脊柱多发硬化性上皮样纤维肉瘤一例报告
宇尧 刁小莉 杜心如 骆辉 徐子
脊柱多发硬化性上皮样纤维肉瘤一例报告
宇尧 刁小莉 杜心如 骆辉 徐子
脊柱;纤维肉瘤;诊断,鉴别;骨肿瘤;外科手术
硬化性上皮样纤维肉瘤 ( sclerosing epithelioid fibrosarcoma,SEF ) 是一种罕见的特殊类型纤维肉瘤[1]。由Meis-Kindblom 等[2]于 1995 年首次报道。迄今国内外文献共报道 100 余例[3-4]。多发生在四肢等软组织,原发于脊柱骨质的 SEF 目前未见报道。现将我院经治的 1 例脊柱多发 SEF 报道如下。
资料与方法
一、一般资料
患者,男,62 岁,因“肩背痛 2 个月,伴双下肢麻木 1 个月,伴双下肢无力 6 天”入院。初步诊断为“双下肢无力麻木原因待查,脊髓病不除外”,收入神经内科,给予激素及脱水治疗,症状无好转。入院查体:T5以下感觉减退;双上肢正常;左下肢肌力 IV 级、右下肢肌力III 级;肌张力高、腱反射活跃、双侧病理征未引出。化验检查:CEA 3.47 ng / ml;Ca 2.09 mmol / L。超声:肝胆胰脾双肾未见明显占位病变。 影像学检查:颈椎 X 线片示 C2椎板及附件骨质破坏;CT 示 C2椎板占位病变,椎管管径明显减小;MRI 示 C2椎板附件破坏,肿块突入椎管,颈髓受压。胸椎 X 线片示 T1、T4~6多发性骨质破坏;MRI 示胸段 T1、T4~6椎体及附件多发占位并侵入椎管,胸髓受压。全身骨显像示颅骨、右侧第 7、8 肋,双侧肩胛骨、脊柱、骨盆多发骨质破坏,考虑为恶性病变。初步诊断:颈胸椎多发骨破坏,脊髓压迫症,Frankle 分级D 级,二便失禁。
二、治疗
第一期全麻下行胸椎后路肿瘤切除+椎板减压+椎弓根螺钉内固定术,术中所见,肿瘤侵犯至 T4~6椎板并突入椎管,脊髓硬膜囊受压。侵蚀骨质且局部骨皮质膨胀,可见硬化边缘,未见包膜,肿瘤灰白,大小 7.5 cm× 3.5 cm×3 cm,切面灰白质硬,可见部分骨化。将肿瘤完全切除直至正常骨组织内,侵及软组织部分切除至正常软组织内 3 cm。第一期术后 2 周行颈椎后路肿瘤切除+椎板减压+C1~3椎弓根螺钉+侧块螺钉内固定术,术中所见肿瘤破坏 C2椎板,突入椎管,压迫颈髓后部,肿瘤肉眼所见同前,术后患者症状减轻、神经功能逐步恢复。第二期手术范围同第一期手术。术后未行放化疗,随访 2 年,手术部位无复发,Frankle E 级,二便功能恢复,生活自理( 图 1 )。
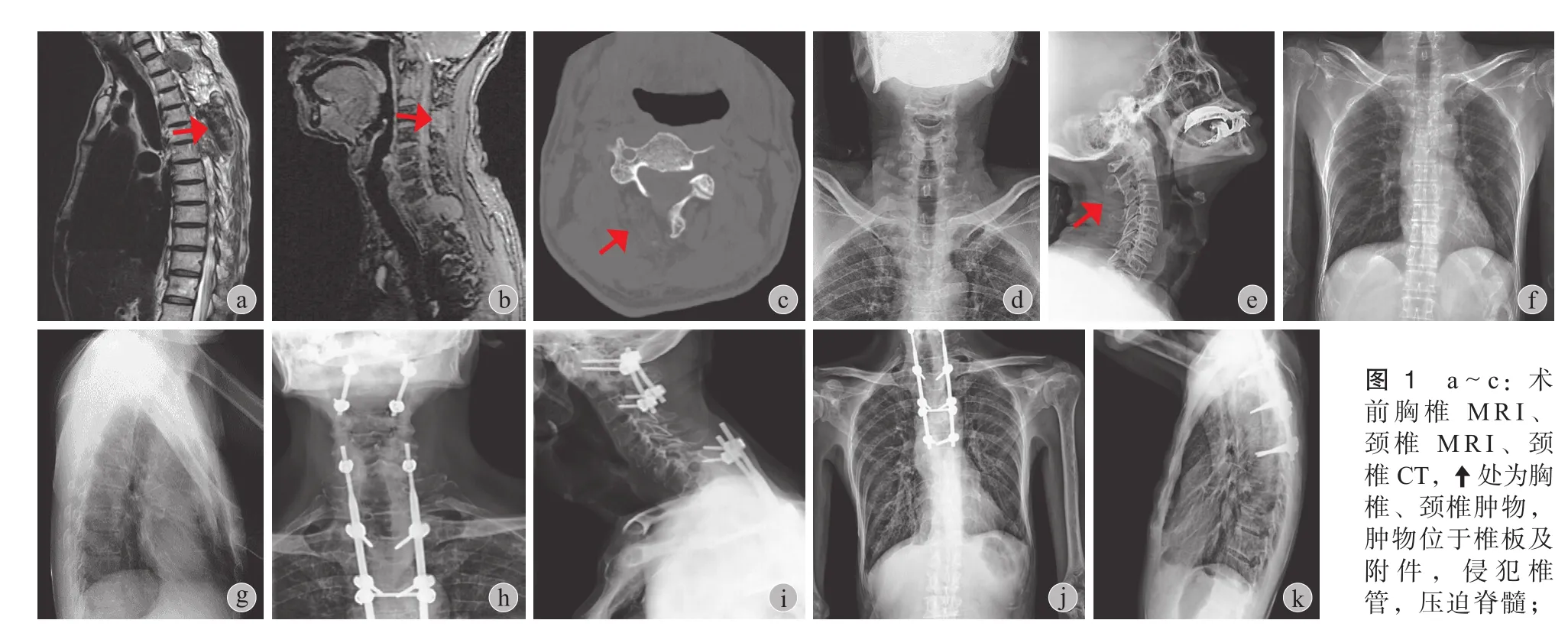
图 1 a~c:术前胸椎 MRI、颈椎 MRI、颈椎 CT,处为胸椎、颈椎肿物,肿物位于椎板及附件,侵犯椎管,压迫脊髓;d~g:术前颈椎、胸椎 X 线片,C2、T1、T4~6多发性骨质破坏;h~k:术后颈椎、胸椎术后正侧位 X 线片:可见内固定位置良好,术前软组织肿物影消失Fig.1 a-c: CT and MRI images before surgical treatment. Theshowed the tumor in the cervical and thoracic vertebra, invading the canal and compressing the spinal cord; d-g: