Friction welding of AA6061 to AISI 4340 using silver interlayer SURESH D. MESHRAM*, G. MADHUSUDHAN REDDY
Defence Metallurgical Research Laboratory, Kanchanbagh, Hyderabad 500058, India Received 27 March 2015; revised 17 May 2015; accepted 19 May 2015 Available online 26 June 2015
Friction welding of AA6061 to AISI 4340 using silver interlayer SURESH D. MESHRAM*, G. MADHUSUDHAN REDDY
Defence Metallurgical Research Laboratory, Kanchanbagh, Hyderabad 500058, India Received 27 March 2015; revised 17 May 2015; accepted 19 May 2015 Available online 26 June 2015
Abstract
The present work pertains to the study on joining of AA6061 and AISI 4340 through continuous drive friction welding. The welds were evaluated by metallographic examination, X-ray diffraction, electron probe microanalysis, tensile test and microhardness. The study reveals that the presence of an intermetallic compound layer at the bonded interface exhibits poor tensile strength and elongation. Mg in AA6061 near to the interface is found to be favourable for the formation and growth of Fe2Al5intermetallics. Introduction of silver as an interlayer through electroplating on AISI 4340 resulted in accumulation of Si at weld interface, replacing Mg at AA6061 side, thereby reducing the width of intermetallic compound layer and correspondingly increasing the tensile strength. Presence of silver at the interface results in partial replacement of Fe—Al based intermetallic compounds with Ag—Al based compounds. The presence of these intermetallics was confirmed by X-ray diffraction technique. Since Ag—Al phases are ductile in nature, tensile strength is not deteriorated and the silicon segregation at weld interface on AA6061 in the joints with silver interlayer acts as diffusion barrier for Fe and further avoids formation of Fe—Al based intermetallics. A maximum tensile strength of 240 MPa along with 4.9% elongation was obtained for the silver interlayer dissimilar metal welds. The observed trends in tensile properties and hardness were explained in relation to the microstructure.
Copyright©2015, China Ordnance Society. Production and hosting by Elsevier B.V. All rights reserved.
Keywords:Dissimilar metal weld; Interlayer; Friction welding; Intermetallics; Microstructure; Tensile strength
E-mail addresses: suresh_uor@yahoo.co.in (S.D. MESHRAM), gmreddy_ dmrl@yahoo.co.in (G. MADHUSUDHAN REDDY).
Peer review under responsibility of China Ordnance Society.
http://dx.doi.org/10.1016/j.dt.2015.05.007
2214-9147/Copyright©2015, China Ordnance Society. Production and hosting by Elsevier B.V. All rights reserved.
1. Introduction
Several situations arise in industrial practices which call for joining of dissimilar metals. The joining of dissimilar metals is commonly done to satisfy different requirements for effective and economic utilization of the special properties of each material for enhanced performance. Dissimilar metal joints can be made successfully if there is a mutual solubility between the two metals. Otherwise, using interlayer/layers that is/are compatible with each other is required to produce joint. For dissimilar metals having widely different coefficients of thermal expansion, the joint may fail due to thermal fatigue either during solidification or soon thereafter. This is because the internalstressesaresetupintheintermetalliczone,whichtendsto be extremely brittle. In the case of two metals having different melting temperatures or thermal conductivities, the process of welding is complicated because one metal is molten before the other. There is a continuing demand for reliable methods of joining dissimilar metals and alloys. For example, welding is irreplaceable in the manufacture of vacuum system made of dissimilar metals for cryogenic engineering. Recently, the range of combinations of the dissimilar metals, used in welded structures, has greatly increased and is continuous to increase. Joiningofaluminiumwithsteelfindsitsapplicationinthefields of cryogenic engine, space craft and automobile. However, the fusion welding of aluminium to steel is difficult due to the formation of brittle intermetallic compounds at the interface [1—4]. The solid state joining of steel directly to aluminium also gives an unsatisfactory product because of incompatibility of physico-chemical properties of the two metals to be bonded [5]. The interdifussion of Al and Fe often yields a series of brittle intermetallic compounds at the interface. Such brittle layers cannot sustain the strain of subsequent metal workingoperation which results in the internal rupture of the bond. The intermetallic formation rate at the interface is diffusion-driven and is a function of time, temperature and alloying element [6]. Satisfactory mechanical properties can be achieved by reducing the thickness ofintermetalliccompoundlayer [7].The thickness of intermetallic compound layer can be reduced by controlling the process parameters and composition of weld metal[2],controllingtheheatflowintoweld[8]andbyusingan interlayer which exhibits improved diffusion resistance to both Al and Fe [9,10].
The techniques available for joining the dissimilar incompatiblemetalcombinationsaregenerallylimitedtotheprocesses which do not result in the melting and solidification of metals to be joined [11—16]. Among the various solid-state welding processes currently available, the friction welding is probably the most proven and established welding technique. The material combination (high strength low alloy steel and aluminium) is reportedtobeweldedbysolid-stateprocesses[9,15,17]and,toa limited extent, by friction welding without interlayer [18].
The present work is aimed at studying the effect of silver in the form of electroplating as interlayer to produce the joint between low alloy steel and aluminium alloy and to understand the role of silver in preventing the formation of brittle intermetallics. The joints were characterized through optical microscopy, scanning electron microscopy (SEM), X-ray diffraction (XRD), scanning electron probe microanalysis (SEPMA), and the mechanical properties were evaluated in terms of microhardness and tensile strength.
