Effects of GLP on Intestinal Mucosal Injury and the Change of TNF-α Content in Hemorrhagic Shock Rabbits*
YANG Hong-mei,CHEN Jie,LI Yi-pei,WANG Li
(Henan Medical College,Zhengzhou Henan 450003,China)
Intestinal mucosal injury and bacterial/endotoxin translocation of blood from gut have been implicated as the initial triggering events that contribute to systemic inflammatory response syndrome(SIRS)and the secon-dary multiple organ dysfunction syndrome(MODS)in the course of hemorrhagic shock and its resuscitation(HS-R)[1],so the protection of intestinal mucosa is important to prevent the occurrence and development of MODS.LIU[2]found that tumor necrosis factor-α(TNF-α)could be the starting factor of inflammatory response in early stage of shock.Using rabbit model of HS-R,we studied the effects of GLP on intestinal mucosal injury and the change of TNF-α content,to provide experimental basis for clinical treatment of hemorrhagic shock.
Materials and methods
Drugs GLP,which was extracted from cultivated ganoderma lucidum by ultrasonic-enzyme methods,was bought from Baoding Shidake Bio-engineering technology Ltd.Its main components are monosaccharide polymer with physiological activity,such as L-fucose,D-galactose ,D-mannose,D-xylose and D-dextran.1 g GLP equals to 22.2 g crude drug.GLP was dissolved in heated(37.0℃)normal saline and 1%GLP was gotten,then asepticly filtrated and stored at 4.0℃.
Animal experimental model and grouping
30 Japanese white rabbits[2.5 ~3.0 kg,BCXK(YU)2003-2002]were divided by random number table into 3 groups:sham operation group(Sham group),reperfusion with NS group(NS group),reperfusion with 1%GLP group(LS group).There were 10 rabbits in each group.All rabbits were anesthetized by injecting amobarbital sodium(30 mg/kg)through ear vein.Tracheal intubation was performed for ventilation.The right external jugular vein was cannulated for fluid infusion and drug delivery.The left carotid artery was cannulated and linked with BL-420 computer collecting and disposing system of organic signal for monitoring arterial pressure.The left femoral artery was cannulated for blood letting and blood sample drawing.The rabbits were heparinized(3.0mg/kg)and bled to the mean arterial pressure(MAP)of 40 mmHg within 10 min and kept for 40 mins.The shed blood was collected in glass syringes with heparin and reinfused during resuscitation.Then,the rabbits in NS group were resuscitated with reinfusion of their shed blood and twice as much of NS in 30 mins.The blood samples were drawn at the time before shock(BI),40 min after shock(S40),40 min(R40)and 90 min after reperfusion(R90).The rabbits in LS group were delt with the same means except for NS replacing by 1%GLP.The rabbits in Sham group were only operated without bloodletting.
Preparation of specimens and measurements
Bacterial culture and identification Under aseptic condition,the blood samples were drawn from femoral artery at each time point and were put into sodium acetate liquid culture medium,then cultured for 48 h at 37.0℃.The positive samples were cultured isolatedly for aerobe or anaerobe,and were identificated by G-,G+or anaerobe bacilli identification cards.The results were determinated by automatic microbiology analysis system(ATB,French MEILIE).
Morphological analysis of gut After experiment,all rabbits were killed by air embolism through ear vein.Two centimetres of the terminal ileum was fixed in 10%buffered formalin,processed,stained with hematoxylin-eosin(HE),and examined by light microscopy.The damage of intestinal mucosa was evaluated by the criteria of Chiu’s method[3].
Detection of TNF-α content in serum and intestinal mucosa Serum were obtained from blood samples after centrifugation.Intestinal mucosa were made into 10%homogenate with iced normal saline and centrifuged at 3,000 r/min.The serum and supernatants were respectively transferred into fresh tubes for evaluation TNF-α content(by ELISA method).ELISA bit were bought from Shanghai Ruicong Technology Development Ltd,the A values were read at 450 nm.
Statistical analysis
Data were expressed as mean±SD and the analysis of variance was performed with SPSS 10.0 software.One-way analysis of variance was used for multiple comparison and least significant difference test(LSD-t)was used for intra-group comparison.The test standard was α=0.05.
