Stress corrosion cracking behaviour of gas tungsten arc welded super austenitic stainless steel joints M. VINOTH KUMARa,*, V. BALASUBRAMANIANb,1, S. RAJAKUMARb, SHAJU K. ALBERTc
Deprtment of Mnufturing Engineering, Annmli University, Annmli Ngr, Tmil Ndu 608002, IndiCenter for Mterils Joining nd Reserh (CEMAJOR), Deprtment of Mnufturing Engineering, Annmli Ngr, Tmil Ndu 608002, IndiMterils Tehnology Division, Indir Gndhi Centre for Atomi Reserh (IGCAR), Klpkkm, Tmilndu 603102, IndiReeived 13 My 2015; epted 15 My 2015 Aville online 2 July 2015
Stress corrosion cracking behaviour of gas tungsten arc welded super austenitic stainless steel joints M. VINOTH KUMARa,*, V. BALASUBRAMANIANb,1, S. RAJAKUMARb, SHAJU K. ALBERTc
aDepartment of Manufacturing Engineering, Annamalai University, Annamalai Nagar, Tamil Nadu 608002, IndiabCenter for Materials Joining and Research (CEMAJOR), Department of Manufacturing Engineering, Annamalai Nagar, Tamil Nadu 608002, India
cMaterials Technology Division, Indira Gandhi Centre for Atomic Research (IGCAR), Kalpakkam, Tamilnadu 603102, India
Received 13 May 2015; accepted 15 May 2015 Available online 2 July 2015
Abstract
Super 304H austenitic stainless steel with 3% of copper posses excellent creep strength and corrosion resistance, which is mainly used in heat exchanger tubing of the boiler. Heat exchangers are used in nuclear power plants and marine vehicles which are intended to operate in chloride rich offshore environment. Chloride stress corrosion cracking is the most likely life limiting failure with austenitic stainless steel tubing. Welding may worsen the stress corrosion cracking susceptibility of the material. Stress corrosion cracking susceptibility of Super 304H parent metal and gas tungsten arc (GTA) welded joints were studied by constant load tests in 45% boiling MgCl2solution. Stress corrosion cracking resistance of Super 304H stainless steel was deteriorated by GTAwelding due to the formation of susceptible microstructure in the HAZ of the weld joint and the residual stresses. The mechanism of cracking was found to be anodic path cracking, with transgranular nature of crack propagation. Linear relationships were derived to predict the time to failure by extrapolating the rate of steady state elongation.
Copyright©2015, China Ordnance Society. Production and hosting by Elsevier B.V. All rights reserved.
Keywords:Super 304H; Chloride stress corrosion cracking; Constant load test; Gas tungsten arc welding
E-mail addresses: vinothmecho@gmail.com (M. VINOTH KUMAR), visvabalu@yahoo.com (V. BALASUBRAMANIAN), srkcemajor@gmail. com (S. RAJAKUMAR), shaju@igcar.gov.in (S.K. ALBERT).
Peer review under responsibility of China Ordnance Society.
1Tel.: +91 9443412249 (mobile).
http://dx.doi.org/10.1016/j.dt.2015.05.009
2214-9147/Copyright©2015, China Ordnance Society. Production and hosting by Elsevier B.V. All rights reserved.
1. Introduction
Austenitic stainless steels are the desired material for use in high temperatures under highly corrosive environment. Heat exchangers are used in nuclear power plants and marine vehicles which are intended to operate in chloride rich offshore environment. The efficiency of the power cycle is function of the operating temperature and pressure. Development and selection of materials with required high temperature strength and corrosion resistance is vital in further improvement in efficiency of the power cycle [1,2]. Recently developed Super 304H austenitic stainless steel with excellent creep strength and corrosion resistance is mainly used in heat exchanger tubing of the boiler. The addition of 3 wt.% Cu to Super 304H enhances the precipitation strengthening of the alloy by precipitating out fine, stable and coherent Cu rich particles at elevated temperatures [3].
Stainless steels resist general corrosion but are susceptible to localized corrosion such as pitting, and stress corrosion cracking (SCC) in chloride environments [4]. SCC is the most likely life limiting failure in boilers with austenitic stainless steel tubing [5]. SCC is caused by the synergic and simultaneous action of tensile stress, environment and susceptible microstructure [6]. The microstructure depends on the chemical composition and manufacturing methods. Welding is considered as the major manufacturing method for pressure equipments in power plants [7]. Welding may alter thefavorable parent metal microstructure and induce residual stresses in the joints. In some cases the residual stress may exceed the tensile stress of the material, resulting in worsening of SCC susceptibility of the material [4,8]. The addition of Nb to the steel and weld metal is beneficial for stabilizing C in the matrix to avoid sensitization, while the effect of nitrogen on SCC in parent metal is considered beneficial, and for the welds it remains complicated due to their inhomogenous dendritic cast structure [9].
In the absence of analytical approaches to predict SCC, testing becomes vital. In actual conditions, SCC tends occur over long periods of time; hence the SCC tests are accelerated byusinghighly aggressive environments, constantly increasing the load/strain. The results of accelerated tests can be extrapolated to predict the long term service life of the structure [10]. The test methods for SCC are classified as constant load tests, constant strain tests and slow strain rate tests based on mode of specimen loading [11]. Recent works in Refs. [12,13] on SCC of Super 304H using constant strain method revealed the SCC susceptibility of the Super 304H under larger strain and improper heat treatment conditions.