2. Experimental procedure
Aluminium alloy AA6061 in T6 condition and low alloy steel AISI 4340 in quenched and tempered condition in the form of 16 mm diameter rods were used for friction welding. Friction welding was carried out on a continuous drive friction welding machine with friction force of 3 kN, upsetting force of 6 kN, rotational speed of 2400 RPM, and burn-off length of 2 mm. Friction welding parameters were selected based on the optimization studies carried out earlier on joining of dissimilar metal combinations [19]. It was recommended that the incompatible materials should be welded at low burn-off length (length loss during friction stage of welding). Low burn-off length results in lower heat input and consequently less time availability for formation of intermetallics. Silver was electroplated on the faying surface of AISI 4340 using standard commercial technique by subcontracted plating firm. The detailed electroplating procedures are not given because they are confidential to the various electroplating firms. A 20 μm-thick silver was electroplated on AISI 4340 since it does not undergo deformation as compared to AA6061. Fig. 1 shows the friction welded joints obtained with AA6061 and AISI 4340 without interlayer and with silver interlayer.
The weld specimens were sectioned and metallographically polished. The specimens were etched with Keller's reagent for AA6061 side and with Nital solution for AISI 4340. Tensile testing was performed employing standard specimen configuration confirming to ASTM standard E8-04, having gauge length of 25 mm with weld interface located at the centre of the specimen. Tensile test specimen used for testing is shown along with its dimensions in Fig. 2. Tensile tests were performed on an Instron 1185 universal testing machine. The cross head speed during the tensile test was maintained at 1 mm/min. Three tensile test specimens were tested and the average of the tensile test results are reported. The failed tensile specimens were subjected to fractographic examination using LEO scanning electron microscope.