X-ray images of the cervical and thoracic vertebra before surgical treatment, tumors were found in the vertebra of C2, T1and T4-6. h-k: X-ray images of the cervical and thoracic vertebra after surgical treatment: good fxation, preoperative soft tissues disappeared
三、病理结果
梭形细胞肿瘤,细胞密集,肿瘤边界不清,局部边缘可见骨壳样结构形成。细胞界限清楚,部分呈上皮样,核分裂象可见。细胞间可见胶原纤维沉积,软骨化、骨化,部分区域可见地图样坏死。免疫组化:Vimentin( 3+ ),CD99 ( 3+ ),Bc1-2 ( 2+ ),KI-67-LI ( 密集区约15%+ ),CD117 部分细胞胞浆弱阳;SMA ( - ),Desmin( - ),NF ( - ),CgA ( - ),S-100 ( - ),HMB-45 ( - ),CD34 ( - ),EMA ( - ),CK8 ( - ),CK19 ( - ),CK7 ( - ),p53 ( - ),CK ( - ),CK5 ( - ),GFAP ( - ),CD68 ( - ),CD21 ( - ),CD163 ( - ),CD1a ( - ),S-100 ( - )。肿瘤分级:依据 FNCLCC 分级系统[5]评分:胸椎原发肿瘤免疫组化:Vimentin ( 3+ ),CD99 ( 3+ ),Bc1-2 ( 2+ ),KI-67-LI ( 密集区约 15%+ ),余均阴性,胸椎原发肿瘤为 I 级3 分;颈椎原发肿瘤免疫组化:Vimentin ( 3+ )、Ki-67 指数密集区约 15%,余均阴性。为 I 级 2 分,确诊:脊柱原发 SEF,恶性程度较低 ( 图 2 )。
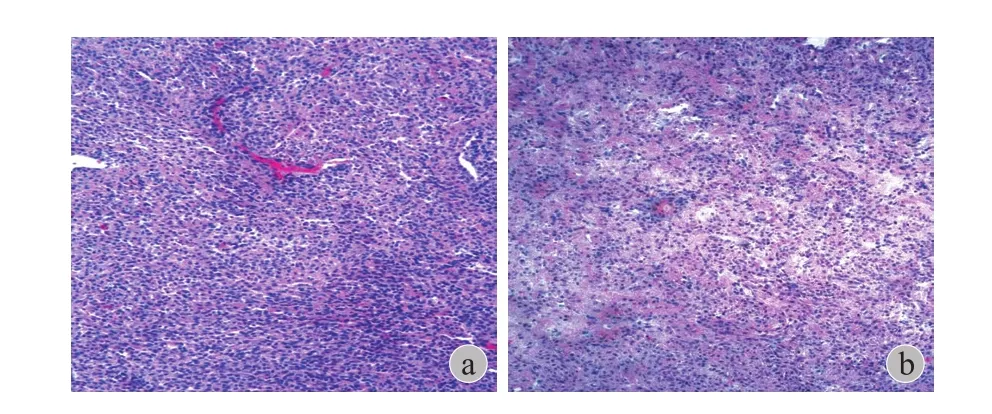
图 2 a:胸椎病灶病理 ( HE ×10 ):梭形肿瘤细胞密集,部分呈上皮样。细胞间可见胶原纤维沉积;b:颈椎术后病理图像( HE ×10 ):短梭形肿瘤细胞异型性不明显,密度较大。细胞间可见胶原纤维沉积,骨化Fig.2 a: Pathology of the case ( H&E stain, ×10 ). The tumor was composed of small to medium size epithelioid cells with pale or clear cytoplasm. The tumor cells were arranged in nests, cords and clusters, and were embedded in dense hyalinized collagenous matrix;b: ( H&E stain, ×10 ). No severe atypia in short spindle cells. The tumor cells were also embedded in dense hyalinized collagenous matrix. Collagen deposition and ossifcation were showed