In this present work, the SCC susceptibility of Super 304H parent metal and gas tungsten arc welded (GTA) joints were studied by recording the“corrosion—elongation curves”during constant load tests in boiling MgCl2solution.

Table 1Chemical composition (wt.%) of parent metal (PM) and filler metal (FM).
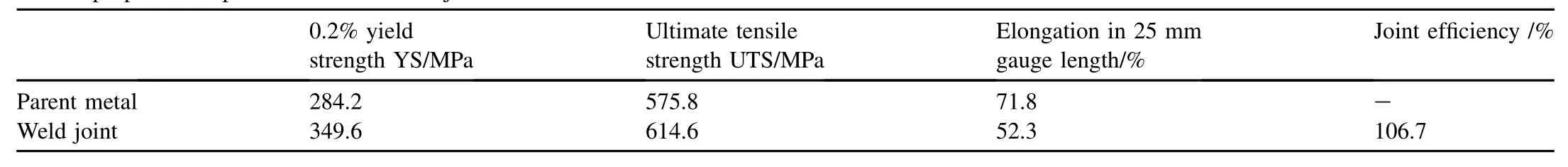
Table 2Tensile properties of parent metal and weld joint.
2. Experimental details
The parent metal used in this investigation was Super 304H austenitic stainless steel with distinct addition of 3 wt% of copper. Super 304H was received in annealed condition (1145°C), in the form of tubes with outer diameter of 57.1 mm and wall thickness of 3.5 mm. For GTAwelding, the joints with single‘V’butt configuration were welded with addition of filler metal. Filler metal composition was suitably modified to achieve delta ferrite free weld metal by increasing the Ni content; the resultant weld metal microstructure was fully austenitic, as preferred in high temperature applications [9]. Mo was added to avoid the risk of hot cracking in the fully austenitic weld metal by modifying the S inclusions and enhance the resistance to pitting corrosion [14,15]. The chemical compositions of the parent metal and filler metal are presented in Table 1. The welding was carried out with average heat input of 0.68 kJ/mm, in which argon was used as the shielding and purging gas.
The specimens for transverse tensile test and SCC tests were extracted from the parent metal and weld joints using wire-cut electric discharge machining. The tensile properties of as-received parent metal and weld joints are listed in Table 2. In order to reveal the susceptibility of Super 304H parent metal and weld joint to intergranular corrosion (IGC), the specimens were subjected to oxalic acid etch test as per ASTM A262 practice A. The specimens were probed under light microscope to reveal the level of Super 304H's susceptibility to IGC before and after welding.
The SCC test was carried out using the smooth tensile specimen (shown in Fig. 1) in a custom-built constant load setup with maximum loading capacity of 10 kN. The applied loads are measured using a load cell with an accuracy of ±10 N. The strain measurements were done using an LVDT with measurable range of±5 mm and an accuracy of <1 μm. The schematic representation of the SCC constant load setup is shown in Fig. 2. The environment for SCC testing of Super 304H was chosen as 45% MgCl2boiling at 155°C, and thetests were conducted in accordance with ASTM G36. The constant load SCC tests were conducted for parent metal and weld joints of Super 304H at stress levels of 100%, 80%, 60% and 40% of the parent metal's yield strength. The specimens for microstructure analysis were polished and etched using Glyceregia. The fracture surfaces of the SCC specimens were ultrasonically cleaned and analyzed using scanning electron microscopy (SEM) to reveal the modes of failure. Energy dispersive spectroscopy (EDS) attached with SEM was used to reveal the elemental composition of interested spots in fracture surface.
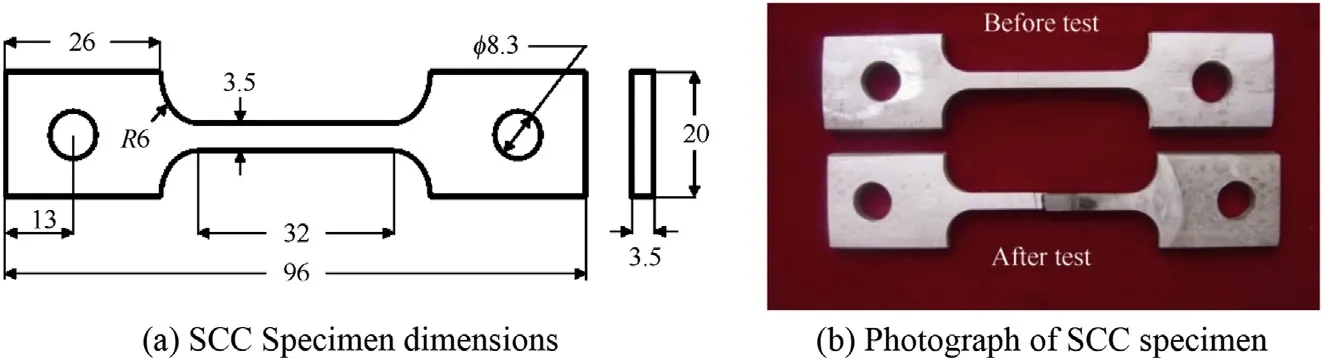
Fig. 1. Schematic representation of the SCC constant load setup.
