BmNPVorf98对家蚕核型多角体杆状病毒复制、转录及包装的影响
史利利,蒋彩英,于威,陈琛,蒋磊,巩成见,童富淡
(浙江理工大学生命科学学院,浙江省家蚕生物反应器和生物医药重点实验室,杭州310018)
BmNPVorf98对家蚕核型多角体杆状病毒复制、转录及包装的影响
史利利,蒋彩英,于威,陈琛,蒋磊,巩成见,童富淡*
(浙江理工大学生命科学学院,浙江省家蚕生物反应器和生物医药重点实验室,杭州310018)
为了研究家蚕核型多角体病毒(Bombyx mori nucleopolyhedrovirus,BmNPV)基因orf98的功能,通过λRed重组系统定点敲除BmNPVorf98基因,构建缺失型重组病毒Bm98-ko-Bacmid;以Bac-to-Bac系统补回BmNPVorf98基因,构建补回型重组病毒Bm98-re-Bacmid;将野生型病毒(wtBacmid)、缺失型病毒(Bm98-ko-Bacmid)和补回型病毒(Bm98-re-Bacmid)分别转染家蚕细胞BmN.病毒滴度检测结果显示,Bm98-ko-Bacmid可形成侵染性的病毒粒子,但数量显著降低(P<0.05).透射电子显微镜观察发现,Bm98-ko-Bacmid只产生游离的杆状病毒粒子,数量明显减少,而wtBacmid和Bm98-re-Bacmid产生大量具有囊膜结构的成熟病毒粒子.荧光定量聚合酶链反应分析结果表明,BmNPVorf98基因缺失对BmNPV病毒复制没有影响,而早期基因lef3、晚期基因vp39和极晚期基因p10的转录水平显著降低(P<0.05).综上所述,BmNPVorf98基因对病毒复制是非必需的,但显著影响病毒的繁殖速度和包装(P<0.05);对病毒各个时期的基因转录也具有重要影响.
家蚕核型多角体病毒;λRed重组系统;Bac-to-Bac系统;病毒复制;基因转录
Journal of Zhejiang University(Agric.&Life Sci.),2015,41(6):623-630
SummaryBaculoviruses have been considered as the powerful vectors to express the exogenous gene.And the representative vectors in baculovirus expression vector system is Autographa californica multinucleocapsid nucleopolyhedrovirus(AcMNPV)and Bombyx mori nucleopolyhedrovirus(BmNPV).The AcMNPV expression system has been widely applied in American and European countries.However,the BmNPV expression reaches a higher level over other systems,because BmNPV can infect silkworm larva or pupa.Moreover,silkworm is pretty normal in China,with lower cost and mature breeding technology,thus it is really popular to use the silkworm as a“biofactory"to produce recombinant protein.The BmNPV genome sequenced in 1999was 128 413nucleotides long with a G+C content of 40%and contained about 136open reading frames(ORFs)encoding predictedproteins of over 60amino acids.
The gene of BmNPVorf98 is found in all GroupⅠand the most of GroupⅡgenomes.It is not a highly conserved gene,as the deletion of this gene in BmNPV,it has no apparent effect on infectivity.The function of BmNPVorf98 has not been reported until now.In order to study the specific function of BmNPVorf98 gene,a BmNPVorf98 knockout bacmid byλRed recombination was constructed,naming Bm98-ko-Bacmid.Additionally,Bm98-re-Bacmid was constructed by Bac-to-Bac system.BmN cells were infected with three kinds of virus DNA from wild-type bacmid(wtBacmid),Bm98-ko-Bacmid and Bm98-re-Bacmid,and the cells were respectively collected in 12h,24h,48hand 72hphases,then virus titer(50%tissue culture infective dose,TCID50)was determinated and virus proliferation curve was drawn,and total DNA was extracted using a eukaryotic DNA extraction kit.After DpnⅠenzyme digestion overnight,the effects of lacking BmNPV orf98 gene on virus replication and transcription were analyzed by fluorescence quantitative polymerase chain reaction(PCR).
The results showed that the knockout bacmid was able to produce viral progeny after transfecting the DNA of Bm98-ko-Bacmid into BmN cells,but the number of viral progeny reduced significantly(P<0.05);meanwhile,the virus infection level of repair was recovered which was similar with that of wild-type virus,indicating that the virus infection level in the incidence of BmN cells could be delayed after the deletion of BmNPVorf98.Assembly of virus was observed by transmission electron microscope in 48hphase.The results indicated that,after 48hof transfecting host cell,wtBacmid and Bm98-re-Bacmid viruses produced a large number of virus-packed capsule membrane except the BmNPVorf98 knockout virus.The BmNPVorf98 deletion didn’t show significantly effects on viral DNA replication,suggesting that it was not essential for viral replication;however,the transcription of early gene lef3,late gene vp39 and very late gene p10 decreased obviously(P<0.05),because of lack of BmNPV orf98 gene.
