck RA, et al. Effects of antipsychotic medications on psychosocial functioning in patients with chronic schizophrenia: findings from the NIMH CATIE study. Am J Psychiatry. 2007; 164(3): 428-436
2. Eack SM. Cognitive remediation: A new generation of psychosocial interventions for people with schizophrenia.Soc Work. 2012; 57(3): 235-246
3. Ziv I, Leiser D, Levine J. Social cognition in schizophrenia:Cognitive and affective factors. Cogn Neuropsychiatry. 2011;16(1): 71-91. doi: http://dx.doi.org/10.1080/13546805.2010.492693
4. Brekke JS, Hoe M, Long J, Green MF. How neurocognition and social cognition influence functional change during community-based psychosocial rehabilitation for individuals with schizophrenia. Schizophr Bull. 2007; 33(5): 1247-1256.doi: http://dx.doi.org/10.1093/schbul/sbl072
5. Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J,Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011; 35(3): 573-588.doi: http://dx.doi.org/10.1016/j.neubiorev.2010.07.001
6. Grynszpan O, Perbal S, Pelissolo A, Fossati P, Jouvent R,Dubal S, et al. Efficacy and specificity of computer-assisted cognitive remediation in schizophrenia: a meta-analytical study. Psychol Med. 2010. 41(01): 163-173. doi: http://dx.doi.org/10.1017/S0033291710000607
7. Statucka M, Walder DJ. Efficacy of social cognition remediation programs targeting facial affect recognition deficits in schizophrenia: a review and consideration of high-risk samples and sex differences. Psychiatry Res.2013; 206(2-3): 125-139. doi: http://dx.doi.org/10.1016/j.psychres.2012.12.005
8. Eack SM, Hogarty GE, Cho RY, Prasad KM, Greenwald DP,Hogarty SS, et al. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia. Arch Gen Psychiatry. 2010; 67(7): 674. doi:http://dx.doi.org/10.1001/archgenpsychiatry.2010.63
9. Sachs G, Winklbaur B, Jagsch R, Lasser I, Kryspin-Exner I, Frommann N, et al. Training of affect recognition(TAR) in schizophrenia – Impact on functional outcome.Schizophr Res. 2012; 138(2-3): 262-267. doi: http://dx.doi.org/10.1016/j.schres.2012.03.005
10. Wölwer W, Frommann N, Halfmann S, Piaszek A, Streit M, Gaebel W. Remediation of impairments in facial affect recognition in schizophrenia: Efficacy and specificity of a new training program. Schizophr Res. 2005; 80(2-3): 295-303. doi:http://dx.doi.org/10.1016/j.schres.2005.07.018
11. Frommann N, Streit M, Wölwer W. Remediation of facial affect recognition impairments in patients with schizophrenia: a new training program. Psychiatry Res. 2003;117(3): 281-284. doi: http://dx.doi.org/10.1016/S0165-1781(03)00039-8
12. Silver H, Goodman C, Knoll G, Isakov V. Brief emotion training improves recognition of facial emotions in chronic schizophrenia. A pilot study. Psychiatry Res.2004. 128(2): 147-154. doi: http://dx.doi.org/10.1016/j.psychres.2004.06.002
13. Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia:methodology and effect sizes. Am J Psychiatry. 2011;168(5): 472-485. doi: http://dx.doi.org/10.1176/appi.ajp.2010.10060855
14. McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT.A meta-analysis of cognitive remediation in schizophrenia.Am J Psychiatry. 2007; 164(12): 1791-802. doi: http://dx.doi.org/10.1176/appi.ajp.2007.07060906
15. Patel A, Knapp M, Romeo R, Reeder C, Matthiasson P, EverittB, et al. Cognitive remediation therapy in schizophrenia: costeffectiveness analysis. Schizophr Res. 2010; 120(1-3): 217-224. doi: http://dx.doi.org/10.1016/j.schres.2009.12.003