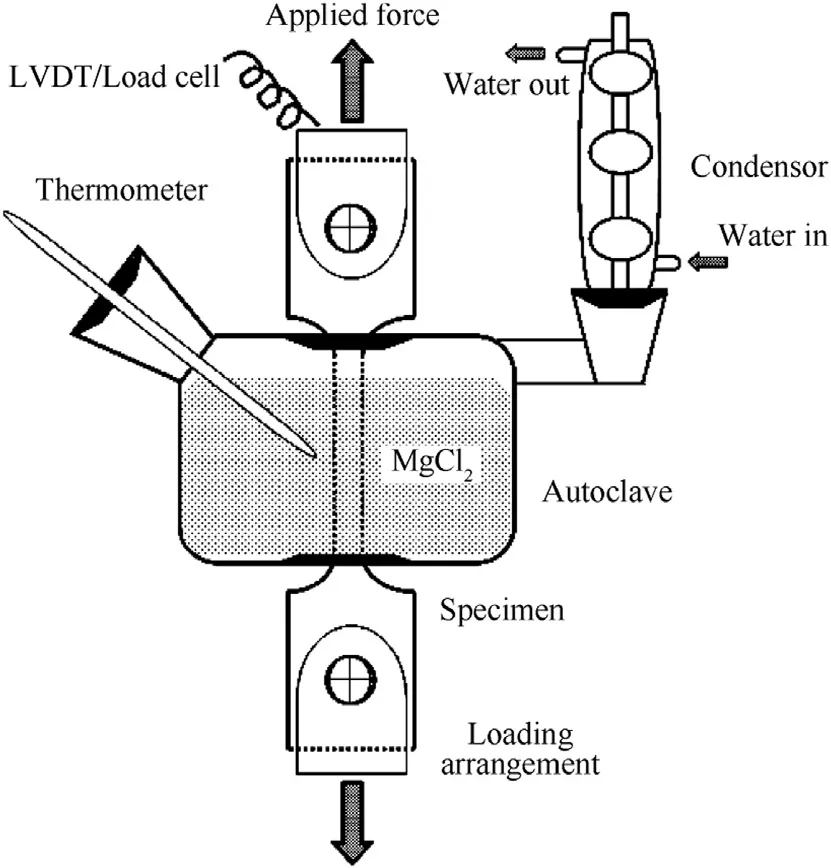
Fig. 2. Schematic representation of the SCC constant load setup with boiling MgCl2.
3. Results
3.1. Microstructure
The SEM micrograph of parent metal is shown in Fig. 3, which cons(a)ists of equiaxed austenitic grains with annealing twins and precipitates (marked by arrows). The precipitates in parent metal were chemically extracted from the austenitematrix, and their XRD pattern is shown in Fig. 3(b). The XRD pattern confirmed the presence of Nb(C,N), M23C6carbides in the parent metal. The SEM micrograph of weldmetal in the center of the joint is shown in Fig. 3(c), which reveals fully austenitic grains with cellular morphology and carbides (NbC, M23C6) precipitated along the boundaries. The fusion line (FL) of the weld joint shown in Fig. 3(d) reveals the epitaxial grain growth. The heat affected zone (HAZ) next to the fusion line shows grain coarsening due to the weld thermal cycles, however no liquation of grain boundaries nor sensitization is observed from SEM micrograph.
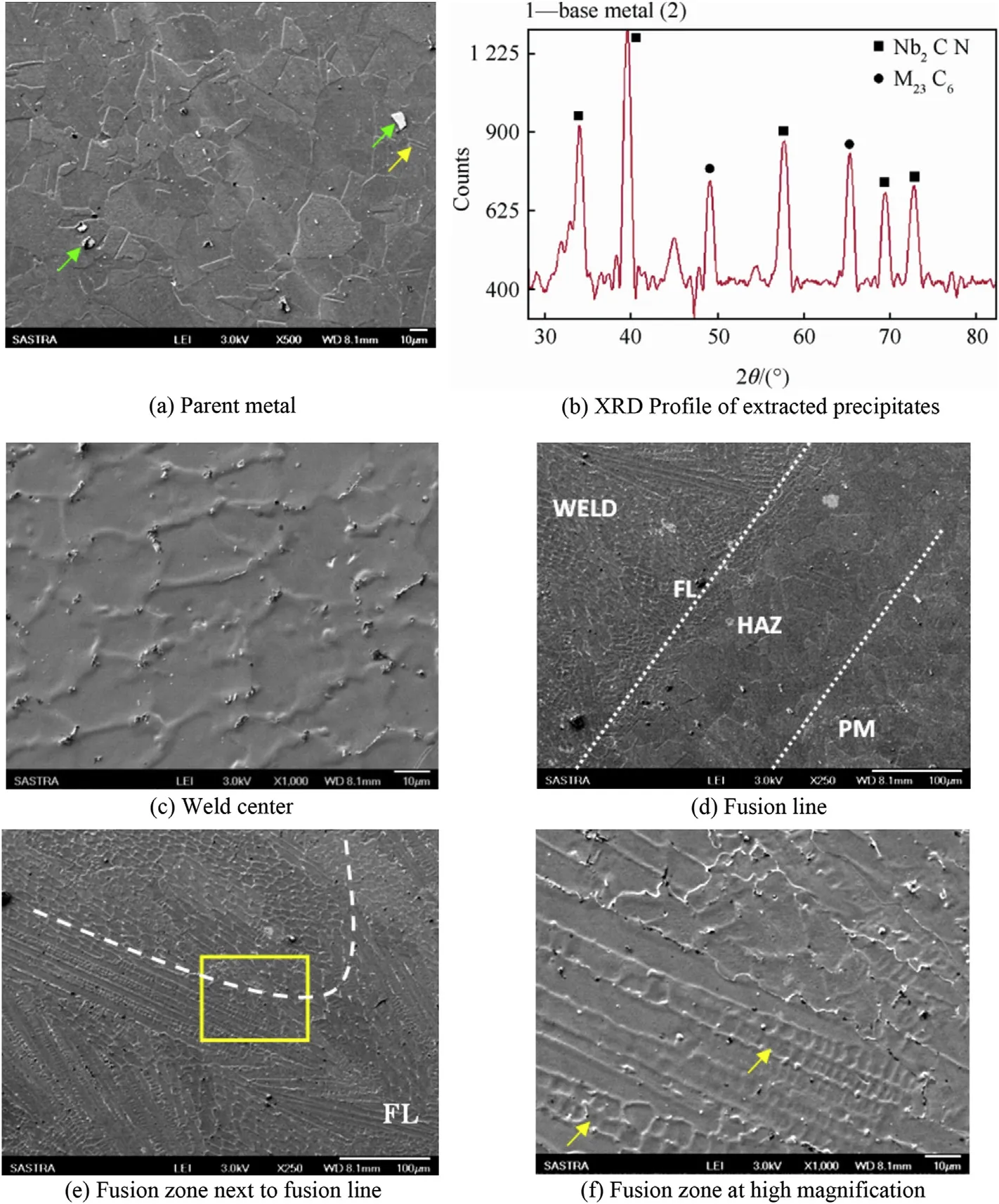
The fusion zone of the weld next to fusion line is shown in Fig. 3(e) which shows the mixed morphology (cellular and dendritic) of the austenite in this zone. The white dotted line marks the transition of the austenitic weld from dendrite near the fusion line to cell morphology towards the weld enter. The magnified image of the area marked in Fig. 3(e) is shown in Fig. 3(f). The marked arrow indicates the primary ferrite dendritic core formed by F-A solidification mode and subsequently transformed to secondary austenite by solid state diffusion. This confirms that both A type (cellular) and F-A type of solidification have prevailed in the fusion zone next to fusion line [16].