All the above results show that BmNPV orf98 gene is non-essential for viral replication,but it can significantly affect viral progeny and assembly,and lack of the gene will lead to significant decline of the transcription level of early gene lef3,late gene vp39 and very late gene p10.
杆状病毒是有囊膜包被的双链闭环超螺旋DNA病毒,在自然环境中专一感染节肢动物,产生的病毒粒子呈杆状,其基因组大小在80~180kb之间[1];根据其形态特征分为颗粒体病毒属(granuloviruses,GVs)和核型多角体病毒属(nucleopolyhedroviruses,NPVs).其中,核型多角体病毒[2-3]主要以2种形式存在:一种为包涵体衍生型病毒(occlusion-derived virus,ODV),它主要存在于环境中的植物表面,被昆虫吞食后在中肠的碱性条件下才会释放出来,并穿过食膜结合到中肠柱状上皮细胞的微小绒毛上[4];另一种是芽生型病毒(budded virus,BV),它主要是病毒侵染个体细胞时由细胞产生的一种芽生病毒,可直接感染邻近细胞[5-6].
家蚕核型多角体病毒(Bombyx mori nucleopolyhedroviruses,BmNPV)是核型多角体病毒属典型代表之一,其基因组大小为128 413bp,能编码蛋白质(60个氨基酸残基以上)的开放阅读框约有136个[7].BmNPVorf98基因编码82个氨基酸[8],其蛋白质相对分子质量为9 500;它不是杆状病毒的核心基因,该基因的插入或缺失对BmNPV病毒侵染性没有明显影响[9],但该基因对病毒复制和转录的影响至今未见报道.因此,为了研究BmNPVorf98基因功能,本文通过λRed重组系统[10-14]和Bac-to-Bac系统[15-16]分别构建了Bm98-ko-Bacmid和Bm98-re-Bacmid;将野生型wtBacmid、缺失型Bm98-ko-Bacmid和补回型Bm98-re-Bacmid病毒分别转染BmN细胞,通过滴度检测和透射电镜扫描分析病毒的增殖和装配,同时以荧光定量聚合酶链反应(polymerase chain reaction,PCR)分析[17]BmNPVorf98基因缺失对BmNPV的复制以及病毒早期基因lef3、晚期基因vp39和极晚期基因p10转录水平的影响.
1 材料与方法
1.1 材料
家蚕细胞BmN、大肠埃希菌(Escherichia coli)
TG1、DH10Bac(含Helper和pKD46质粒)、转移载体pFastBacHTB和pKD46质粒等(均由浙江理工大学生物化学与分子生物学实验室保存);胎牛血清和sf-900TMⅡSFM(1×)(Life Technologies公司);脂质体转染试剂(Roche公司);DpnⅠ酶、KOD高度保真酶、BmHⅠ、XhoⅠ快速内切酶、r Taq酶、T4DNA连接酶和DL15000/2000标志物(Takara公司);SYBR Green实时荧光定量PCR试剂盒(Promega公司).所有特异性引物由上海桑尼生物科技有限公司合成,基因序列由生工生物工程(上海)股份有限公司检测.
1.2 方法
1.2.1扩增打靶片段 根据NCBI提供的BmNPVorf98的开放阅读框,设计扩增引物Bm98-C(5′-ATGAGCATTTTAAACGTTGTAGAAGC GTGCGATTTGGCACACACTGTGTAGGCTGGA GCTGCTTC-3′)和Bm98-N(5′-CATTCTGTAGT TTTTTTGTTTAGCATGACTGCCACTTCCTTT ATGATGGGAATTAGCCATGGTCC-3′).其中,下划线为BmNPVorf98基因的同源臂(45bp),未划线部分为氯霉素的同源区(20bp).以pKD3质粒为模板,扩增特异性打靶片段Bm98-Cm(约1 100 bp).
1.2.2构建缺失型病毒Bm98-ko-Bacmid 利用Primer Premier 5.0设计并合成鉴定引物Bm98-F(5′-CGGGATCCATGAGCATTTTAAACGTT-3′)和Bm98-R(5′-CCGCTCGAGTCATTTGATAGTGT AAAT-3′),氯霉素基因内部的1对引物Vlf-F(5′-CACGTTTAAATCAAAACTGGTG-3′)和Vlf-R(5′-CAATATGGACAACTTCTTCG-3′).
将打靶片段Bm98-Cm转化到含有pKD46质粒的DH10Bac感受态细胞中,以L-阿拉伯糖诱导DH10Bac中pKD46质粒产生重组酶,使打靶片段Bm98-Cm与BmNPVorf98发生重组,利用抗性平板(Kan和Cm)筛选出阳性单克隆菌落,构建缺失型病毒Bm98-ko-Bacmid,并用合成的2对引物Bm98-F/R和vlf-F/R进行PCR鉴定.