Quantitative analysis and X-ray mapping was carried out to know the elemental distribution across the weld interface by scanning electron probe microanalysis (SEPMA). Quantitative analysis was carried out at an interval of 2 μm. The welds were subjected to X-ray diffraction by employing Philips PHL 3020 X-ray diffracto-meter using copper Kαradiation for the identification of various phases. Micro Vickers hardness was measured across the interface of the weld using 100 gm load to determine nature of interface.

Fig. 1. Macrograph of friction welded AA6061-AISI 4340 (a) without silver interlayer and(b) with silver interlayer.
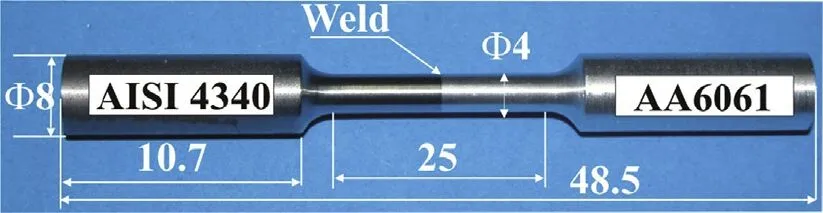
Fig. 2. Tensile test specimen with dimensions (all dimensions in mm).
3. Results
3.1. Optical microscopy
Optical macrograph of a longitudinal section of welds made without interlayer is shown in Fig. 3. From the macrograph it may be observed that aluminium alloy (AA6061) is deformed extensively while no deformation could be noticed on the low alloy steel side during welding. The microstructure near the interface does not show any refinement of grains in steel. However, the aluminium resulted in fine grain structure near interface. The microstructures of the welds without and with interlayer are presented in Fig. 4. The weld interface is straight, a wide dark region is noted on the aluminium alloy beside the weld interface. The joints with silver as interlayer show the replacement of dark region with a bright region.
3.2. Scanning electron probe microanalysis (SEPMA)
The elemental X-ray mappings and quantitative analysis across the weld interface in the central region of weld withoutinterlayer are presented in Fig. 5 and Fig. 6, respectively. Uniform distribution of Mg at weld interface towards Al base metal side of the joint could be observed (Fig. 5). Diffusion of Si from Al to steel could be noticed for direct dissimilar metal weld (without interlayer). Mg is intermittently replaced by Si when silverinterlayerisused(Fig.7).Fig.8showsanaccumulationof Siatthejointinterfacetowardaluminium basemetal withsilver interlayer, indicating impediment of Si migration towards steel.
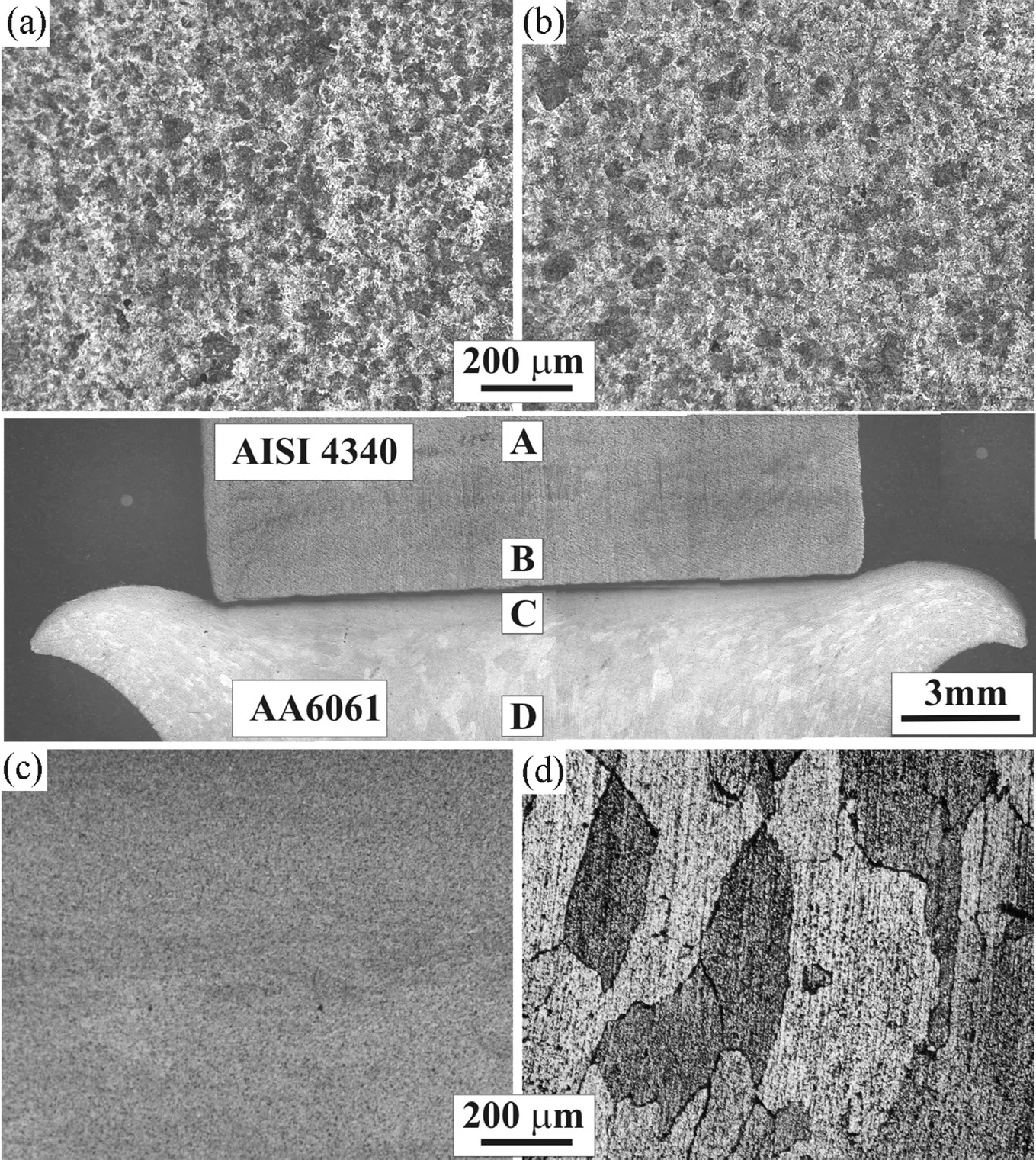
Fig. 3. Optical macrograph and micrograph of friction welded AA6061-AISI 4340 joint.
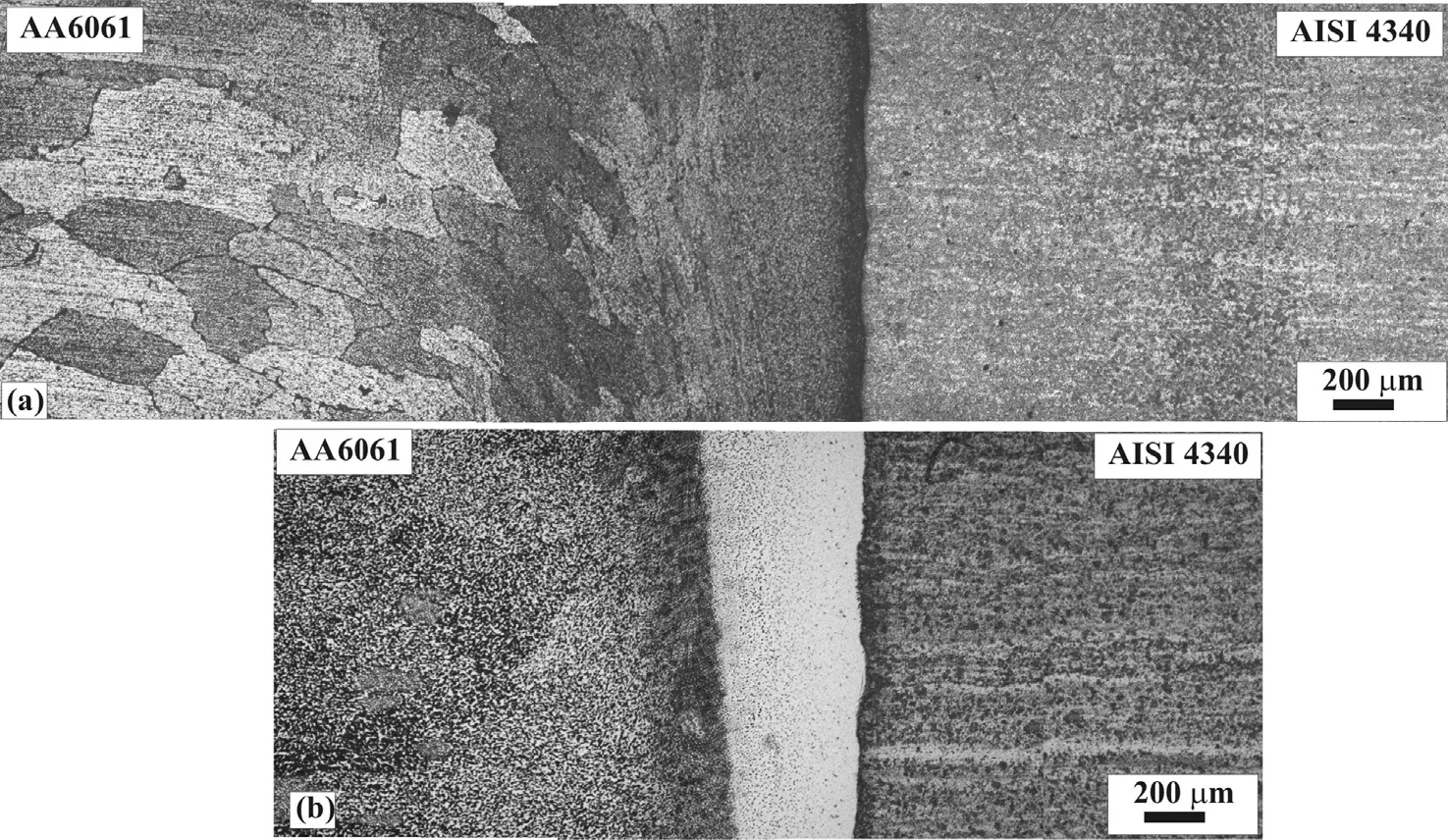
Fig. 4. Micrograph of the cross-section of (a) joint without interlayer and(b) joint with silver interlayer.