讨 论
SEF 形态特殊,病理组织学特点为硬化胶原间质中上皮样肿瘤细胞呈巢状或条索状分布, 类似于分化差的癌、硬化性淋巴瘤或滑膜肉瘤。WHO 软组织肿瘤分类将其归为纤维母细胞 / 肌纤维母细胞肿瘤[6]。过去认为其是低级别变异型纤维肉瘤,但其复发率和致死率高,现认为其是高级别肿瘤[7]。成年人多见,中位年龄 45 岁[8],男性略多[9]。常位于四肢深部临近筋膜、骨膜及神经膜的肌肉组织,极少侵犯骨骼及皮下组织[10]。多位于下肢、躯干、上肢、头颈等部[11-12],少数也可累及额骨、骶骨、颅内、垂体、神经系统、鼻腔、盲肠、腹腔、腹膜后、肾脏、盆腔、卵巢、阴茎根部[13-20]。文献中尚无累及颈椎及胸椎引起脊髓压迫症的报道。平均病程 33 个月,主要表现为局部包块及疼痛[11]。发生机制尚不明确[21]。可能与电离辐射有关[21],纤维母细胞转变为恶性肿瘤细胞时抑癌基因 p53、RB[22]及原癌基因 MDM2、CDK4 发生了改变,MDM2 过度表达,其产物与 p53 结合抑制了 p53 进入胞核并使 p53 泛素化丧失抑制细胞凋亡的能力。CDK4 使RB 磷酸化而失活,导致 G1 期 -S 期失控,细胞呈肿瘤性生长[22-23]。
SEF 无特殊影像学表现,易与其它肿瘤混淆。X 线片常为均匀低密度影,囊性变、小钙化灶及化生骨少见。溶骨性骨破坏时[24],恶性程度低病灶边界清楚且规则,恶性程度高病灶边界模糊不规则并侵犯周围软组织。因病灶中胶原成分多细胞成分少,所以 CT、MRI 上常表现为低密度低信号影。肿瘤分化程度不同增强后肿块内强化的程度也不同,对于难以鉴别的病例可采用增强 CT、MRI。
SEF 的确诊主要依靠病理学表现,其最主要病理学特点为免疫组化仅 Vimeintn 阳性余均阴性。肿瘤一般1~25 cm 大小,平均 8.3 cm[5]。边界清楚,结节状或分叶状,质硬,无包膜或一层假包膜,切面灰白,可见黏液样区及囊性变,坏死钙化少见,边缘可见化生样骨组织。镜下可见上皮样细胞周围有大量玻璃样硬化胶原纤维,可表现骨样、软骨样或黏液样变。瘤细胞呈上皮样,中等大小,呈圆形、卵圆形、多边形或梭形,以条索状、巢状或腺泡状分布。胞浆透亮或淡嗜酸性。核呈圆形、卵圆形或不规则形,淡染,仁居中,体积小,染色质均匀,分裂象少见[2,8],异形型不明显,仅在细胞丰富区域可见,无瘤巨细胞。细胞少间质多区域,呈纤维瘤样改变;细胞多间质少区域,可见经典纤维肉瘤或黏液纤维肉瘤及结节状排列图像。有时可见钙化及血管外皮瘤样血管。肿瘤坏死少见,可表现为小灶凝固性坏死[2,8]或大片坏死[17]。肿瘤组织可浸润周围的软组织、血管、骨骼。淋巴结转移少见,Antonescu 等[11]报道 1 例广泛腋下淋巴结转移 ( 20 / 22 )。本例为多发骨破坏,未发现其它部位病灶,由于颈椎及胸椎病灶分级有一定差别,可能均为原发病灶,其它骨病灶为转移所致。免疫组织化学仅 Vimentin 呈强阳性,CD30、CD31、CD45、CD56、CD57、CD68、SMA、GFAP、LCA、CK7、Desmin、MyoD1、Mygenin、AE1 / AE3、结蛋白、FVIII Rag、actin 及 HMB-45 等均为阴性[17-25]。有报道 Bcl-2、细胞角蛋白、CD34、CD99、CK、MDM2、p53、CAM5.2、MiC2、Fli-1、β2- 微球蛋白、EMA、CK、S-100、NSE、Fascin、INI-1[17-26]可呈阳性或局灶阳性。细胞增殖活性低,MIB-1 平均指数约为5%。肿瘤增生标记物 PCNA、Ki-67 与预后无关[2]。本例符合上述特点。