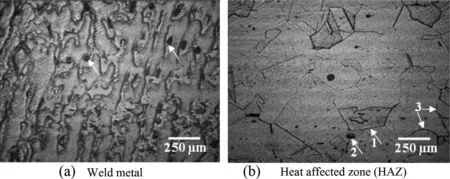
Fig. 4. Oxalic acid etched structure of weld metal and HAZ.
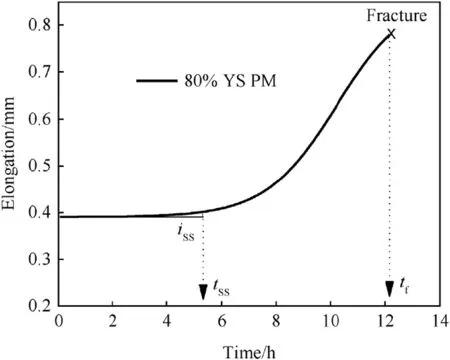
Fig. 5. Typical corrosion elongation curve of Super 304H and its parameters in boiling MgCl2solution.
3.2. Oxalic acid etch structure
The oxalic acid etch structure of weld metal is shown in Fig. 4(a), which reveals the end grain pits in austenitic weld metal. The etch structure of HAZ shown in Fig. 4(b) reveals the step structure among the grains (marked as 1), few deep end grain pits (marked as 2) and dual structure in which a single grain is not completely surrounded by the ditches (marked as 3). The etch structures of both weld metal and HAZ were acceptable as per ASTM A262 and show nosusceptibility to intergranular attack associated with chromium carbide precipitation.

Fig. 6. Corrosion elongation curves for parent metal and weld joints of Super 304H in boiling MgCl2at 100%, 80%, 60% and 40% of the yield strength.
3.3. Corrosion elongation curves
The‘corrosion elongation curve’for unwelded parent metal tested at 80% of its yield strength in boiling MgCl2is shown in Fig. 5, and the parameters such as iss, tssand tfwere derived from this curve. The slope of the curve in the secondary region before the time to transition (tss) from secondary region to tertiary region represents the rate of steady state elongation (iss), where the tfrepresents the time to complete fracture. The corrosion elongation curves of parent metal and weld joint for all test conditions are shown in Fig. 6, and the evaluated parameters are listed in Table 3. The corrosion elongation curves provide information about the SCC cracking mechanisms involved in the parent metal and weld joint of Super 304H austenitic stainless steel [17].