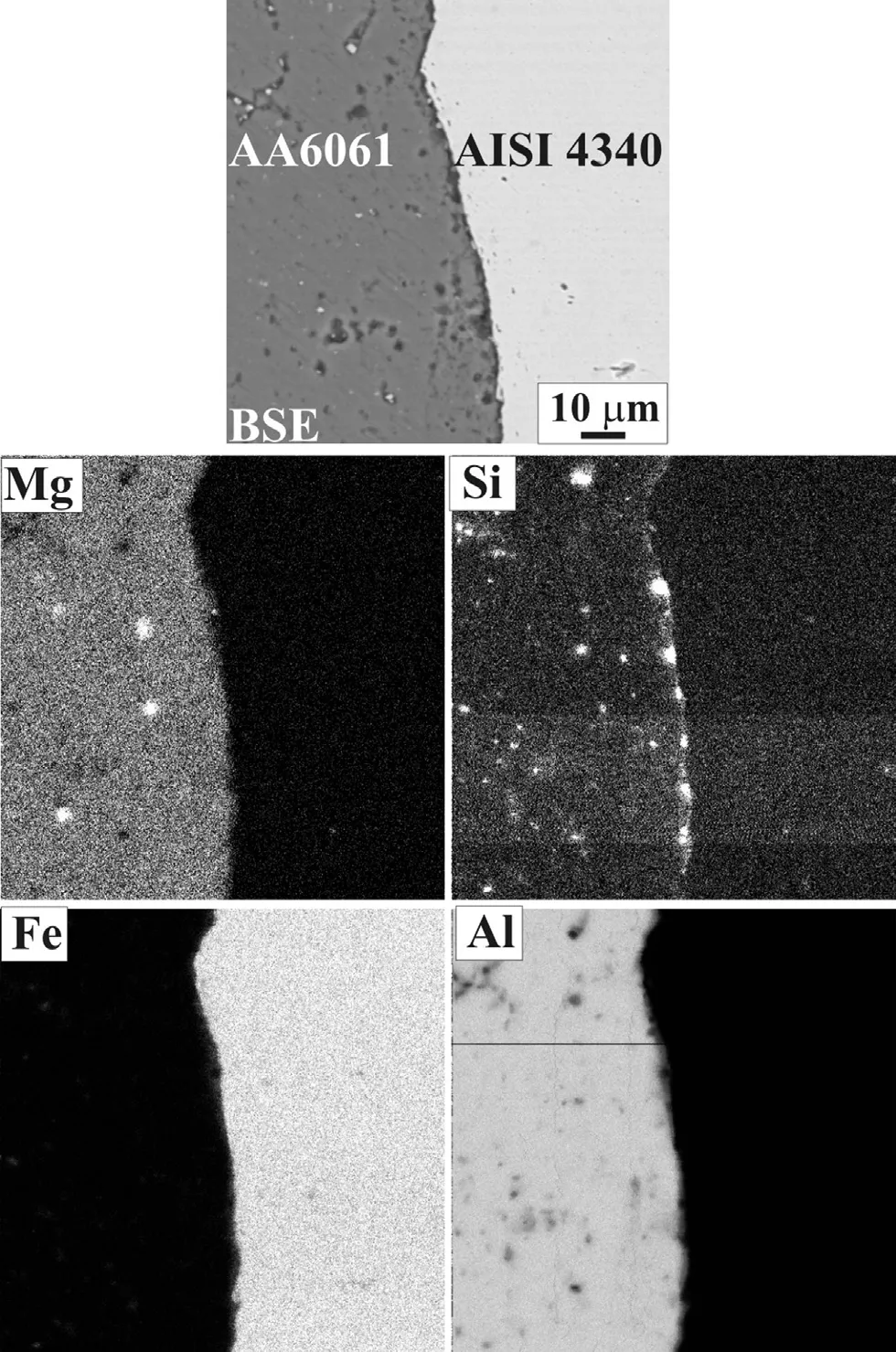
Fig. 5. Elemental X-ray mapping at the weld interface for a joint without interlayer.
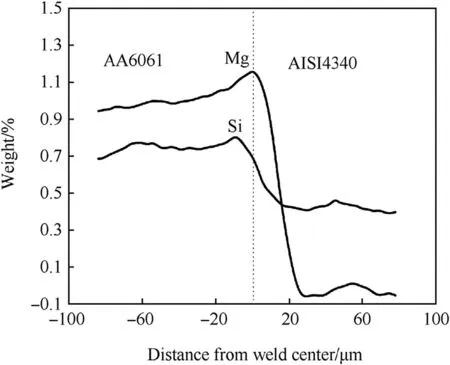
Fig. 6. Quantitative analysis across the weld interface for joint without interlayer.
3.3. Microhardness
Microhardness distribution across the interface of welds made without and with silver interlayer is shown in Fig. 9. It is to be noted that the highest hardness is observed at the weld interface for direct and interlayer weldments. It is observed that the direct weld gives the maximum hardness compared to silver interlayer weld.
3.4. Tensile properties
The tensile properties of parent metal and joints made with and without silver interlayer are detailed in Table 1. Improvement in tensile strength was observed for silver interlayer weld compared to the weld made without interlayer. Failure during tensile testing occurred through the weld interface.