本病发生于脊柱,应与以下几种疾病相鉴别。( 1 )脊柱转移癌:癌症病史,细胞异型性明显、核分裂象多见,SEF 特征性富细胞区排列形态少见,CK ( + );( 2 ) 上皮样平滑肌肉瘤:细胞异型性明显,核分裂象多见。免疫组化表达 CD31、CD34、keratin、EMA、SMA、MSA、desmin 和结蛋白;( 3 ) 中枢神经系统孤立性纤维瘤( solitary fibrous tumor,SFT ):间质胶原形态多样,肿瘤细胞缺乏典型的巢状或腺泡状排列,常表达 CD99、Bcl-2 且CD34 表达较 SEF 弥漫;( 4 ) 滑膜肉瘤 ( synovial sarcoma,SS ):常有双向分化,大多数表达多种 CK ( 包括 AE1 / AE3、CK7、CK19 等 ),可弥漫表达 Bcl-2[27];( 5 ) 低级别纤维黏液样肉瘤:当 SEF 出现纤维瘤样和黏液变性区域时,类似于低级别纤维黏液样肉瘤,但后者瘤细胞没有上皮样细胞的特征;( 6 ) 骨外骨肉瘤:SEF 玻璃样间质类似于骨肉瘤的未成熟性骨样组织,但骨肉瘤的骨样组织有基质硬化现象,多能看到较成熟的骨样组织或骨组织,常伴有恶性软骨组织,且肉瘤细胞多形性、异型性较明显,核分裂象易见,并表达 Oteocalcin;( 7 ) 硬化性淋巴瘤:包括霍奇金淋巴瘤及非霍奇金淋巴瘤:表达 CD45 或存在典型的 R-S 细胞,本病 LCA ( - ),可帮助鉴别诊断。
目前,手术是治疗本病的惟一手段,应彻底切除肿瘤并尽可能扩大切除。术后放疗及化疗能否减低复发率还不明确。虽然 SEF 生物学行为表现为低度恶性,但随着病程延长可进展为高度恶性[27]。如果表现为体积大、远处转移、复发、细胞异型性明显、核分裂活跃、伴有大片坏死、骨及脑脊髓侵犯,提示恶性程度高,预后不佳[28]。Meis-Kindblom 等[2]报道的 25 例中,复发率、转移率、死亡率分别为 53%、43%、25%。Chow 等[27]总结的 44 例随访资料中,复发率、转移率及死亡率分别为 48%、60% 和35%。Antonescu 等[11]报道的 16 例中,复发率、转移率、病死率甚至达到了 50%、86%、57%,但这可能与肿瘤较大和位于颅内等有关。Ossendorf 等[8]报道超过 27% 的患者初诊时即发生远处转移。本例脊柱 ( 颈椎、胸椎 ) SEF患者术后未进行放化疗,随访 2 年,无复发和转移,生活能自理。
[1] Gisselsson D, Andreasson P, Meis-Kindblom JM, et al. Amplification of 12q13 and 12q15 sequences in a sclerosing epithelioid fibrosarcoma. Cancer Genet Cytogenet, 1998,107(2):102-106.