Results
Results of bacterial culture and identification
Before blood letting,the bacterial culture of all animals was negative,and there was no bacterial transloca-tion(P>0.05).There were two positive samples of blood bacterial culture at the time of shock 40 min in NS and LS group respectively.With the extension reperfusion time,the positive rate of blood bacterial increased gradually in NS group,which was significantly higher than that of Sham group and LS group(P<0.05)(Tab 1).Escherichia Coli was the most common positive bacteria.The other positive bacteria were enterococcus,lactobacillus acidophilus and aureus in turn.
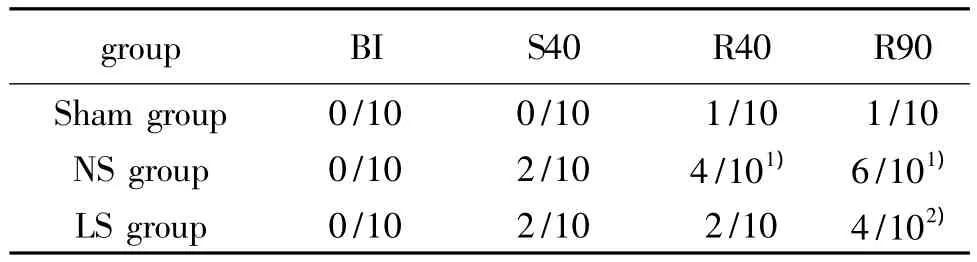
Tab 1 Results of bacterial translocation(n=10)
Morphological analysis of gut by light microscope
The villus and glands were normal and the inflammatory cell infiltration was not observed in mucosal epithelial layer in sham group.The mucosa villus,from NS group showed severe edema and disarranged,the gap under epithelial layer widening,and its desmohemoblast was loose.Extensive denudation and collapse of villi could be found,and the infiltration of neutrophils and lymphocytes were also found in lamina propria.All these indicated that the damage of intestinal mucosa was severe.The damage of intestinal mucosa in LS group obviously relieved.(Fig 1).Chiu’s score of ileal structure was seen in Tab2.

Fig 1 microscopic appearance(HE X 200),
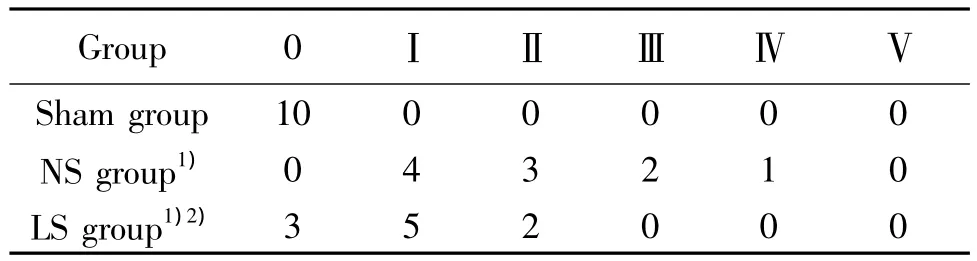
Tab 2 Chiu’s score of ileal structure in different groups(n=10)
Changes of TNF-α content in serum and intestinal mucosa was seen in Tab3.
Discussion
A major function of the gut is to prevent bacteria and endotoxin across the intestinal barrier.Hemorrhagic shock results in blood volume and blood pressuredecreased rapidly,meanwhile the compensative blood re-distribution occurred,all these are prone to causing ischemic hypoxia injury of the gut.Early liquid resuscitation is necessary to hemorrhagic shock,but liquid resuscitation may result in reperfusion injury.The above factors may result in epithelial cells detaching from villi and some regions of villi destroying,thus plenty of bacteria and endotoxin invade into blood cir-culation,then stimulate epithelial cells of gut and related lymphatic system to release IL-1 and IL-6 in a dose-dependent manner.All these cytokines and translocated bacteria and endotoxin may enter into liver by portal vein system and invade other organs,so SIRS and MODS occur[4].Other researches showed that TNF-α could enhance nitric oxide synthase(NOS)activity and increase content of nitric oxide(NO),conversely[5],NO can enhance cytokines releasing.Releasing of TNF-α and NO forms a vicious circle.Meanwhile NO can aggravate SIRS and MODS.The study showed that TNF-α in serum at S40 in NS and LS group were significantly higher than that at the time before shock and in Sham group.When reperfusion with NS,TNF-α in serum increased further,TNF-α in intestinal mucosa was higher than that in Sham group,meanwhile the positive rate of blood bacteria was significantly higher and the degree of intestinal injury was more severe too.These indicated that TNF-α participated in intestinal mucosal injury in the course of hemorrhagic shock and its resuscitation.