16. Wykes T, Spaulding WD. Thinking about the future cognitive remediation therapy – what works and could we do better?Schizophr Bull. 2011. 37(suppl 2): S80-S90. doi: http://dx.doi.org/10.1093/schbul/sbr064
17. Jablensky A. Epidemiology of schizophrenia: the global burden of disease and disability. Eur Arch Psychiatry Clin Neurosci. 2000; 250(6): 274-285
18. Phillips MR. Characteristics, experience, and treatment of schizophrenia in China. Dialogues Clin Neurosci. 2001; 3(2):109-117
19. Byrne LK, Peng D, McCabe M, Mellor D, Zhang J, Zhang T,et al. Does practice make perfect? Results from a Chinese feasibility study of cognitive remediation in schizophrenia.Neuropsychol Rehabil. 2013; 23(4): 580-596. doi: http://dx.doi.org/10.1080/09602011.2013.799075
20. Wong JJ, Cho SY. A local experts organization model with application to face emotion recognition. Expert Systems with Applications. 2009; 36(1): 804-819
21. Prado C, David M, Byrne LK, Wilson C, Xu X, Liu H. Facial emotion recognition: a cross-cultural comparison of Chinese, Chinese living in Australia, and Anglo-Australians.Motiv Emot. 2013; 38(3): 420-428
22. He YL, Zhang MY. [The Positive and Negative Syndrome Scale (PANSS) and its application]. Lin Chuang Jing Shen Yi Xue Za Zhi. 1997; 7(6): 353–355. Chinese
23. Chiu NY, Yang YK, Chen PS, Chang CC, Lee IH, Lee JR.Olanzapine in Chinese treatment-resistant patients with schizophrenia: an open-label, prospective trial. Psychiatry Clin Neurosci. 2003; 57(5): 478–484. doi: http://dx.doi.org/10.1046/j.1440-1819.2003.01151.x
24. Chan HY, Lin WW, Lin SK, Hwang TJ, Su TP, Chiang SC, et al.Efficacy and safety of aripiprazole in the acute treatment of schizophrenia in Chinese patients with risperidone as an active control: a randomized trial. J Clin Psychiatry. 2007;68(1): 29-36
25. Liu J, Guo YQ, Yang XL. [Efficacy and tolerability of olanzapine on childhood-onset schizophrenia].Zhongguo Xin Li Wei sheng Za Zhi. 2005; 19: 71-81. Chinese. doi: http://dx.chinadoi.cn/10.3321/j.issn:1000-6729.2005.02.002
26. Zhang ZJ, Kang WH, Li Q, Wang XY, Yao SM, Ma AQ.Beneficial effects of ondansetron as an adjunct to haloperidol for chronic, treatment-resistant schizophrenia:a double-blind, randomized, placebo-controlled study.Schizophr Res. 2006; 88: 102-110. doi: http://dx.doi.org/10.1016/j.schres.2006.07.010
27. Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101(4): 323-329. doi: http://dx.doi.org/10.1034/j.1600-0447.2000.101004323.x
28. Si TM , Shu L, Su YA, Tian CH, Yan Y, Cheng J, et al. The Chinese version of the Personal and Social Performance scale(PSP): validity and reliability. Psychiatry Res. 2010; 185: 275-279. doi: http://dx.doi.org/10.1016/j.psychres.2010.05.001
29. Chan RC, Wang Y, Deng Y, Zhang Y, Yiao X, Zhang C. The development of a Chinese equivalence version of the letter-number span test. Clin Neuropsychol. 2008; 22: 112-121. doi: http://dx.doi.org/10.1080/13825580601025957
30. Chan AS. The Hong Kong List Learning Test, 2nd Edition.Hong Kong: Chinese University of Hong Kong; 2006
31. Chan AS, Kwok IC, Chiu H, Lam L, Pang A, Chow LY.Memory and organizational strategies in chronic and acute schizophrenia patients. Schizophr Res. 2000;41(3): 431-445. doi: http://dx.doi.org/10.1016/S0920-9964(99)00078-X
32. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardised method for comparing exposure to different drugs. Biol Psychiatry. 2010; 67(3): 255-262. doi: http://dx.doi.org/10.1016/j.biopsych.2009.08.040