1.2.3构建补回型病毒Bm98-re-Bacmid 利用Primer Premier 5.0和Oligo生物信息学软件,设计并合成鉴定引物M13-F(5′-GTTTTCCCAGTCA CGAC-3′)和M13-R(5′-CAGGAAACAGCTATG AC-3′).根据NCBI提供的BmNPVorf98基因以及前后基因序列设计引物Bm98-F1(5′-CGGGATCCTCAGGCGACACGGTGGATT-3′)和Bm98-R1(5′-CCGCTCGAGCCTTACAACAAAC ACGAAG-3′)(下划线处分别为BmHⅠ和XhoⅠ酶切位点).扩增补回片段Bm98(包括BmNPV orf98基因的开放阅读框和自身启动子序列261 bp),将该片段连接到转移载体pFastBacHTB上,构建重组质粒pFastBacHTB-Bm98,并将其转化到缺失型病毒的感受态细胞中,在Bac-to-Bac系统作用下,将BmNPVorf98基因异位补回到多角体启动子下游,用抗性平板(Kan、Gen、Tet、IPTG和X-gal)筛选阳性菌落,构建补回型病毒Bm98-re-Bacmid.用Bm98-F1/R1引物和M13引物进行交叉PCR鉴定.
1.2.4病毒滴度测定 收集4个时相(12、24、48和72h)的3种病毒(wtBacmid、Bm98-ko-Bacmid和Bm98-re-Bacmid),按照1×10-1~1×10-10的梯度进行稀释,分别取100μL加入到96孔板家蚕细胞BmN中,每个稀释度8个重复,以不加任何病毒的正常细胞作为对照.4~5d后根据每个梯度发病数目计算半数组织细胞感染量(50%tissue culture infective dose,TCID50)值[18].
1.2.5电子显微镜观察病毒粒子包装 将3种病毒(wtBacmid、Bm98-ko-Bacmid和Bm98-re-Bacmid)分别转染家蚕细胞BmN,收集48h时相的细胞,通过固定、包埋、切片和染色进行透射电镜观察[19].
1.2.6BmNPVorf98缺失对BmNPV病毒基因组复制的影响 gp41是BmNPV包涵体来源病毒囊膜的结构蛋白基因,是荧光定量PCR检测的标记基因.根据gp41基因序列,利用Primer Premier 5.0设计并合成引物gp41F(5′-CGTAGTGGTAG TAATCGCCGC-3′)和gp41R(5′-AGTCGAGTCG CGTCGCTTT-3′),β-actinF(5′-GCGCGGCTACT CGTTCACT-3′)和β-actinR(5′-TGCCGCAAGCT TCCATACCC-3′).
各取1μg wtBacmid、Bm98-ko-Bacmid和Bm98-re-Bacmid病毒DNA,分别转染家蚕细胞BmN,分别在4个时相(12、24、48和72h)收集细胞,并参照DNA试剂盒说明书提取细胞总DNA,用DpnⅠ酶消化过夜.用β-actin作为内参进行荧光定量PCR,3次重复.
1.2.7BmNPVorf98缺失对家蚕杆状病毒基因转录的影响 利用Primer Premier 5.0和Oligo 6.0软件设计并合成引物β-actinF(5′-GCGCGGC TACTCGTTCACTACC-3′)和β-actinR(5′-TGCCGCAAGCTTCCATACCC-3′),lef-3F(5′-TCGGA TGACCGTTCTACCTCTT-3′)和lef-3R(5′-CTT CCAGCAGCATTGAGATTTG-3′),vp39F(5′-AG ACACCACAAACCCGAACAC-3′)和vp39R(5′-T TGATCGCCAACACCACCT-3′),p10F(5′-TTGA TCGCCAACACCACCT-3′)和p10R(5′-CGATTC TTCCAGCCCGTTT-3′).
各取1μg wtBacmid、Bm98-ko-Bacmid和Bm98-re-Bacmid病毒DNA,分别转染家蚕细胞BmN,收集4个时相(12、24、48和72h)的细胞,用Trizol法提取细胞总RNA,以反转录cDNA为模板进行荧光定量PCR,分析BmNPVorf98基因缺失对早期基因lef3[20]、晚期基因vp39[21]和极晚期基因p10[22]转录水平的影响,3次重复.
2 结果
2.1 缺失型病毒和补回型病毒的鉴定
2.1.1打靶片段的鉴定 以pKD3为模板、Bm98-C/N为引物进行PCR,扩增产物的琼脂糖凝胶电泳结果显示,在1 100bp左右有1条清晰的特异性条带(图1),与理论值一致.将该基因序列送生工生物工程(上海)股份有限公司检测,结果表明该基因序列完全正确,未发生碱基突变.