The relationship between the applied stress and the tffor parent metal and weld joint of Super 304H is shown in Fig. 7, which reveals the significant degradation in SCC resistance of Super 304H subjected to welding, as the tfof weld joint is lower than that of parent metal at their respective stress level. The ratio of tssto tfshown in Table 3 is used to determine the most prominent mode of degradation (corrosive or mechanical) active in their respective test conditions. The typical values of tss/tffor 316 stainless steels in stress corrosion range was reported to be~0.6 [18]. In the case of Super 304H parent metal and weld joint, the value of tss/tftends to increase with the decrease in applied stress typical of N alloyed steels, as reported elsewhere for 0.25% N alloyed austenitic stainless steels [17]. This implies that the time in the tertiary region, i.e. the time for fracture after crack initiation, decreases steadily with the decrease in applied stress level, whereas the steady state elongation rate (iss) decreases with the increase in tfand the decrease in applied stress. Puiggali et al. [18] described the crack growth rate (Vc) as,
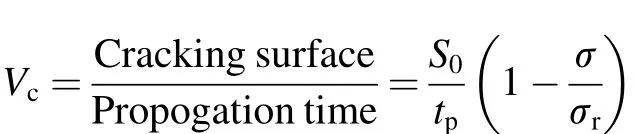
where Sois initial cross section; tpis time for crack propagation after crack initiation (tss);б is initial applied stress; and бris ultimate tensile strength. The crack growth rate in terms of mm2/h was calculated for all the test conditions and listed in Table 3. It was found that, for both parent metal and weld joint, Vcwas higher in the stress dominant region (1.0×YS), while Vcdecreased at 0.8×YS indicating the transition point to SCC dominant region and continues to increase from thereof, till the corrosion dominant region (0.4×YS), where the highest value of Vcwas recorded.

Table 3Corrosion elongation curve parameters for Super 304H in boiling MgCl2solution.

Fig. 7. Relationship between applied stress and time to failure.
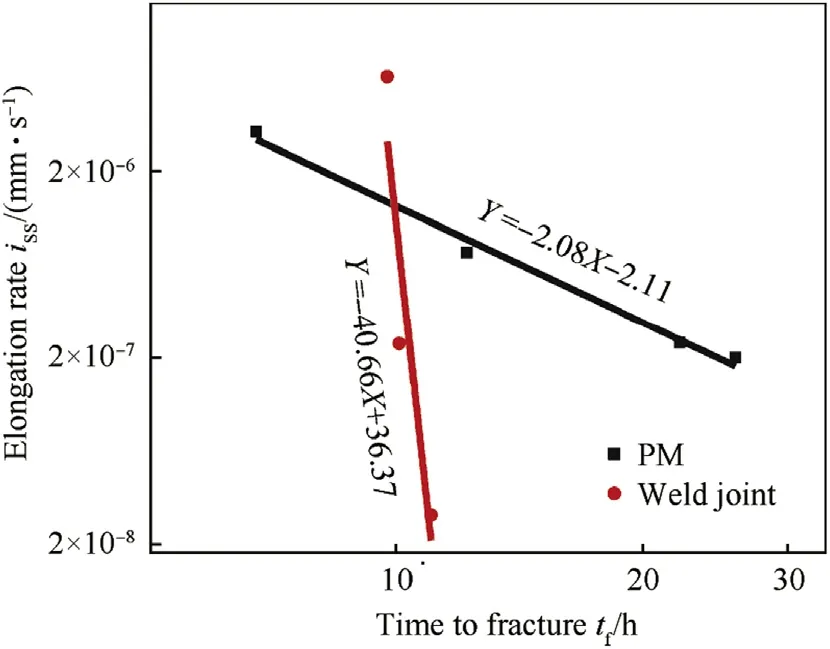
Fig. 8. Prediction of time to failure of Super 304H parent metal and weld joint by SCC in boiling MgCl2.
3.4. Prediction of time to failure
The calculated corrosion elongation curve parameters shown in Table 3 can be used for prediction of time to failure by extrapolation of time scale. The logarithmic relationship between issand tfis always linear and independent of pH, concentration of ion, temperature and material [17,19]. The Fig. 8 reveals that such a linear relationship also exists between the issand tfof parent metal and weld joints of Super 304H. The mathematically expressed linear relationships iss=-2.08tf-2.11(for parent metal) and iss= -40.66 tf+ 36.37 (for weld joint) can be used for prediction of time to failure.

Fig. 9. Fracture characteristics of Super 304H parent metal SCC specimens tested in boiling MgCl2solution at different stress levels.
3.5. Fracture surfaces
The SCC characteristics in the directions parallel and normal to loading, for the parent metal and weld joint areshown in Figs. 9 and 10 respectively. The cracks are oriented normal to the loading direction, and the number of cracks and crack width decreases with the decrease in applied stress for both parent metal (refer Fig. 9(a)) and weld joint (refer Fig. 10(a)). The fracture surfaces of the parent metal and weld joint at low magnification (refer Figs. 9(b) and 10(b)) reveal brittle mode of failure, with no reduction in cross sectional area. The Figs. 9(c) and 10(c) confirms the transgranular mode of crack propagation for both parent metal and weld joint at all stress levels, with de-cohesion of grains at the grain boundaries.

Fig. 10. Fracture characteristics of Super 304H GTAW joints SCC specimens tested in boiling MgCl2solution at different stress levels.
The microstructural characteristic features of SCC in parent metal and weld joint are shown in Fig. 11. In parent metal, the interfacebetweenthematrixandcoarsecarbidesareidentifiedas the sourceof crack initiation, asindicated by arrow in Fig. 11(a). Theothercrackinitiationsitesarethehighnumberofslipstepsat the edges of the specimen, which promotes the formation and growth of corrosion pits in large numbers by anodic dissolution, asshownin Fig.11(c).Theparentmetalwithlesserappliedstress levelprovideslessercrackinitiationsitesbyslipsattheedgesand hence the crack propagates by branching and interconnecting other probable cracks (Fig. 11(e)).