3.5. X-ray diffraction analysis
X-ray diffraction analysis of fractured surface of tensile specimen for welds with and without interlayer is shown in Fig. 10. Examination of the joint made without interlayer revealed an interfacial layer of Fe—Al intermetallics (Fe2Al5and FeAl3). However, introducing silver as an interlayer results in the formation of Ag3Fe2, Ag2Al and Ag3Al in addition to Fe2Al5and FeAl3. Fig. 7. Elemental X-ray mapping at the weld interface for a joint with silver interlayer.

Fig. 8. Quantitative analysis across the weld interface for joint with silver interlayer.
3.6. Fractography
Thefractographfeaturesoftensilesamplesofweldswithand without interlayer are shown in Fig. 11. The fracture is predominantly by cleavage. However, in the case of joints without silver interlayer, a weak bonding can be observed as indicated by arrows where the steel surface is exposed, indicating either nobondinghasoccurredorthebondissoweakthatithasleftno mark of aluminium on steel surface. In the case of joint with silver as an interlayer, no such unbounded/weak zone is observed. This gives an indication of good joint integrity.
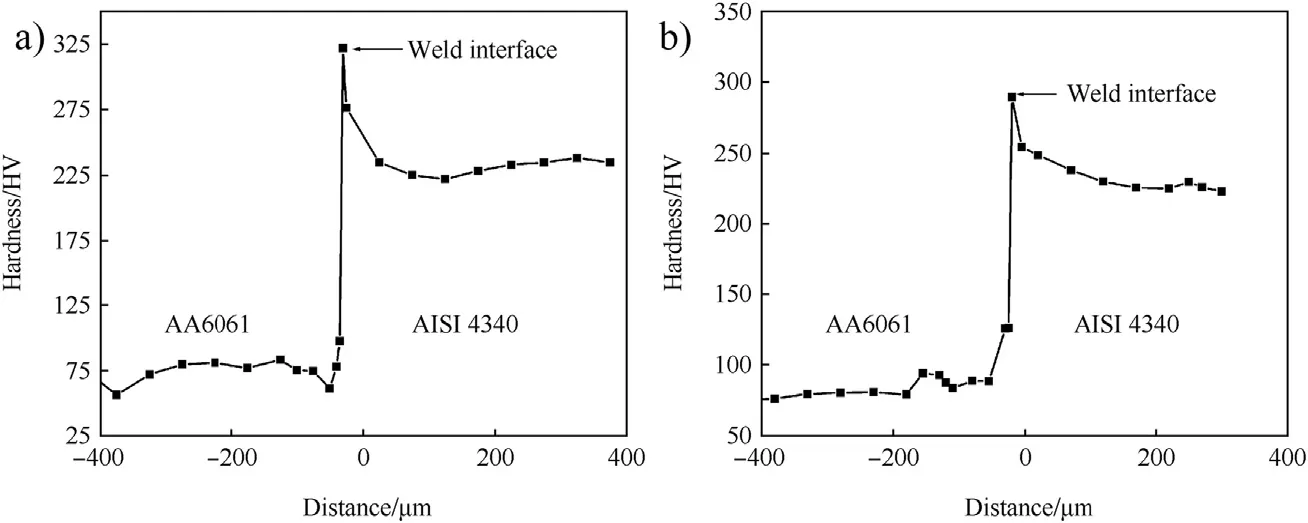
Fig. 9. Microhardness distribution across the weld interface (a) without interlayer and (b) with silver interlayer.
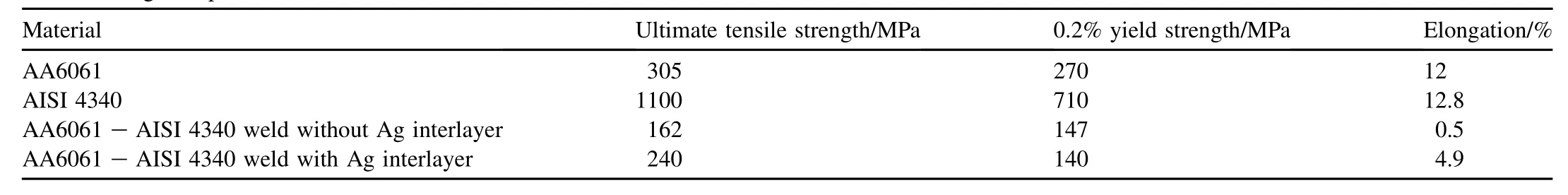
Table 1Tensile strength of parent metal and welds.
4. Discussion
The friction welding process can be used to produce a metallurgical bond through the interaction of frictional heating and simultaneous deformation along the interface separating the material to be joined. Heat generated along the interface flows either axially away from the interface or radially along the interface as material is upset from the joint forming the characteristic flash. The different thermal and physical properties of the materials welded in dissimilar metal welding, including heat capacity, thermal conductivity, relation between hardness and temperature, generally results in asymmetrical deformation. The formation of flash on aluminium side and no flash on low alloy steel (Fig. 3) can be attributed to lower thermal conductivity and higher hardness of low alloy steel at elevated temperatures compared to aluminium alloys. The same phenomenon has been reported during friction welding of dissimilar welds namely Al to Cu [20], Al to stainless steel [21] and titanium to steel [22]. From the metallographic study it is observed that direct welding of AISI 4340 to AA6061 is not feasible due to the presence of continuous intermetallic layer across the weld width (Fig. 4).
A correlation of strength data of welds with microstructure at the interface suggests that the direct welding of AISI 4340 to AA6061 aluminium alloy results in the formation of continuous intermetallic zone, and therefore exhibits very poor strength and almost nil ductility (Table 1).