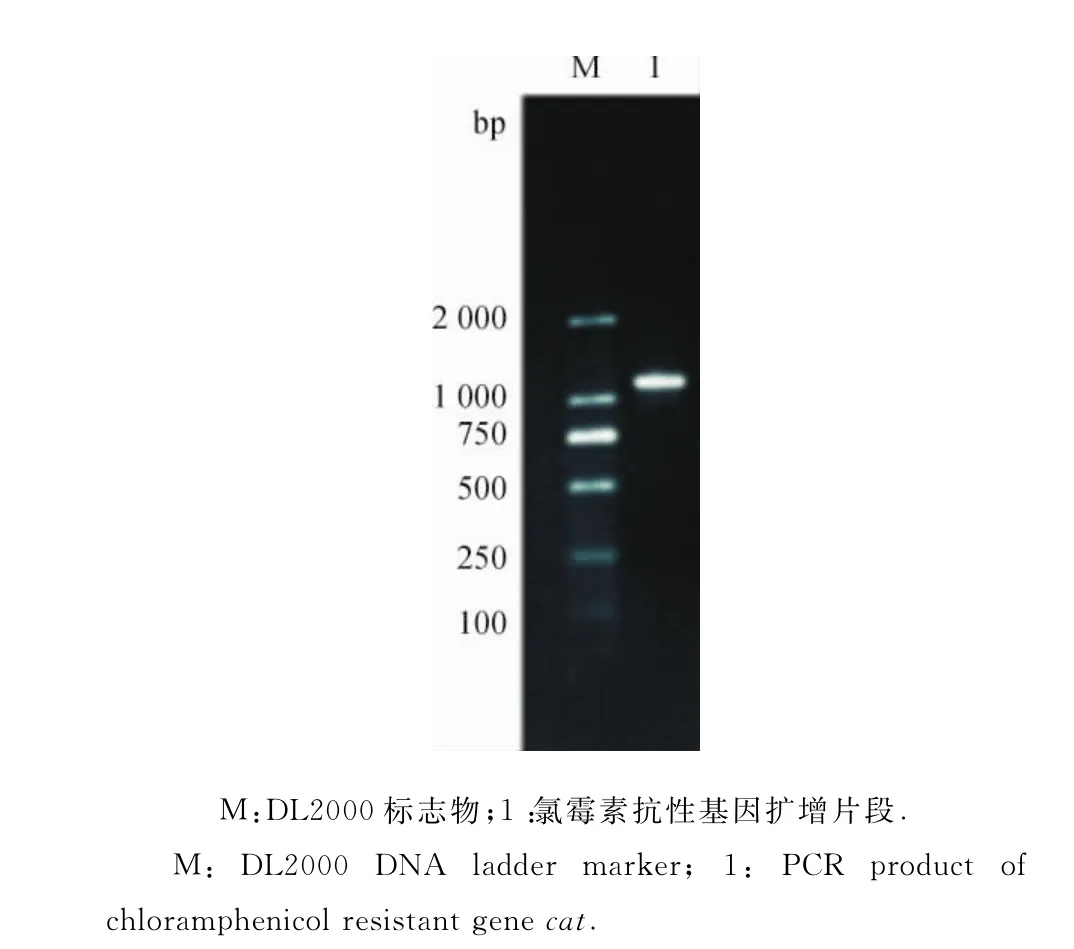
图1 打靶片段扩增Fig.1 Amplification of target gene
2.1.2Bm98-ko-Bacmid的鉴定 将抗性平板(Kan和Cm)筛选出的阳性单克隆菌落用2对引物进行交叉扩增,引物组合Vlf-F/Bm98-R、Bm98-F/ Vlf-R和Bm98-F/R扩增产物的1%琼脂糖凝胶电泳结果显示,在1 172、325和887bp处均出现特异性条带 (图2),与理论值一致.表明成功构建了Bm98-ko-Bacmid.

图2 Bm98-ko-Bacmid的PCR鉴定Fig.2 Identification of Bm98-ko-Bacmid by PCR
2.1.3Bm98-re-Bacmid的鉴定 将抗性平板(Kan、Gen、Tet、IPTG和X-gal)筛选出的阳性单克隆菌落用2对引物M13-F/R和Bm98-F1/R1进行交叉PCR鉴定,引物组合M13-F/R、M13-F/Bm98-R1和Bm98-F1/M13-R扩增产物的1%琼脂糖凝胶电泳结果显示,在3 100、2 178和1 128bp处均出现单一特异性条带(图3),与理论值一致.表明成功构建了Bm98-re-Bacmid.