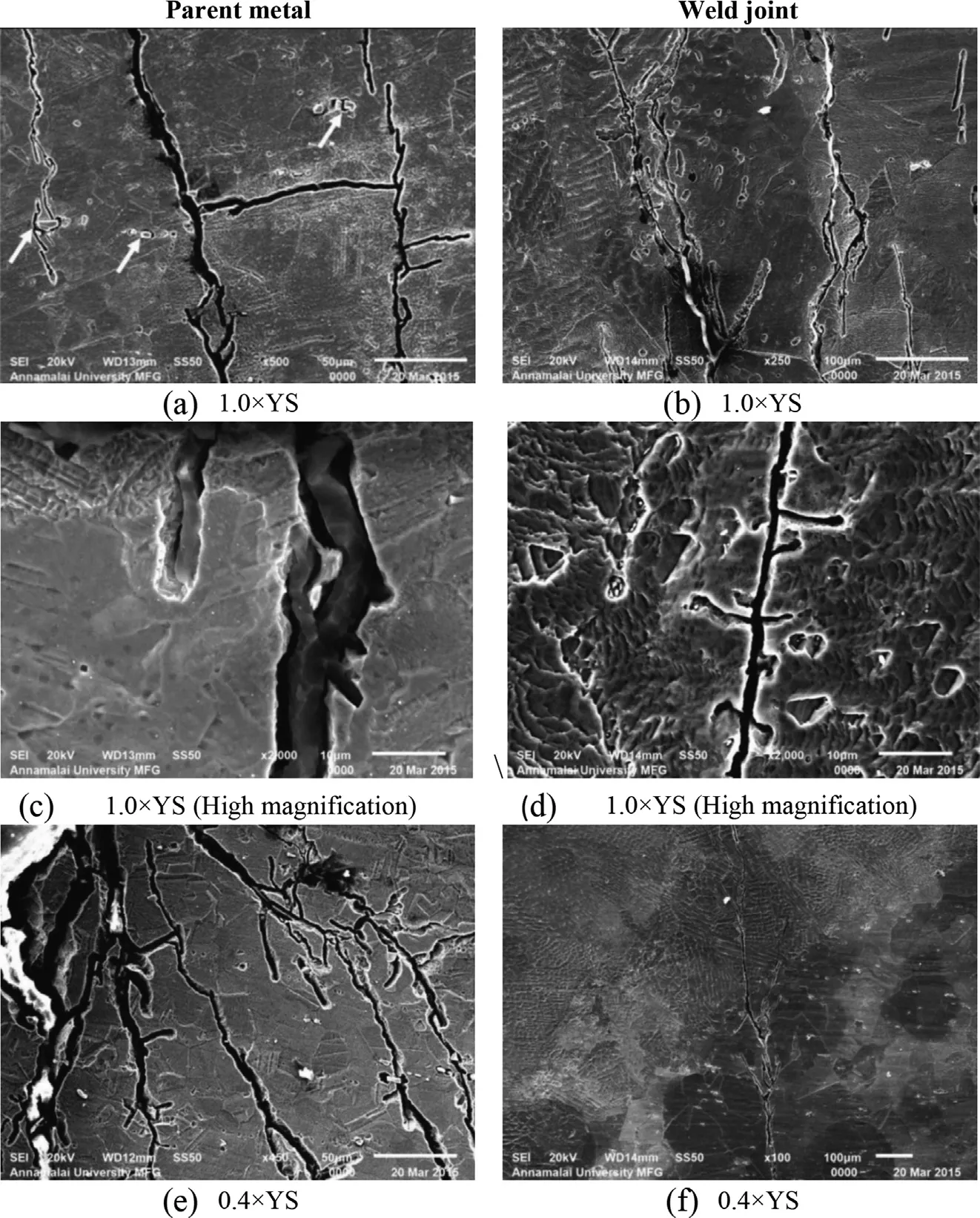
Fig. 11. Crack propagation characteristics in SCC specimen tested in boiling MgCl2solution at different applied stress levels.
In the weld joint the cracks initiate and propagate in the HAZ and fusion line/HAZ region, which are more susceptible to SCC, as shown in Fig. 11(b). The weld metal posses better resistance to SCC (refer Fig. 11(e) and (f)), however the presence of carbides results in initiation of the crack and these cracks gets interconnected to the main crack propagating in to the weld metal, as shown in Fig. 11(d).
The crack propagation micrograph of weld joint tested at lower applied stress is shown in Fig. 11(f), which reveals the initiation of crack in HAZ region and its transgranular propagation into the weld metal. However, no crack is observed in the weld metal of the joint tested at lower applied stress (refer Fig. 11(f)). The EDS spectra of fracture surfaces of parent metal and weld joint are shown in Fig. 12(a) and (b),respectively. The presence of chloride in the fracture surfaces confirms the interaction of chloride environment with the test specimens to cause SCC failure.