X-ray diffraction data of fractured tensile samples of direct weld contains highly brittle intermetallics such as Fe2Al5and FeAl3(Fig. 10). Incorporation of silver interlayer in the form of electroplating has been observed to be a solution to realize the welding of low alloy steel to aluminium alloy. Silver interlayer isfoundtobemostusefulasitimplantsgoodductilityaswellas strength. Silver as an interlayer results in the formation of Ag3Fe2, Ag2Al and Ag3Al in addition to Fe2Al5and FeAl3(Fig. 10). Silver, presenting in the form of interlayer, acts as a barrier for the direct interaction between aluminium and steel, resulting in partial replacement of Fe—Al based intermetallic compound with Al—Ag based intermetallic compound.
Mg and Si are the major alloying elements in AA6061. Mg is reported to be favourable for increasing the width of Fe—Al based intermetallic compound layer [23], particularly Fe2Al5.Uniform distribution of Mg at weld interface towards Al base metal side of the joint can be observed in the joint without silver interlayer (Fig. 5), which is a favourable condition for the formation of Fe—Al based intermetallics. However the joints with silver interlayer show that Mg is intermittently replaced by Si at the weld interface (Fig. 7). The absence of Mg near interface for joint with silver interlayer results in less favourable condition for the formation of Fe2Al5, and the situation is more favorable for the silver/aluminium intermetallic formation at the interface. This intermetallic compound is reported to be significantly softer than that formed between iron and aluminium and hence much greater thickness can be tolerated in the attainment of good quality welds between aluminium and its alloy to low alloy steel [24].
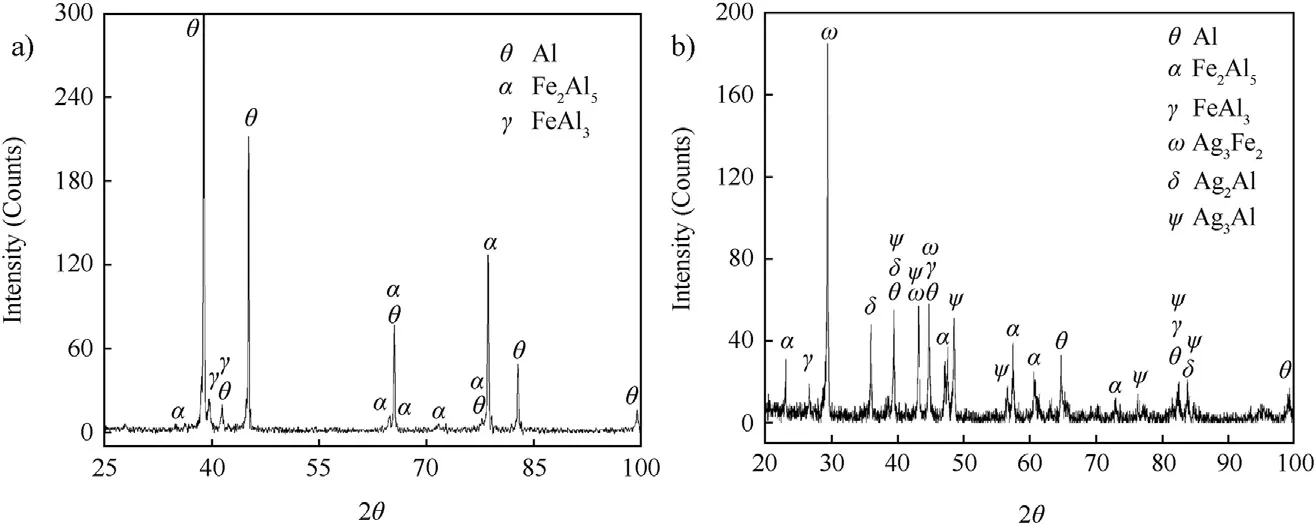
Fig. 10. X-ray diffraction analysis of fractured tensile samples (a) without interlayer and (b) with silver interlayer.
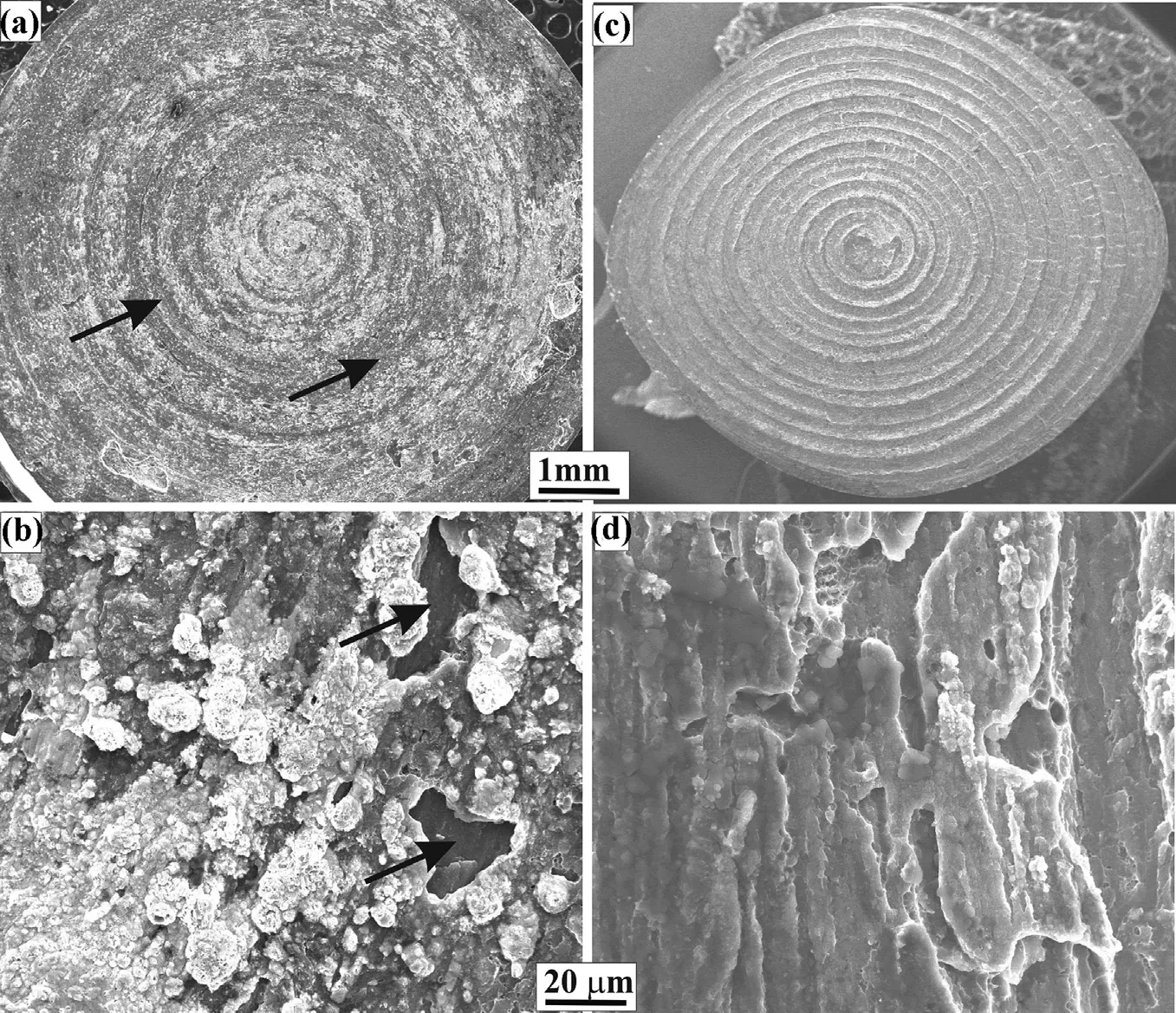
Fig. 11. Fractograph of tensile samples at weld interface (a) (b) without interlayer and (c) (d) with silver interlayer.
Heating of aluminium alloy at high temperature results in diffusing Si out of the lattice [25]. Since Si—Ag is an eutectic system which is practically immiscible in solid state, the diffusion of Si across the silver interlayer is restricted, resulting in higher concentration of Si at interface of aluminium base metal and silver (Fig. 7). This observation is supported by quantitative analysis of Si across the weld interface for joints withsilverinterlayer(Fig.8).Higherconcentrationof Siretards the formation of Fe2Al5at weld interface by acting as a barrier to Fe diffusion [6,18,26,27]. Silicon segregation at the weld interface is not observed in a joint without silver interlayer, which allows more Fe to diffuse towards Al, resulting in Fe2Al5formation and poor tensile property.