2.2 BmNPVorf98缺失对BmNPV病毒增殖和包装的影响
病毒滴度检测结果(图4)显示:在12h时相,3种病毒的滴度都为0;随着时间延长,3种病毒的TCID50随之增大,缺失型的病毒滴度显著低于补回型和野生型的病毒滴度(P<0.05),补回型与野生型的病毒滴度差异无统计学意义(P>0.05).说明BmNPVorf98基因的缺失影响了病毒BV的产生量,推迟了细胞的发病时间.在72h时相,野生型与补回型病毒滴度一致,说明这种缺陷可以通过基因补回而得到恢复,所以BmNPVorf98基因不是病毒增殖的必需基因,但显著影响病毒的增殖速度.
收集48h的病毒转染细胞并制样,通过透射电子显微镜观察发现,野生型和补回型病毒产生大量具有囊膜包裹的成熟病毒粒子,而缺失型病毒仅形成游离态杆状病毒粒子,囊膜包装的成熟病毒粒子少见(图5).
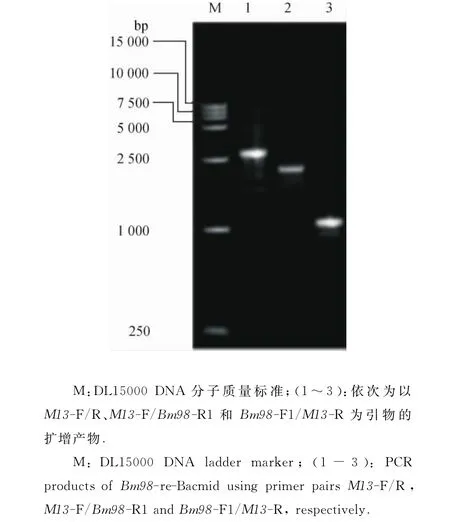
图3 Bm98-re-Bacmid的PCR鉴定Fig.3 Identification of Bm98-re-Bacmid by PCR

图4 病毒增殖曲线Fig.4 Virus growth curve in the transfected BmN cells
2.3 BmNPVorf98缺失对BmNPV病毒复制的影响
荧光定量PCR结果显示,3种病毒基因组复制水平均随转染时间延长而提高,但在同一时相3种病毒基因组复制水平差异无统计学意义(P>0.05)(图6).表明BmNPVorf98的缺失不影响病毒基因组的复制,该基因不是病毒复制的必需基因.
2.4 BmNPVorf98缺失对BmNPV病毒不同时期基因转录的影响
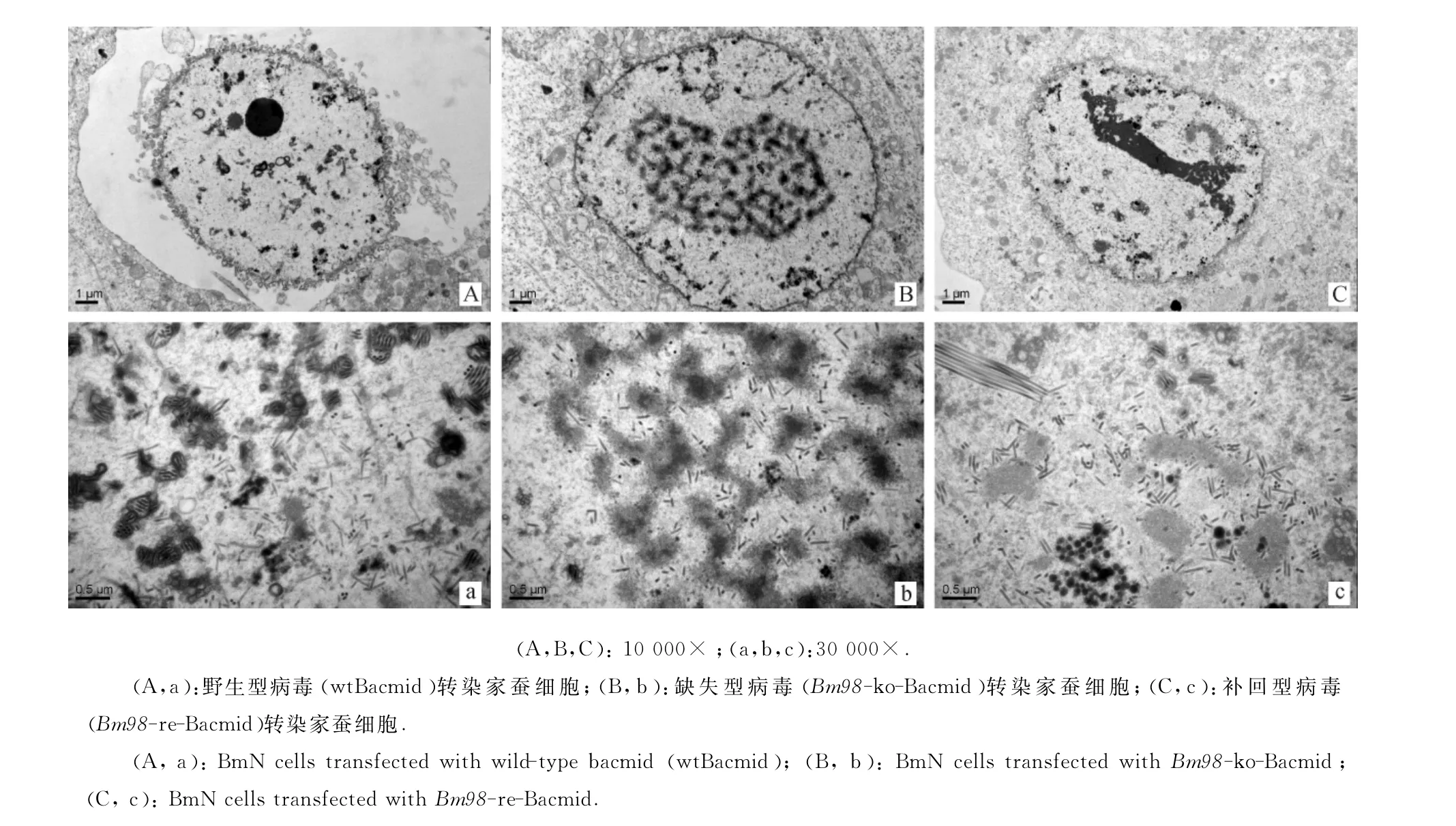
图5 病毒转染家蚕细胞48h后的装配情况Fig.5 Assembly of BmN cells transfected with three kinds of viruses for 48h

图6 病毒复制水平的实时荧光定量PCR分析Fig.6 Fluorescent quantitative PCR analysis of viral DNA replication
从图7可以看出:wtBacmid、Bm98-ko-Bacmid和Bm98-re-Bacmid病毒分别转染家蚕细胞后,这3种病毒的转录水平随着转染时间延长而增大;在转染48和72h时相,BmNPVorf98基因的缺失导致早期基因lef3、晚期基因vp39和极晚期基因p10转录水平显著下降(P<0.05),但补回型与野生型基因转录水平基本一致,差异无统计学意义(P>0.05).说明该种缺陷可以通过基因补回予以恢复.