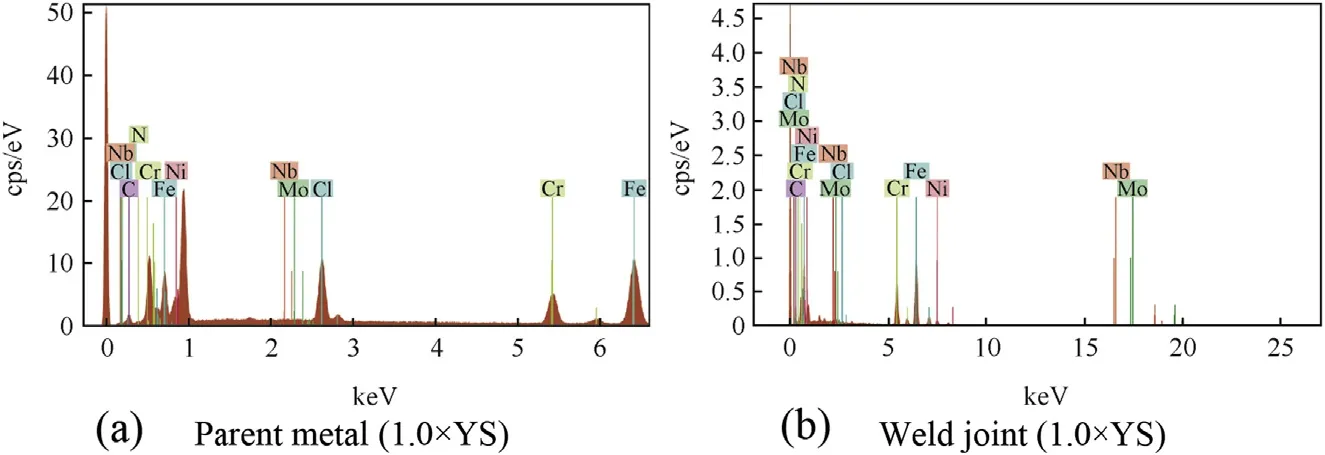
Fig. 12. EDS elemental analysis of fracture surface.
4. Discussion
The SCC was transgranular at all the stress levels i.e. SCC-dominated and stress-dominated regions, in parent metal and weld joint, which is characteristic of the higher steady elongation observed [20]. Out of the numerous mechanisms proposed to explain SCC behaviour the anodic path cracking mechanism (APC) belongs to the cracking of austenitic stainless steel in chloride environment [21]. The mechanism proposed by Nishimura for transgranular SCC is also valid for this work, where the entire cracking is based on a cyclic event of passive film formation and rupture, dealt elsewhere in detail [22,4]. The anodic metal dissolution and passive film formation at the crack tip result in dislocation pile-up at the crack tip, resulting in an increase in local stresses higher than the applied local stress at the vicinity of the crack tip. The film ruptures when the local stress exceeds a critical value and a crack propagates by exposing fresh metal to repeat the process. Such event of crack propagation was recorded as noise peaks in corrosion elongation curves until tss, resulting in steady state elongation (refer Fig. 13). The downward arrows indicate the critical stress at which the film ruptures and the upward arrows indicate the applied mean stress.
Inweldjoints,HAZisthemostsusceptibleregionto SCCdue to the metallurgical changes caused by weld thermal cycles. The oxalic acid etch test reveals a dual structure in the HAZ, confirming the deterioration in the corrosion resistance of the zone. Intheworstcase,theweldingresidualstresscanbesuperimposed and may be as high as yield strength of the material [15,4]. The microstructure examination of the SCC weld joints reveals that the failure occurred between the weld interface and HAZ, indicating the susceptibility of the zone to SCC. At lower applied stress(0.4×YS),thecracksare found toinitiate inthe HAZ and propagate in to the weld metal. The crack initiation sites are not observedintheweldmetalatlowerstress(referFig.11(f)),which confirms the enhanced pitting resistance of weld metal by Mo addition [15]. The cracks initiated in the weld metal at higher stress (refer Fig. 11(d)) are attributed to the local strain field around the interface of matrix and precipitates, caused by the misfit between matrix and precipitates.
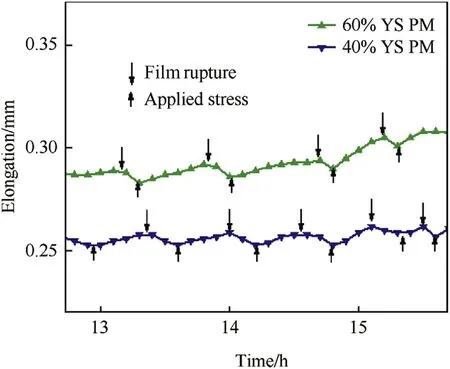
Fig. 13. Magnified view of serrations observed in corrosion elongation curves of constant load SCC specimens tested in boiling MgCl2solution.
5. Conclusions
1) The SCC resistance of Super 304H stainless steel is deteriorated by GTA welding due to the detrimental microstructural changes in the HAZ of the joint and the residual stresses caused by the weld thermal cycles.
2) Linear relationships are derived from the elongation rate and time to failure, from which it is possible to extrapolate and predict the time to failure from the corrosion elongation curves.
3) The stress corrosion cracking is transgranular in nature under all the test conditions, and the cracking mechanism is found to be anodic path cracking.
4) Molybdenum is found to improve the stress corrosion cracking resistance of weldmetal due to its enhanced resistance for pitting corrosion.
Acknowledgements
The authors wish to express their sincere thanks to M/s Mailam India Ltd, Pondicherry, India for providing the funds to carry out this research work through Mailam India Research (MIR) Fellowship, M/s Salzgitter Mannesmann Stainless Tubes Italia Srl, Italy for supplying the Super 304H tubes and Department of Science and Technology (DST-SERB), Government of India, for providing the stress corrosion cracking setup wide project no. SB/FTP/ETA-281/2012.
References
[1] Viswanathan R, Bakker W. Materials for ultra supercritical coal power plants—boiler materials: Part 1. J Mater Eng Perform 2001;10:81—95.