Higher value of microhardness in a joint without silver interlayercanbeattributedtotheformationof Fe2Al5and FeAl3[28]. Slight reduction in hardness with silver interlayer can be observed due to the presence of silver/aluminium intermetallic compounds which are soft in nature. Low tensile strength and elongation of joints withoutsilver interlayer can be attributed to the formation of Fe2Al5and FeAl3intermetallics which arebrittle in nature. Introduction of silver interlayer results in improvement in tensile strength and elongation since, and Fe—Al based intermetallic compound are partially replaced by Al—Ag based compounds which are ductile in nature.
5. Conclusions
A friction welding technique has been developed for joining aluminium (AA6061) to low alloy steel (AISI 4340) using an interlayer of silver. Silver as an interlayer partially reduces the formation of Fe—Al based intermetallic and replaces it with Al—Ag based intermetallic, such as Ag3Fe2, Ag2Al and Ag3Al, resulting in better tensile strength and ductility of welds. Presence of silver as an interlayer reduces Mg concentration at the weld interface by intermittently replacing it with Si on AA6061 side, which restricts the interaction of Fe with aluminium. The higher strength and ductility of aluminium to low alloy steel dissimilar metal welds with silver as an interlayer was attributed to the formation of ductile phases like Ag3Fe2, Ag2Al and Ag3Al.
Acknowledgement
The authors express their gratitude to Defence Research and Development Organization for the financial support to carry out this program and are thankful to Dr. Amol A. Gokhale, Distinguished scientist, Director, Defence Metallurgical Research Laboratory, India for his continued encouragement and support.
References
[1] Agudo L, Eyidi D, Schmaranzer CH, Arenholz E, Jank N, Bruckner J, et al. Intermetallic FexAly-phases in a steel/Al-alloy fusion weld. J Mater Sci 2007;42(24):4205—14.
[2] Zhang H, Liu J. Microstructure characteristics and mechanical property of aluminum alloy/stainless steel lap joints fabricated by MIG weldingbrazing process. Mater Sci Eng A 2011;528(19—20):6179—85.
[3] Zhang HT, Feng JC, He P, Hackl H. Interfacial microstructure and mechanical properties of aluminium-zinc-coated steel joints made by a modified metal inert gas welding brazing process. Mater Charact 2007;58(7):588—92.
[4] Donga H, Yanga L, Dong C, Kou S. Improving arc joining of Al to steel and Al to stainless steel. Mater Sci Eng A 2012;534(1 Feb):424—35.
[5] Dieter GE. ASM handbook, materials selection and design. 1st ed. Materials Park, OH: ASM International; 1997.
[6] Kobayashi S, Yakou T. Control of intermetallic compound layers at interface between steel and aluminum by diffusion-treatment. Mater Sci Eng A 2002;338(1—2):44—53.
[7] Cao R, Yu G, Chen JH, Wang PC. Cold metal transfer joining aluminum alloys-togalvanizedmildsteel. JMaterProcessTechnol 2013;213(10):1753—63.