3 讨论
传统BmNPV载体构建和筛选主要通过将外源基因插入到杆状病毒转移载体病毒启动子的多克隆位点上,然后将其与病毒DNA同步转入昆虫细胞,经过空斑鉴定和分析进行病毒纯化.该过程不仅过程烦琐,耗时长,并且重组率低下,同时筛选时也需要丰富的经验和专业知识[23-25].Red重组系统需设计2对引物,即带同源臂的抗性基因扩增引物和鉴定引物.带同源臂的抗性基因扩增引物可以扩增两侧带有FRT位点的抗性基因片段,并在FRT位点的外侧加入同源臂,其扩增产物通过同源重组将抗性片段替换以敲除目的基因,构建缺失型病毒Bm98-ko-Bacmid;在Bac-to-Bac系统中穿梭质粒Bacmid包含mini-F复制子、卡那霉素抗性筛选标记和细菌转座子Tn7的靶位点以及编码β-半乳糖苷酶A肽的部分DNA片段.将含有外源基因的供体质粒转化DH10Bac感受态细胞(该细胞含有杆状病毒穿梭载体、复制子以及LacZ肽段编码基因),辅助质粒编码转座酶使供体质粒中的外源基因通过转座插入到杆状病毒基因组中,破坏LacZ的表达.用含有卡那霉素、庆大霉素、四环素和X-gal的培养板筛选,重组病毒基因组转化体菌落呈白色,即补回型病毒Bm98-re-Bacmid.该过程历时较短,成功率高,与传统方法比有着较大的优势.

图7 不同时期基因转录水平分析Fig.7 Transcription level analysis of lef-3,vp39 and p10 genes in different periods
文献报道lef-9[26]和gp88[27]是BmNPV形成有感染活性病毒粒子BV的必需基因,lef-9或gp88缺失病毒都不能产生有感染活性的BV,补回型病毒感染活性恢复.而本研究表明:BmNPV orf98基因缺失后BmNPV病毒仍能够形成侵染性的病毒粒子,但数量降低.透射电镜观察发现,lef-9或gp88基因的缺失都影响了病毒的装配能力,不产生具有侵染性的BV,而BmNPVorf98的缺失仅抑制病毒粒子增殖和病毒粒子的包装,这种缺陷都可以通过基因的补回来弥补,BmNPVorf98的抑制作用机制有待深入研究.gp88基因的缺失使病毒基因组DNA的复制水平明显降低,而BmNPV orf98和lef-9的缺失不影响病毒基因组的DNA复制,BmNPVorf98和lef-9都不是病毒复制的必需基因.BmNPVorf98、lef-9和gp88缺失都导致BmNPV病毒早期基因lef3、晚期基因vp39和极晚期基因p10转录水平显著降低(P<0.05),三者对BmNPV基因组转录的作用相似,但它们的作用机制是否相同还有待进一步研究.