[2] Viswanathan R, Sarver J, Tanzosh JM. Boiler materials for ultrasupercritical coal power plants—steamside oxidation. J Mater Eng Perform 2006;15:255—74.
[3] Li Xin-mei, Zou Yong, Zhang Zhong Wen, Zou Zeng-da. Microstructure evolution of a novel Super304H steel aged at high temperatures. J Mater Trans 2010;51:305—9.
[4] Lu BT, Chen ZK, Luo JL, Patchett BM, Xu ZH. Pitting and stress corrosion cracking behavior in welded austenitic stainless steel. J ElectrochimActa 2005;50:1391—403.
[5] SaadAbouelazm A, El Mahallawi I, Abdel karim R, Rashad R. Failure investigation of secondary super-heater tubes in a power boiler. J Eng Fail Anal 2009;16:433—48.
[6] Antunes PD, Correa EO, Barbosa RP, Silva EM, Padilha AF, Guimaraes PM. Effect of weld metal chemistry on stress corrosion cracking behavior of AISI 444 ferritic stainless steel weldments in boiling chloride solution. J Mater Corros 2013;64:415—21.
[7] Pettersson CO, Boellinghaus T, Kannengiesser T. Corrosion testing of welds a review of methods. J Weld World 2007;51:79—106.
[8] Schvartzman Monica Maria de Abreu Mendonca, Quinan Marco Antonio Dutra, Campos Wagner Reis da Costa, Lima Luciana Iglesias Lourenco.Stress corrosion cracking of the AISI 316L stainless steel HAZ in a PWR nuclear reactor environment. J Weld Int 2011;25:15—23.
[9] Nage Deepashri D, Raja VS. Effect of nitrogen addition on the stress corrosion cracking behavior of 904 L stainless steel welds in 288°C deaerated water. J Corros Sci 2006;48:2317—31.
[10] Dietzel Wolfgang. Rising displacement stress corrosion cracking testing. J Metall Mater Trans A 2011;42:365—72.
[11] Gnanamoorthy JB. Corrosion of austenitic stainless steels in aqueous environments. Proc Indian Acad Sci—Chem Sci 1986;97:495—511.
[12] Prabha B, Sundaramoorthy P, Suresh S, Manimozhi S, Ravishankar B. Studies on stress corrosion cracking of Super 304H austenitic stainless steel. J Mater Eng Perform 2009;18:1294—9.
[13] Gao Yan, Zhang Chunlei, Xiong Xiahua, Zheng Zhijun, Zhu Min. Intergranular corrosion susceptibility of a novel Super 304H stainless steel. J Eng Fail Anal 2012;24:26—32.
[14] Lauro A, Mandina M. Welding and weldability of the super austenitic and super martensitic stainless steels. J Weld Int 2003;17:710—20.
[15] Kaneko M. Stress corrosion cracking of stainless steels. J Weld Int 2007;21:95—9.
[16] Suutala N. Effect of manganese and nitrogen on the solidification mode in austenitic stainless steel welds. J Metall Trans A 1982;13A:1982—2121.
[17] Vehovar L, Vehovar A, MetikosHukovic M, Tandler M. Investigation into the stress corrosion cracking of stainless steels alloyed with nitrogen. J Mater Corros 2002;53:316—27.
[18] Puiggali M, Desjardins D, Ajana L. A critical study of stress corrosion cracking testing methods for stainless steels in hot chloride media. J Corros Sci 1987;27:585—94.
[19] Nishimura R, Kudo K. Stress corrosion cracking of AISI 304 and AISI 316 austenitic stainless steels in HCl and H2SO4solutions prediction of time-to-failure and criterion for assessment of SCC susceptibility. J Corros 1989;45:308.
[20] Alyousif Osama M, Nishimura R. The stress corrosion cracking behavior of austenitic stainless steels in boiling magnesium chloride solutions. J Corros Sci 2007;49:3040—51.
[21] Lu BT, Qiao LJ, Luo JL, Gao KW. Role of hydrogen in stress corrosion cracking of austenitic stainless steels. J Philos Mag 2011;91:208—28.
[22] Nishimura Rokuro, Sulaiman Achmad, Maeda Yasuaki. Stress corrosion cracking susceptibility of sensitized type 316 stainless steel in sulphuric acid solution. J CorrosSci 2003;45:465—84.
* Corresponding author. Tel.: +91 9751014430 (mobile).
- Defence Technology的其它文章
- Gas tungsten arc welding of ZrB2
—SiC based ultra high temperature ceramic composites R.V. KRISHNARAO*, G. MADHUSUDHAN REDDY - Influence of tool pin profile on microstructure and corrosion behaviour of AA2219 Al
—Cu alloy friction stir weld nuggets Ch. VENKATA RAOa, G. MADHUSUDHAN REDDYb, K. SRINIVASA RAOa,* - Optimization of process parameters of aluminum alloy AA 2014-T6 friction stir welds by response surface methodology Ramanjaneyulu KADAGANCHIa, Madhusudhan Reddy GANKIDIb,*, Hina GOKHALEb
- Effect of hot-humid exposure on static strength of adhesive-bonded aluminum alloys
- Numerical modeling of friction stir welding using the tools with polygonal pins M. MEHTAa, G.M. REDDYb, A.V. RAOb, A. DEc,*
- Microstructure and pitting corrosion of shielded metal arc welded highnitrogen stainless steelRAFFI MOHAMMEDa, G. MADHUSUDHAN REDDYb, K. SRINIVASA RAOa,*