[8] Borrisutthekul R, Yachi T, Miyashita Y, Mutoh Y. Suppression of intermetallicreactionlayerformationbycontrollingheatflowindissimilarjoiningof steel and aluminum alloy. Mater Sci Eng A 2007;467(1—2):108—13.
[9] Madhusudhan Reddy G, Sambasiva Rao A, Mohandas T. Role of electroplated interlayer in continuous drive friction welding of AA6061 to AISI 304 dissimilar metals. Sci Technol Weld Join 2008;13(7):619—28.
[10] Sun X, Stephens EV, Khaleel MA, Shao H, Kimchi M. Resistance spot welding of aluminum alloy to steel with transition material-from process to performance-part I: experimental study. Weld J 2004;83(7):188—95.
[11] Date H, Kobayakawa S, Naka M. Microstructure and bonding strength of impact-welded aluminum-stainless steel joints. J Mater Process Technol 1999;85(1—3):166—70.
[12] Shubhavardhan RN, Surendran S. Friction welding to join stainless steel and aluminium materials. Int J Metall Mater Sci Eng (IJMMSE) 2012;2(3):53—73.
[13] Tsujino J, Hidai K, Hasegawa A, Kanai R, Matsuura H, Matsushima K, et al. Ultrasonic butt welding of aluminum, aluminum alloy and stainless steel plate specimens. Ultrasonics 2002;40(1—8):371—4.
[14] He P, Yue X, Zhang JH. Hot pressing diffusion bonding of a titanium alloy to a stainless steel with an aluminum alloy interlayer. Mater Sci Eng A 2008;486(1—2):171—6.
[15] Acarer M, Demir B. An investigation of mechanical and metallurgical properties of explosive welded aluminum-dual phase steel. Mater Lett 2008;62(25):4158—60.
[16] Dehghani M, Amadeh A, Akbari Mousavi SAA. Investigations on the effects of friction stir welding parameters on intermetallic and defect formation in joining aluminum alloy to mild steel. Mater Des 2013;49(1):433—41.
[17] Patel VK, Bhole SD, Chen DL. Ultrasonic spot welding of aluminum to high-strength low-alloy steel: microstructure, tensile and fatigue properties. Metall Mater Trans A 2014;45A(1):2055—66.
[18] Fuji A. Friction welding of Al—Mg—Si alloy to Ni—Cr—Mo low alloy steel. Sci Technol Weld Join 2004;9(1):83—9.
[19] Meshram SD, Mohandas T, Madhusudhan Reddy G. Friction welding of dissimilar pure metals. J Mater Process Technol 2007;184(1—3):330—7.
[20] Yilbas BS, Sahin AZ, Kahraman N, Al-Garni AZ. Friction welding of St—Al andAl—Cumaterials. JMaterProcessTechnol 1995;49(3—4):431—43.
[21] Kimura M, Ishii H, Kusaka M, Kaizu K, Fuji A. Joining phenomena and joint strength of friction welded joint between aluminium magnesium alloy (AA5052) and low carbon steel. Sci Technol Weld Join 2009;14(7):655—61.
[22] Dey HC, Ashfaq M, Bhaduri AK, Rao KP. Joining of titanium to 304L stainless steel by friction welding. J Mater Process Technol 2009;209(18—19):5862—70.
[23] Hwang IH, Watanabe T, Doi Y. Dissimilar metal welding of steel to Al-MgAlloybyspot resistancewelding. AdvMater Res 2007;15(17):381—6.
[24] Rosalie JM, Bourgeois L, Muddle BC. Precipitate assemblies formed on dislocationloopsinaluminium-silveralloys. PhilosMag 2009;89(15):1267—78.
[25] Fujikawa SI, Hirano KI, Yoshiaki Fukushima Y. Diffusion of silicon in aluminum. Metall Mater Trans A 1978;9(12):1811—5.
[26] Yin FC, Zhao MX, Liu YX, Han W, Li Z. Effect of Si on growth kinetics of intermetallic compounds during reaction between solid iron andmoltenaluminum. TransNonferrMet SocChina 2013;23(2):556—61.
[27] Springer H, Kostka A, Payton EJ, Raabe D, Kaysser-Pyzalla A, Eggeler G. On the formation and growth of intermetallic phases during interdiffusion between low carbon steel and aluminum alloys. Acta Mater 2011;59(4):1586—600.
[28] Rathod MJ, Kutsuna M. Joining of aluminum alloy 5052 and low-carbon steel by laser roll welding. Weld J 2004;83(1):16s—26s.
* Corresponding author. Tel.: +91 4024586433; fax: +91 4024342697.
- Defence Technology的其它文章
- Gas tungsten arc welding of ZrB2
—SiC based ultra high temperature ceramic composites R.V. KRISHNARAO*, G. MADHUSUDHAN REDDY - Influence of tool pin profile on microstructure and corrosion behaviour of AA2219 Al
—Cu alloy friction stir weld nuggets Ch. VENKATA RAOa, G. MADHUSUDHAN REDDYb, K. SRINIVASA RAOa,* - Optimization of process parameters of aluminum alloy AA 2014-T6 friction stir welds by response surface methodology Ramanjaneyulu KADAGANCHIa, Madhusudhan Reddy GANKIDIb,*, Hina GOKHALEb
- Effect of hot-humid exposure on static strength of adhesive-bonded aluminum alloys
- Numerical modeling of friction stir welding using the tools with polygonal pins M. MEHTAa, G.M. REDDYb, A.V. RAOb, A. DEc,*
- Microstructure and pitting corrosion of shielded metal arc welded highnitrogen stainless steelRAFFI MOHAMMEDa, G. MADHUSUDHAN REDDYb, K. SRINIVASA RAOa,*