[2] Meis-Kindblom JM, Kindblom LG, Enzinger FM. Sclerosing epithelioid fbrosarcoma. A variant of fbrosarcoma simulating carcinoma. Am J Surg Pathol, 1995, 19(9):979-993.
[3] Wojcik JB, Bellizzi AM, Dal Cin P, et al. Primary sclerosing epithelioid fibrosarcoma of bone: analysis of a series. Am J Surg Pathol, 2014, 38(11):1538-1544.
[4] 刘坦坦, 王云峰, 李静, 等. 甲状腺原发性硬化性上皮样纤维肉瘤伴颈内静脉癌栓和淋巴结转移1例并文献复习. 临床与实验病理学杂志, 2012, 28(8):930-933.
[5] Albertson DG. Gene amplification in cancer. Trends Genet,2006, 22(8):447-455.
[6] Jo VY, Fletcher CD. WHO classifcation of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology, 2014,46(2):95-104.
[7] Folk GS, Williams SB, Foss RB, et al. Oral and maxillofacial sclerosing epithelioid fbrosarcoma: report of fve cases. Head Neck Pathol, 2007, 1(1):13-20.
[8] Ossendorf C, Studer GM, Bode B, et al. Sclerosing epithelioid fbrosarcoma: case presentation and a systematic review. Clin Orthop Relat Res, 2008, 466(6):1485-1491.
[9] 甘梅富, 余春开, 张建伟, 等. 硬化性上皮样纤维肉瘤一例报道及文献分析. 临床与试验病理杂志, 2006, 22:742.
[10] 郑锦阳, 郭滟, 金晓龙. 硬化性上皮样纤维肉瘤临床病理分析. 临床与试验病理杂志, 2006, 22:409-410.
[11] Antonescu CR, Rosenblum MK, Pereira P, et al. Sclerosing epithelioid fbrosarcoma: a study of 16 cases and confrmation of a clinicopathologically distinct tumor. Am J Surg Pathol,2001, 25(6):699-709.
[12] Folk GS, Williams SB, Foss RB, et al. Oral and maxillofacial sclerosing epithelioid fbrosarcoma: report of fve cases. Head Neck Pathol, 2007, 1(1):13-20.
[13] Ossendorf C, Studer GM, Bode B, et al. Sclerosing epithelioid fbrosarcoma: case presentation and a systematic review. Clin Orthop Relat Res, 2008, 466(6):1485-1491.
[14] Abdulkader I, Cameselle-Teijeiro J, Fraga M, et al. Sclerosing epithelioid fbrosarcoma primary of the bone. Int J Surg Pathol,2002, 10(3):227-230.
[15] Hindermann W, Katenkamp D. Sclerosing epithelioid fibrosarcoma. Pathologe, 2003, 24(2):103-108.
[16] Massier A, Scheithauer BW, Taylor HC, et al. Sclerosing epithelioid fbrosarcoma of the pituitary. Endocr Pathol, 2007,18(4):233-238.
[17] Battiata AP, Casler J. Sclerosing epithelioid fibrosarcoma: a case report. Ann Otol Rhinol Laryngol, 2005, 114(2):87-89.
[18] Bilsky MH, Schefler AC, Sandberg DI, et al. Sclerosing epithelioid fbrosarcomas involving the neuraxis: report of three cases. Neurosurgery, 2000, 47(4):956-959.