33. Judd CM, McClelland GH, Culhane SE. Data analysis:continuing issues in the everyday analysis of psychological data. Annu Rev Psychol. 1995; 46(1): 433-465. doi: http://dx.doi.org/10.1146/annurev.ps.46.020195.002245
34. Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013; 4: 863. doi: http://dx.doi.org/10.3389/fpsyg.2013.00863
35. Correard N, Mazzola-Pomietto P, Elissalde SN, Viglianese-Salmon N, Fakra E, Azorin JM. [What perspectives for cognitive remediation in schizophrenia?]. Encephale.2011; 37(Suppl 2): S155-60. French. doi: http://dx.doi.org/10.1016/S0013-7006(11)70044-6
36. Medalia A, Choi J. Cognitive remediation in schizophrenia.Neuropsychol Rev. 2009; 19(3): 353-364
37. Pan L, Mellor D, McCabe M, Hill Briony, Tan W, Xu Y.An evaluation of the Shanghai Mental Health Service Schizophrenia Rehabilitation Program. Am J Psychiatr Rehabil. 2011; 14(4): 287-306. doi: http://dx.doi.org/10.10 80/15487768.2011.622150
38. Bediou B, Krolak-Salmon P, Saoud M, Henaff MA, Burt M, Dalery J, et al. Facial expression and sex recognition in schizophrenia and depression. Can J Psychiatry. 2005;50(9): 525-533
39. Bellack AS, Mueser KT, Wade J, Sayers S, Morrison RL. The ability of schizophrenics to perceive and cope with negative affect. Br J Psychiatry. 1992; 160: 473-480
40. Kohler CG, Turner TH, Bilker WB, Brensinger CM, Siegel SJ,Kanes SJ, et al. Facial emotion recognition in schizophrenia:intensity effects and error pattern. Am J Psychiatry. 2003;160(10): 1768-1774
41. Souto T, Baptista A, Tavares D, Queirós C, Marques A.[Facial emotional recognition in schizophrenia: preliminary results of the virtual reality program for facial emotional recognition]. Rev Psiquiatr Clín. 2013. 40(4): 129-134.Portuguese. doi: http://dx.doi.org/10.1590/S0101-60832013000400001
42. Bediou B, Asri F, Brunelin J, Krolak-Salmon P, D'Amato T, Saoud M, et al. Emotion recognition and genetic vulnerability to schizophrenia. Br J Psychiatry. 2007; 191:126-130.
43. Bellucci DM, Glaberman K, Haslam N. Computer-assisted cognitive rehabilitation reduces negative symptoms in the severely mentally ill. Schizophr Res. 2003; 59(2-3): 225-232.
44. Sartory G, Zorn C, Groetzinger G, Windgassen K.Computerized cognitive remediation improves verbal learning and processing speed in schizophrenia.Schizophr Res. 2005; 75(2-3): 219-223. doi: http://dx.doi.org/10.1016/j.schres.2004.10.004
45. Ueland T, Rund BR. A controlled randomized treatment study: the effects of a cognitive remediation program on adolescents with early onset psychosis. Acta Psychiatr Scand. 2004; 109(1): 70-74. doi: http://dx.doi.org/10.1046/j.0001-690X.2003.00239.x
(received, 2015-08-25; accepted, 2015-09-25)

Dr. Linda K Byrne graduated with a master’s degree of Clinical Neuropsychology from Macquarie University, Sydney, Australia in 2006 and received her PhD from the University of Southern Queensland, Queensland, Australia in 2008. She began work in Deakin University in Melbourne in 2006 and currently is a senior lecturer in the School of Psychology, Faculty of Health. Dr. Byrne’s major area of research is the understanding and remediation of neuro- and social cognitive deficits in clinical disorders, including ADHD, schizophrenia, and neurodegenerative disorders. She has published three book chapters and a number of journal articles in the area of neuropsychiatric disorders. She has an ongoing collaboration with the Shanghai Mental Health Center in China. More recently, Dr. Byrne’s research has been focusing on agents that may protect or improve cognitive functioning in both healthy and compromised populations.