4 结论
病毒滴度检测和透射电子显微镜观察结果表明,BmNPVorf98缺失可抑制BmNPV病毒的增殖和包装.荧光定量PCR分析结果表明:BmNPV orf98的缺失不影响BmNPV的病毒基因组复制;而早期基因lef3、晚期基因vp39和极晚期基因p10的转录水平因BmNPVorf98基因的缺失而显著降低(P<0.05),将BmNPVorf98基因补回后恢复到野生型水平,说明该种缺陷可以通过基因的补回来弥补.
(References):
[1] Nie Y,Theilmann D A.Deletion of AcMNPV AC16and AC17results in delayed viral gene expression in budded virus infected cells but not transfected cells.Virology,2010,404(2):168-179.
[2] Hamajima R,Ito Y,Ichikawa H,et al.Degradation of rRNA in BM-N cells from the silkwormBombyx mori during abortive infection with heterologous nucleopolyhedroviruses.Journal of General Virology,2013,94(Pt 9):2102-2111.
[3] Ishihara G,Shimada T,Katsuma S.Functional characterization of Bombyx mori nucleopolyhedrovirus CG30 protein.Virus Research,2013,174(1/2):52-59.
[4] Tang Q,Li G H,Yao Q,et al.Bm91 is an envelope component of ODV but is dispensable for the propagation of Bombyx mori nucleopolyhedrovirus.Journal of Invertebrate Pathology,2013,113(1):70-77.
[5] Dickison V L,Willis L G,Sokal N R,et al.Deletion of AcMNPVac146 eliminates the production of budded virus.Virology,2012,431(1/2):29-39.
[6] Chen H,Li M,Mai W,et al.Analysis of BmNPVorf101 disruption:orf101 is essential for mediating budded virus production.Cytotechnology,2014,66(6):1021-1029.
[7] Zhang M J,Cheng R L,Lou Y H,et al.Disruption of Bombyx mori nucleopolyhedrovirus ORF71(Bm71)results in inefficient budded virus production and decreased virulence in host larvae.Virus Genes,2012,45(1):161-168.
[8] Gomi S,Majima K,Maeda S.Sequence analysis of the genome of Bombyx mori nucleopolyhedrovirus.Journal of General Virology,1999,80(Pt 5):1323-1337.
[9] Ono C,Kamagata T,Taka H,et al.Phenotypic grouping of 141BmNPVs lacking viral gene sequences.Virus Research,2012,165(2):197-206.
[10] Katsuma S,Shimada T.Bombyx mori nucleopolyhedrovirus ORF34is required for efficient transcription of late and very late genes.Virology,2009,392(2):230-237.
[11] Pacholska-Bogalska J,Myga-Nowa M,Ciepluch K,et al.Analysis of the coding sequence and expression of the coiledcoil alpha-helical rod protein 1gene in normal and neoplastic epithelial cervical cells.International Journal of Molecular Medicine,2012,29(4):669-676.
[12] Wang Q,Guo Z J,Yao Q,et al.Rapid disruption of Bombyx mori nucleopolyhedrovirus orf60 by Red recombination system.Chinese Journal of Biotechnology,2007,23(5):801-805.
[13] Okano K,Vanarsdall A L,Rohrmann G F.Characterization of a baculovirus lacking the alkaline nuclease gene.Journal of Virology,2004,78(19):10650-10656.
[14] Hou S W,Chen X W,Wang H Z,et al.Efficient method to generate homologous recombinant baculovirus genomes in E.coli.Preparative Biochemistry,2002,32(4):783-784,786,788.
[15] Li X H,Wang D,Zhou F,et al.Cloning and expression of a cellulase gene in the silkworm,Bombyx mori by improved Bac-to-Bac/BmNPV baculovirus expression system.Molecular Biology Reports,2010,37(8):3721-3728.
[16] Xiang X W,Yang R,Yu S F,et al.Construction of a BmNPV polyhedrin-plus Bac-to-Bac baculovirus expression system for application in silkworm,Bombyx mori.Applied Microbiology and Biotechnology,2010,87(1):289-295.
[17] Yu W,Du C Y,Quan Y P,et al.Characterization of late gene expression factor LEF-10from Bombyx mori nucleopolyhedrovirus.Virus Research,2013,175(1):45-51.
[18] McIntosh A H,Ignoffo C M,Andrews P L.In vitro host range of five baculoviruses in lepidopteran cell lines.Intervirology,1985,23(3):150-156.
[19] Xiang X W,Chen L,Guo A Q,et al.The Bombyx mori nucleopolyhedrovirus(BmNPV)ODV-E56envelope proteinis also a per os infectivity factor.Virus Research,2011,155(1):69-75.