[19] Smith PJ, Almeida B, Krajacevic J, et al. Sclerosing epithelioid fbrosarcoma as a rare cause of ascites in a young man: a case report. J Med Case Rep, 2008, 2:248.
[20] Puerta Roldán P, Rodríguez Rodríguez R, Bagué Rossell S,et al. Sclerosing epithelioid fbrosarcoma of the paravertebral column. Case report and literature review. Neurocirugia (Astur),2013, 24(4):178-182.
[21] Tomimaru Y, Nagano H, Marubashi S, et al. Sclerosing epithelioid fibrosarcoma of the liver infiltrating the inferior vena cava. World J Gastroenterol, 2009, 15(33):4204-4208.
[22] Hoos A, Lewis JJ, Antonescu CR, et al. Characterization of molecular abnormalities in human fibroblastic neoplasms: a model for genotype-phenotypeassociation in soft tissue tumors. Cancer Res, 2001, 61(7):3171-3175.
[23] 范钦和. 软组织病理学. 南昌: 江西科技出版社. 2003: 76-77.
[24] Antonescu CR, Baren A. Spectrum of low-grade fbrosarcomas: a comparative ultrastructural analysis of low-grade myxofbrosarcoma andfibromyxoid sarcoma. Ultrastruct Pathol, 2004,28(5-6):321-332.
[25] Guillou L, Benhattar J, Gengler C, et al. Translocationpositive low-grade fibromyxoid sarcoma: clinicopathologic and molecular analysis of a seriesexpanding the morphologic spectrum and suggesting potential relationship to sclerosing epithelioidfibrosarcoma: a study from the French Sarcoma Group. Am J Surg Pathol, 2007, 31(9):1387-1402.
[26] 王昀, 王殿军, 等. 一例硬化性滑膜肉瘤的临床病理观察. 军医进修学院学报, 2012, 33(5):530-533.
[27] Chow LT, Lui YH, Kumta SM, et al. Primary sclerosing epithelioid fibrosarcoma of the sacrum: a case report and review of the literature. J Clin Pathol, 2004, 57(1):90-94.
[28] Kanno A, Hatori M, Hosaka M, et al. Multiple bone metastasis of sclerosing epithelioid fibrosarcoma 12 years after initial surgery-increasing ki-67 labeling index. Sarcoma, 2009, 2009: 953750.
( 本文编辑:王萌 )
Multiple sclerosing epithelioid fibrosarcoma in the spine: 1 case report
YU Yao, DIAO Xiao-li, DU Xin-ru, LUO Hui, XU Zi-yu. Department of Orthopedics, Chaoyang Hospital, Capital University of Medical Sciences, Beijing,100020, PRC
DU Xin-ru, Email: duxinru@163.com
Objective To study pathological features, diagnosis and differential diagnosis, and treatment of sclerosing epithelioid fibrosarcoma ( SEF ). Methods Clinical manifestations, pathological morphology,immunohistochemical staining, and treatment of cervicothoracic vertebrae SEF in 1 patient were analyzed with related literature review. Results The case ( male, 62-year-old ) of cervicothoracic vertebrae SEF was frst reported. Primary tumors located in cervical vertebrae and thoracic vertebrae, inducing spinal cord compression. A large amount of irregular cord-like trabecular collagen was found in tumors. Rarefaction of tumor cells and spindle cells around the collagen was observed, among which were epithelial cells. Calcifcation and ossifcation were found in certain areas of the tumor. Immunohistochemical staining showed that the tumors were positive in Vimentin ( 3+ ), CD99 ( 3+ ),Bcl-2 ( 2+ ), KI-67-LI ( 15%+ ), while negative in SMA, Desmin, NF, CgA, S-100, HMB-45, CD34, EMA, CK8,CK19, CK7, p53, CK, CK5, GFAP, CD68, CD21, CD163, CD1a and S-100. Conclusions SEF is an extremely rare type of fibrosarcoma. It can be confirmed by pathological examination and immunohistochemical study. Surgical treatment is the main option.
Spine; Fibrosarcoma; Diagnosis, differential; Bone neoplasms; Surgical procedures, operative
10.3969/j.issn.2095-252X.2015.12.020
R738.1
100020 北京,首都医科大学附属北京朝阳医院骨科 ( 宇尧、杜心如、骆辉、徐子),病理科 ( 刁小莉 )
杜心如,Email: duxinru@163.com
2015-02-28 )