对一项为期六周、基于计算机的慢性精神分裂症社会认知矫正项目的评估
Byrne LK, Pan LY, McCabe M, Mellor D, 徐一峰
精神分裂症;认知康复;工作记忆;社会认知;面部表情识别;对照试验;中国随机对照试验;中国
Background:Programs to remediate cognitive deficits have shown promising results in schizophrenia, but remediation of social cognition deficits is less well understood. Social cognitive deficits may cause more disability than the widely recognized neurocognitive deficits, suggesting that this is an area worthy of further investigation.Aim:Implement and evaluate a brief computerized cognitive remediation program designed to improve memory, attention, and facial affect recognition (FAR) in outpatients with chronic schizophrenia.Methods:Baseline assessments of FAR and of clinical, cognitive, and psychosocial functioning were completed on 20 males with schizophrenia enrolled in an outpatient rehabilitation program at the Shanghai Mental Health Center (the intervention group) and on 20 males with schizophrenia recruited from among regular outpatients at the Center (the control group). Both groups received treatment as usual, but the intervention group also completed an average of 12.7 sessions of a computer-based remediation program for neurocognitive, social, and FAR functioning over a 6-week period. The baseline measures were repeated in both groups at the end of the 6-week trial.Results:There were no statistically significant differences in the changes in clinical symptoms (assessed by the Positive and Negative Syndrome Scale, PANSS) or cognitive measures (assessed using the Hong Kong List Learning Test and the Letter-Number Sequencing Task) between the intervention and control groups over the 6-week trial, but there were modest improvements on the PANSS for the intervention group between baseline and after the intervention. There was a significantly greater improvement in the social functioning measure (the Personal and Social Performance scale, PSP) in the intervention group than in the control group. The pre-post change in the total facial recognition score in the intervention group was statistically significant (paired t-test=-2.60, p=0.018), and there was a statistical trend of a greater improvement in facial recognition in the intervention group than in the control group (F(1,37)=2.93; p=0.092).Conclusions:Integration of FAR training with a short, computer-administrated cognitive remediation program may improve recognition of facial emotions by individuals with schizophrenia, and, thus, improve their social functioning. But more work on developing the FAR training modules and on testing them in larger, more diverse samples will be needed before this can be recommended as a standard part of cognitive remediation programs.
[Shanghai Arch Psychiatry. 2015, 27(5): 296-306.
http://dx.doi.org/10.11919/j.issn.1002-0829.215095]
1School of Psychology, Deakin University, Melbourne, Australia
2Shanghai Mental Health Centre, Shanghai, China
*correspondence: linda.byrne@deakin.edu.au
A full-text Chinese translation of this article will be available at http://dx.doi.org/10.11919/j.issn.1002-0829.215095 on February 26, 2016.
背景:在精神分裂症患者中,认知缺陷矫正项目已经取得可喜成果,但人们对社会认知缺陷矫正知之甚少。神经认知缺陷造成的功能受损已广为知晓,而与之相比社会认知缺陷所致损害可能会更明显,这表明社会认知缺陷值得深入研究。目的:在门诊慢性精神分裂症患者中,开展一个简明的计算机化认知矫正项目,旨在改善患者的记忆力、注意力和面部表情识别 (facial affect recognition, FAR) 能力。方法:干预组为20例在上海市精神卫生中心参加门诊康复计划的男性精神分裂症患者,对照组为20例在该中心普通门诊招募的男性精神分裂症患者。基线时评估两组患者FAR能力、临床症状、认知和社会心理功能。两组均接受常规治疗,与此同时干预组在6周内完成了平均12.7次的对于神经认知功能、社会功能和FAR能力的计算机化矫正项目。6周研究结束时两组均用基线时的测验再次进行功能评估。结果:在6周的研究期间,干预组和对照组的临床症状(采用阳性和阴性症状量表评估)变化、认知功能(采用香港列表学习测验和字母——数字排序任务评估)变化的组间差异均无统计学意义。然而,干预组的社会功能评定结果改善比对照组显著(采用个人和社会功能量表评估)。治疗前后干预组面部识别测验总分的变化有统计学意义(配对t检验,t=-2.60,p=0.018),干预组面部识别能力的改善比对照组的改善有更明显的统计学趋势 (F(1,37)=2.93; p=0.092)。结论:采用短期的、计算机化认知矫正模式与面部表情识别训练整合可能会改善精神分裂症患者对面部情感的识别能力,进而改善他们的社会功能。但还需要做很多工作来完善面部表情识别训练的模式,并且需要在更大规模、更多样化的样本中测试验证,才可以将这一模式作为认知矫正计划的一个标准组成部分进行推广。
本文全文中文版从2016年2月26日起在
http://dx.doi.org/10.11919/j.issn.1002-0829.215095可供免费阅览下载
- 上海精神医学的其它文章
- Meta-analysis of the relationship of peripheral retinal nerve fiber layer thickness to Alzheimer’s disease and mild cognitive impairment
- Adjunctive treatment with high frequency repetitive transcranial magnetic stimulation for the behavioral and psychological symptoms of patients with Alzheimer’s disease:a randomized, double-blind, sham-controlled study
- Effectiveness of Traditional Chinese Medicine (TCM) treatments on the cognitive functioning of elderly persons with mild cognitive impairment associated with white matter lesions
- Heredity in comorbid bipolar disorder and obsessive-compulsive disorder patients
- The effect of simple imputation on inferences about population means when data are missing in biomedical research due to detection limits
- Infanticide by a mother with untreated schizophrenia