[20] Yu M,Carstens E B.Identification of a domain of the baculovirus Autographa californica multiple nucleopolyhedrovirus single-strand DNA-binding protein LEF-3essential for viral DNA replication.Journal of Virology,2010,84(12):6153-6162.
[21] Danquah J O,Botchway S,Jeshtadi A,et al.Direct interaction of baculovirus capsid proteins VP39and EXON0 with kinesin-1in insect cells determined by fluorescence resonance energy transfer-fluorescence lifetime imaging microscopy.Journal of Virology,2012,86(2):844-853.
[22] Liang X,Lu Z L,Wei B X,et al.Phylogenetic analysis of Bombyx mori nucleopolyhedrovirus polyhedrin and p10 genes in wild isolates from Guangxi Zhuang Autonomous Region,China.Virus Genes,2013,46(1):140-151.
[23] 曹翠平.家蚕新型高效杆状病毒表达系统的开发和应用研究.杭州:浙江大学,2007:11-14.Cao C P.Development of a novel BmNPV expression vector system using silk worm as bioreactor and the application.Hangzhou:Zhejiang University,2007:11-14.(in Chinese with English abstract)
[24] 邵纯君.重组杆状病毒高效筛选方法的建立.辽宁,大连:大连理工大学,2008:8-10,45.Shao C J.The establishment of a high-effective select strategy for recombinant baculovirus.Dalian,Liaoning:Dalian University of Technology,2008:8-10,45.(in Chinese with English abstract)
[25] 费伟强,陈琴,陈倩,等.复制缺陷型BmNPV载体的构建及初步应用.蚕业科学,2013(3):514-521.Fei W Q,Chen Q,Chen Q,et al.Construction and preliminary application of replication-deficient BmNPV vectors.Science of Sericulture,2013(3):514-521.(in Chinese with English abstract)
[26] 石杨辉.家蚕杆状病毒晚期表达因子Lef-9的功能研究.杭州:浙江理工大学,2013:30-31,42.Shi Y H.Functional studies on Bombyx mori nucleopolyhedrovirus polyhedrin late expression factor of Lef-9.Hangzhou:Zhejiang Sci-Tech University,2013:30-31,42.(in Chinese with English abstract)
[27] 张臣.BmNPV DNA结合蛋白GP88对病毒复制和转录调控机制的研究.杭州:浙江理工大学,2013:31,39,47,56.Zhang C.Study on the viral replication and transcription of Bombyx mori nucleopolyhedrovirus DNA-binding protein GP88.Hangzhou:Zhejiang Sci-Tech University,2013:31,39,47,56.(in Chinese with English abstract)
作者投稿时请提供开放研究者与贡献者身份识别码
开放研究者与贡献者身份识别码(Open Researcher and Contributor Identifier,ORCID)是由汤森路透和自然出版集团等单位于2009年共同发起创建的,其意义与科学文献领域的数字对象唯一标识符(Digital Object Identifier,DOI)类似.DOI为科技文献的身份证,一文一证;ORCID为科研人员的学术身份证,一人一证.作者使用ORCID可促进其研究成果的可视化,有利于研究成果的传播.
若尚未获取ORCID的作者请先登录http://orcid.org,进入网站注册后免费获取ORCID.本刊从2015年第4期起在作者信息栏添加ORCID,即http://orcid.org/后16位数字.如某作者ORCID为0000-0002-5115-1154,请将该ORCID添加到本刊在线投稿系统的作者信息备注栏中,并在投稿论文首页页脚作者姓名后加上http://orcid.org/0000-0002-5115-1154.
——浙江大学学报(农业与生命科学版)编辑部
Influence of BmNPVorf98 on DNA replication,transcription and virus package of Bombyx morinucleopolyhedrovirus.
Shi Lili,Jiang Caiying,Yu Wei,Chen Chen,Jiang Lei,Gong Chengjian,Tong Fudan*
(Key Laboratory of Silkworm Bioreactor and Biological Medicine in Zhejiang,College of Life Sciences,Zhejiang Sci-Tech University,Hangzhou 310018,China)
Bombyx mori nucleopolyhedrovirus;λRed recombination system;Bac-to-Bac system;viral replication;gene transcription
Q 78;S 884.5
A
10.3785/j.issn.1008-9209.2015.01.191
国家高技术研究发展计划(863计划)项目(2011AA100603).
童富淡(http://orcid.org/0000-0003-0813-0072),Tel:+86-571-86843193,E-mail:fdtong@zstu.edu.cn
联系方式:史利利(http://orcid.org/0000-0002-4587-7426),E-mail:15757184377@163.com
2015-01-19;接受日期(Accepted):2015-03-26;< class="emphasis_bold">网络出版日期
日期(Published online):2015-11-18
URL:http://www.cnki.net/kcms/detail/33.1247.s.20151118.1648.010.html