Tab 3 Changes of TNF-α content in serum and intestinal mucosa(n=10,)

Tab 3 Changes of TNF-α content in serum and intestinal mucosa(n=10,)
Notes:vs the same time of Sham group,1)P<0.05;vs BI of the same group,2)P<0.05;vs S40 of the same group,3)P <0.05;vs the same time of NS group,4)P<0.05.
intestinal mucosa Sham group 0.006±0.001 0.008±0.002 0.006±0.Group serum BI S40 R40 R90002 0.008±0.001 20.43±0.03 NS group 0.002±0.000 15.39±3.241)2) 43.81±10.751)2)3) 85.08±4.331)2)3) 167.73±12.321)LS group 0.005±0.002 17.14±5.861)2) 29.28±4.161)2)3)4) 33.35±7.951)2)3)4) 96.38±8.591)4)
Ganoderma lucidum belongs to basidiomycete fungi,which is typically traditional anti-aging herb in China.Its main effective component is GLP.Studies showed that GLP had the function of anti-oxidant,antiaging[6],promoting blood circulation and removing blood stasis[7].In the experiment,compared with NS group,TNF-α content in serum and intestinal mucosa reduced evidently in LS group,meanwhile the positive rate of blood bacteria decreased distinctly too,the degree of intestinal mucosa injury obviously relieved.These showed that GLP have protective effect on intestinal mucosal injury in the course of hemorrhagic shock and its resuscitation,which is related to the decreasing of TNF-α.
[1]Sori AJ,Rush BF Jr,Lysz TW,Smith S,Machiedo GW.The gut as source of sepsis after hemorrhagic shock[J].Am J Surg,1988,155(2):187-192.
[2]LIU Yong-jun,MAO En-qiang,LI Lei,LIU Wei,TANG Yao-qing,JI Yu-bao,et al.Expression and release-phases of pro-inflammatory cytokines in the intestine,liver,and lung in hemorrhagic shock rats[J].Journal of Surgery Concepts & Practice(Chin),2007,12(1):58-62.
[3]Chiu CT.Intestinal mucosal lesion in low flow states[J].Arch Surg,1970,101(4):478-485.
[4]ZHANG Liang-cheng,Wang Hong-geng,Zhou Lin-ying,YUAN Shi-ying,GUO Yong-zheng,ZENG Bang-xiong.Alterations of intestinal morphology and barrier function after two projects ressusciation for traumatic/hemorrhagic shock[J].The Chinese Journal of Modern Applied Pharmacy,2004,21(6):438-441.
[5]Singh U,Kumar A,Sinha R,Manral S,Arora S,Ram S.Calreticulin transacetylase catalyzed modification of the TNF-alpha mediated pathway in the human peripheral blood mononuclear cells by polyphenolic acetates[J].Chemico-Biological Interactions,2010,185(3):263-270.
[6]YANG Li-juan,YOU Yu-hong ,LIN Zhi-bin,LIN Yun-feng.Protective effects of ganoderma lucidum polysaccharides peptide on human umbilical vein endothelial cells injury by reactive oxygen species[J].Chinese Pharmacological Bulletin,2010,26(5):657-660.
[7]XIE Shao-qiong,LIAO Wan-qing.Ganoderma Polysaccharides and H2O2-induced Kerotinocytes Oxidative Stress.The Chinese Journal of Dermatovenereology ,2006,20(2):77-79.

